Abstract
Purpose
A multicenter phase II study was conducted to assess the efficacy of rituximab, methotrexate, procarbazine, and vincristine (R-MPV) followed by consolidation reduced-dose whole-brain radiotherapy (rdWBRT) and cytarabine in primary CNS lymphoma.
Patients and Methods
Patients received induction chemotherapy with R-MPV (five to seven cycles); those achieving a complete response (CR) received rdWBRT (23.4 Gy), and otherwise, standard WBRT was offered (45 Gy). Consolidation cytarabine was given after the radiotherapy. The primary end point was 2-year progression-free survival (PFS) in patients receiving rdWBRT. Exploratory end points included prospective neuropsychological evaluation, analysis of magnetic resonance imaging (MRI) white matter changes using the Fazekas scale, and evaluation of the apparent diffusion coefficient (ADC) as a prognostic factor.
Results
Fifty-two patients were enrolled, with median age of 60 years (range, 30 to 79 years) and median Karnofsky performance score of 70 (range, 50 to 100). Thirty-one patients (60%) achieved a CR after R-MPV and received rdWBRT. The 2-year PFS for this group was 77%; median PFS was 7.7 years. Median overall survival (OS) was not reached (median follow-up for survivors, 5.9 years); 3-year OS was 87%. The overall (N = 52) median PFS was 3.3 years, and median OS was 6.6 years. Cognitive assessment showed improvement in executive function (P < .01) and verbal memory (P < .05) after chemotherapy, and follow-up scores remained relatively stable across the various domains (n = 12). All examined MRIs (n = 28) displayed a Fazekas score of ≤ 3, and no patient developed scores of 4 to 5; differences in ADC values did not predict response (P = .15), PFS (P = .27), or OS (P = .33).
Conclusion
R-MPV combined with consolidation rdWBRT and cytarabine is associated with high response rates, long-term disease control, and minimal neurotoxicity.
INTRODUCTION
The optimal treatment strategy for newly diagnosed primary CNS lymphoma (PCNSL) remains controversial.1–4 There is wide acceptance that methotrexate-based chemotherapy achieves high response rates and improves survival in this disease, but the optimal chemotherapy regimen and the role of whole-brain radiotherapy (WBRT) are less well defined. Most studies have suggested that regimens using WBRT result in prolonged progression-free survival (PFS), although improvements in overall survival (OS) remain to be demonstrated.5–9 A major deterrent to the use of WBRT is the high risk for late-delayed neurotoxicity in patients who achieve long-term disease control.10,11 This complication manifests as progressive cognitive deterioration that often leads to severe dementia and death. In an attempt to minimize neurotoxicity, some investigators have opted to defer radiotherapy until tumor progression, although disease control may be compromised.12–14
In this multicenter phase II study, we sought to improve outcomes in newly diagnosed PCNSL through two innovative approaches: (1) incorporation of rituximab, a humanized monoclonal antibody to CD20 shown to increase response rates and prolong survival in non-Hodgkin lymphoma15; and (2) use of reduced-dose WBRT (rdWBRT) as consolidation treatment in patients with radiographic complete response (CR), with the goal of improving disease control as well as decreasing neurotoxicity, in an attempt to achieve the optimal balance between antilymphoma activity and deleterious effects on normal brain tissue.
The results from the initial phase of this trial have been reported previously,16 demonstrating feasibility and short-term outcomes of this chemotherapy regimen in 30 patients and describing rituximab CSF pharmacokinetics. We are now reporting the final results of this study including 2-year PFS in patients receiving rdWBRT (primary end point), long-term outcomes, and neurotoxicity in all 52 enrolled patients, including correlative neurocognitive function and magnetic resonance imaging (MRI) biomarkers.
PATIENTS AND METHODS
Patients
Immunocompetent patients with newly diagnosed PCNSL were eligible for this prospective phase II trial, conducted at five institutions. Inclusion criteria consisted of age ≥ 18 years; histologic confirmation of PCNSL; negative HIV serology; no evidence of systemic non-Hodgkin lymphoma as demonstrated by computed tomography scan of the chest, abdomen, and pelvis and bone marrow aspirate and biopsy; leukocytes ≥ 4,000/μL; platelets ≥ 100,000/μL; and creatinine ≤ 1.5 mg/dL or creatinine clearance ≥ 50 cm3/min/1.73 m2. Exclusion criteria consisted of any other active primary malignancy (exception: basal cell carcinoma of the skin and cervical carcinoma in situ), preexisting immunodeficiency, and prior treatment for PCNSL. There were no eligibility restrictions based on performance status. Pretreatment evaluations included baseline ophthalmologic examination to assess for ocular involvement, lumbar puncture to assess for leptomeningeal involvement, baseline MRI of the brain within 14 days before treatment start, and hepatitis B screening. The study was approved by the institutional review board of all participating institutions and all patients or guardians signed an institutional review board–approved written informed consent.
Treatment and Response Evaluation
After registration, five 14-day cycles of induction chemotherapy with rituximab, methotrexate, procarbazine, and vincristine (R-MPV) were given as follows: day 1, rituximab 500 mg/m2; day 2, methotrexate 3.5 g/m2 (over 2 hours), vincristine 1.4 mg/m2; days 1 through 7, procarbazine 100 mg/m2/d (odd cycles only). Hydration and leucovorin rescue were given as per institutional guidelines. There was no methotrexate dose adjustment according to creatinine clearance. Owing to grade 4/5 neutropenia in two of the first five patients enrolled, all subsequent patients received prophylactic filgrastim (5 μg/kg/d subcutaneously for 3 to 5 days) after each cycle. Patients with CSF evidence of malignancy received 12 mg of intra-Ommaya methotrexate between cycles.
Response was assessed using International PCNSL Collaborative Group criteria, based on imaging, corticosteroid use, and CSF cytology and slit lamp examination in case of CSF or ocular involvement.17 Patients who achieved a CR after five cycles received rdWBRT (23.40 Gy in 1.8-Gy fractions × 13) 3 to 5 weeks after chemotherapy completion (Fig 1). Opposed lateral radiation fields were used to include the whole brain down to the level of C2 (so-called German helmet shape) and excluded the anterior two thirds of the orbit. Patients who obtained a partial response (PR) after five cycles received two additional cycles of R-MPV, and if a CR was achieved, then rdWBRT was given as previously described. Otherwise, a standard dose of WBRT (45 Gy in 25 fractions) was offered. Patients with stable disease or progressive disease were offered standard WBRT. Patients with ocular involvement were irradiated without orbital shielding to the full dose of 23.40 Gy (patients in CR) or to a dose of 36 Gy (patients with less than a CR).
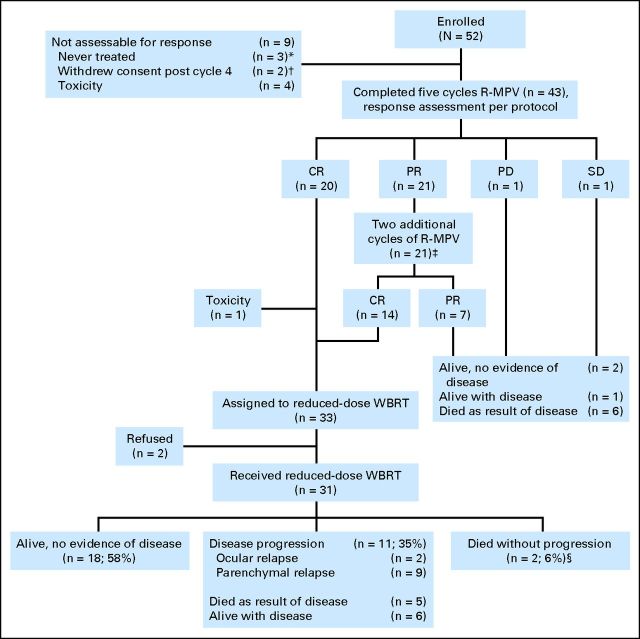
Fig 1.
CONSORT diagram. (*) One patient was ineligible because of evidence of systemic non-Hodgkin lymphoma, and two had rapid progression of disease before initiation of any protocol therapy. (†) One patient had a CR and received reduced-dose WBRT off protocol; the other had a PR and received standard-dose WBRT. (‡) Includes one patient who developed renal impairment and only received six cycles of treatment. He achieved a CR, completed the rest of treatment per protocol, and is alive and progression-free 4.5 years later. (§) One patient died as a result of toxicity and one as a result of unknown causes. CR, complete response; PD, progressive disease; PR, partial response; R-MPV, rituximab, methotrexate, procarbazine, and vincristine; SD, stable disease; WBRT, whole-brain radiotherapy.
After radiotherapy, all patients received two consolidation high-dose cytarabine cycles (one cytarabine cycle = 28 days), given at 3 g/m2/d on days 1 and 2 of each cycle. Prophylactic filgrastim/pegfilgrastim was given after each cycle of cytarabine.
Statistics
The primary end point was 2-year PFS in patients treated with rdWBRT. The target accrual was 30 evaluable patients in CR who received rdWBRT, allowing for an estimation of the 95% CI at ± 18%. Enrollment was to continue until this target was achieved (approximately 50 patients). Secondary end points included objective response rate to R-MPV, overall response rate, OS, and neurocognitive outcome. Time to an event was calculated from the initiation of chemotherapy until death (OS), progression or death (PFS), or date of last follow-up for patients without an event. Survival was assessed by the Kaplan and Meier-method; potential prognostic factors were compared using the log-rank test. The Wilcoxon rank sum test was used to evaluate whether the measures of apparent diffusion coefficient (ADC) varied by best response after five R-MPV cycles. Logistic regression was used to further address whether these variables were associated with CR after five cycles. Neuropsychological test results were summarized using descriptive statistics, and t tests were used for longitudinal comparisons.
Exploratory Neuropsychological and Neuroimaging Correlates
Prospective comprehensive neuropsychological evaluations18–20 were offered to patients treated at Memorial Sloan-Kettering Cancer Center (MSKCC) and performed by a neuropsychologist or a research assistant under direct supervision. Evaluations were conducted at baseline, after induction chemotherapy (before rdWBRT), and at 6-month intervals after completion of rdWBRT and cytarabine. The present analysis reports on patients who remained progression-free and underwent evaluations up to 48 months after treatment. Raw cognitive test scores were compared with published normative values according to age and education and converted into z-scores to characterize the presence and severity of cognitive difficulties. Composite scores were calculated by adding the z-scores for each test within a cognitive domain and dividing the sum by the number of tests.18 Three cognitive domains were evaluated: executive (Trail Making Test; Brief Test of Attention), verbal memory (Hopkins Verbal Learning Test), and motor speed (Grooved Pegboard Test). Quality of life was examined using the Functional Assessment of Cancer Therapy–Brain Cancer, and mood was assessed with the Beck Depression Inventory. To further evaluate the biologic effects of the rdWBRT in the patients who completed serial cognitive evaluations, baseline and follow-up MRIs conducted within 3 months of each cognitive assessment were analyzed for the development of white matter changes. Fluid attenuated inversion recovery MRI sequences were reviewed for white matter changes and rated as per the modified Fazekas scale21–23 as follows: grade 0, no white matter change; grade 1, minimal patchy white matter foci; grade 2, start of confluence of white matter disease; grade 3, large confluent areas; grade 4, confluence of white matter changes with cortical and subcortical involvement; grade 5, diffuse leukoencephalopathy with widespread and diffuse white matter disease.
In a separate post hoc analysis, the prognostic impact of diffusion MRI parameters was evaluated. All available baseline MRIs were analyzed using commercially available software (FuncTool v4.0, GE Healthcare, Little Chalfont, United Kingdom) to determine ADC parameters by processing diffusion-weighted images (DWI), guided by postcontrast axial images, as previously described.24 Areas of contrast-enhancing tumor were identified from axial images, DWI scans were aligned to the same location, and ADC maps were calculated on a voxel-by-voxel basis for each region of interest. In MRIs with multiple enhancing lesions, regions of interest were placed around all areas of enhancement, producing ADC measurements derived from the entire enhancing tumor burden. The mean, 25th percentile, and minimum ADC values for all regions of interest were calculated for all transaxial sections for each patient. Patient outcome (response and PFS) was compared by ADC measurements.
RESULTS
Patient Characteristics
From October 2002 to February 2009, 52 patients were enrolled (22 women and 30 men). The median age was 60 years (range, 30 to 79 years), with a total of 27 (52%) ≥ 60 years of age. At baseline, median Karnofsky performance score (KPS) was 70 (range, 50 to 100), five patients had ocular involvement, and 11 (21%) had positive CSF cytology. The MSKCC recursive partitioning analysis (RPA) prognostic class was as follows: 10 patients (19%), class 1; 24 patients (46%), class 2; 14 patients (27%), class 3; in four patients (8%), the RPA class was missing.25
Response to Induction Chemotherapy
A total of 43 patients were evaluable for response as per protocol (Fig 1). After five cycles of chemotherapy, 20 patients (47%) achieved a CR and 21 (49%) achieved a PR. With two additional cycles of R-MPV, 14 of the patients in PR achieved a CR. Therefore, at the end of induction chemotherapy, 34 patients (79%) were in CR and seven (16%) were in PR, for an objective response rate (ORR) of 95% (41 of 43). Among patients ≥ 60 years of age, the ORR was 100% (22 of 22).
Among the 34 patients eligible for rdWBRT, one patient died from febrile neutropenia after the last cycle of chemotherapy, and two patients refused radiation. Therefore, 31 patients received rdWBRT. The median age of these patients was 59 years (range, 30 to 79 years), and 15 (48%) were age ≥ 60 years.
PFS and OS With rdWBRT
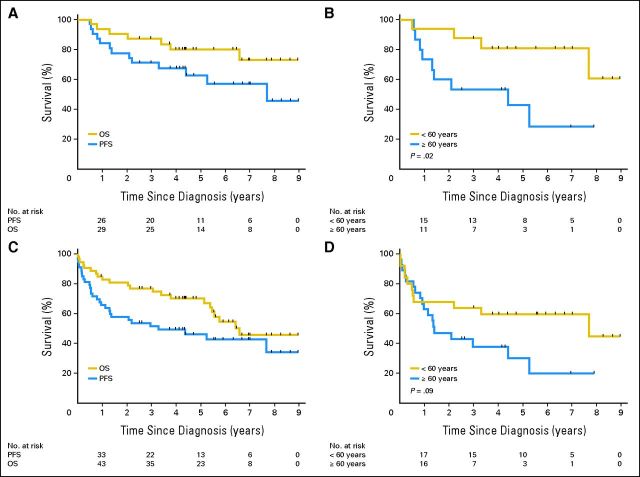
Median follow-up was 5.9 years for survivors (Fig 1). The median PFS in the cohort of patients who received rdWBRT (n = 31) was 7.7 years, and the median OS has not been reached (Fig 2A). The 2-year PFS (primary study end point) was 77% (95% CI, 63% to 92%). The 5-year OS was 80% (95% CI, 66% to 94%). Strikingly, median PFS was not reached in patients younger than 60 years and was 4.4 years in patients ≥ 60 years (P = .02; Fig 2B). Median OS was not reached in either patients younger than 60 years or those aged ≥ 60 years (P = .17).
Fig 2.
Progression-free survival (PFS) and overall survival (OS). (A) PFS and OS in patients who received reduced-dose radiotherapy (n = 31). The 1-, 2-, and 3-year PFS was 84% (95% CI, 71% to 97%), 77% (95% CI, 63% to 92%), and 71% (95% CI, 55% to 87%), respectively. The 1-, 2-, 3-, and 5-year OS was 94% (95% CI, 85% to 100%), 90% (95% CI, 80% to 100%), 87% (95% CI, 75% to 99%), and 80% (95% CI, 66% to 94%), respectively. (B) PFS by age in patients who received reduced-dose radiotherapy (n = 31). The 1-, 2-, and 3-year PFS for patients younger than 60 years was 94% (95% CI, 82% to 100%), 94% (95% CI, 82% to 100%), and 88% (95% CI, 71% to 100%), respectively. The 1-, 2-, and 3-year PFS for patients ≥ 60 years of age was 73% (95% CI, 51% to 96%), 60% (95% CI, 35% to 85%), and 53% (95% CI, 28% to 79%), respectively. (C) Intent-to-treat PFS and OS in entire cohort (n = 52). The 1-, 2-, and 3-year PFS was 65% (95% CI, 52% to 78%), 57% (95% CI, 44% to 71%), and 51% (95% CI, 38% to 65%), respectively. The 1-, 2-, 3-, and 5-year OS was 85% (95% CI, 75% to 94%), 81% (95% CI, 70% to 91%), 77% (95% CI, 65% to 88%), and 70% (95% CI, 57% to 83%), respectively. (D) Intent-to-treat PFS by age in entire cohort (n = 52). The 1-, 2-, and 3-year PFS for patients younger than 60 years was 68% (95% CI, 50% to 86%), 64% (95% CI, 45% to 83%), and 64% (95% CI, 45% to 83%), respectively. The 1-, 2-, and 3-year PFS for patients ≥ 60 years of age was 63% (95% CI, 45% to 81%), 47% (95% CI, 28% to 66%), and 38% (95% CI, 19% to 57%), respectively.
Intent-to-Treat PFS and OS
Survival of the entire cohort (n = 52) was examined in an intent-to-treat analysis. At median follow-up of 5.6 years for survivors, the median PFS and OS were 3.3 and 6.6 years, respectively (Fig 2C). Median PFS was 7.7 years in patients younger than 60 years and 1.4 years in those ≥ 60 years of age (Fig 2D), although this difference did not reach statistical significance (P = .09). Median OS was not reached in patients younger than 60 years and was 5.5 years in those aged ≥ 60 years (P = .14). Of the 22 assessable patients aged ≥ 60 years, nine (40%) are alive without disease progression, 10 (45%) have had disease progression, and three (14%) died without progression (toxicity, n = 2; unknown, n = 1). No deaths from neurotoxicity have been reported. The favorable regimen performance in patients expected to have a poor prognosis (elderly and low KPS) abrogated the predictive value of the MSKCC RPA class, and no differences were seen in PFS or OS according to methotrexate pharmacokinetic parameters.26
Acute Toxicity
The median number of R-MPV cycles received was five (range, 0 to seven). As previously described, neutropenia was the main dose-limiting toxicity.16 Four patients came off study for toxicity before completion of five cycles of R-MPV; one had renal impairment (grade 3) after cycle 1, one had ileus (grade 3) after cycle 1, another developed febrile neutropenia (grade 5) after cycle 2, and one had a perforated diverticulum (grade 4) after cycle 4. Despite filgrastim, one patient had grade 5 neutropenia after the fifth cycle of R-MPV, and one patient had grade 5 febrile neutropenia/pneumonitis after the second cycle of cytarabine. Therefore, the total number of deaths possibly/probably related to toxicity was three (6%).
Exploratory Neuropsychological and Imaging Correlates
Among 31 patients who received rdWBRT, 12 patients (median age, 58 years, including three patients age ≥ 60 years) were progression-free and completed neuropsychological evaluations up to 48 months. At baseline, cognitive impairment was present in several domains. After induction chemotherapy, there was a significant improvement in executive (P < .01) and verbal memory (P < .05). There was no evidence of significant cognitive decline during the follow-up period, except for motor speed (P < .05). Minor fluctuations were observed on memory performance over time. There was no evidence of depressed mood, and self-reported quality of life remained stable during the follow-up period (Table 1).
Table 1.
Neuropsychological Test Results in Patients Treated With Reduced-Dose Whole-Brain Radiotherapy
| Test | Baseline (n = 12) | Post R-MPV (n = 11) | 1 Year (n = 12) | 2 Years (n = 12) | 3 Years (n = 12) | 4 Years (n = 12) |
|---|---|---|---|---|---|---|
| Executive function | ||||||
| TMTA | ||||||
| Mean z-score* | −1.2 | −0.3 | −0.3 | 0.2 | −0.03 | −0.1 |
| SD | 1.2 | 1.2 | 1 | 0.9 | 0.9 | 1.1 |
| 95% CI | −1.9 to −0.5 | −1 to 0.4 | −0.9 to 0.3 | −0.3 to 0.7 | −0.5 to 0.5 | −0.7 to 0.5 |
| TMTB | ||||||
| Mean z-score* | −1.2 | −0.7 | −0.4 | −0.3 | −0.3 | −0.4 |
| SD | 1.0 | 1.3 | 0.8 | 1.2 | 0.8 | 1.2 |
| 95% CI | −1.8 to −0.6 | −1.5 to 0.1 | −0.9 to 0.1 | −1 to 0.4 | −0.8 to 0.2 | −1.1 to 0.3 |
| BTA | ||||||
| Mean z-score* | −1.6 | −0.6 | −0.2 | −0.2 | −0.3 | 0.1 |
| SD | 1.5 | 1.6 | 0.8 | 1.1 | 1.0 | 0.8 |
| 95% CI | −2.4 to −0.8 | −1.5 to 0.3 | −0.7 to 0.3 | −0.8 to 0.4 | −0.9 to 0.3 | −0.4 to 0.6 |
| Memory | ||||||
| HVLT-R-TL | ||||||
| Mean z-score* | −2.0 | −1.5 | −1.2 | −1.3 | −0.8 | −1.0 |
| SD | 0.8 | 1.3 | 1.2 | 1.4 | 1 | 1.4 |
| 95% CI | −2.5 to −1.5 | −2.3 to −0.7 | −1.9 to −0.5 | −2.1 to −0.5 | −1.4 to −0.2 | −1.8 to −0.2 |
| HVLT-R-DEL | ||||||
| Mean z-score* | −2.3 | −1.5 | −1.3 | −1.5 | −0.9 | −1.3 |
| SD | 1.1 | 1.4 | 1.5 | 1.3 | 1.4 | 1.6 |
| 95% CI | −2.9 to −1.7 | −2.3 to −0.7 | −2.1 to −0.5 | −2.2 to −0.8 | −1.7 to −0.1 | −2.2 to −0.4 |
| HVLT-R-DI | ||||||
| Mean z-score* | −1.3 | −0.9 | −0.6 | −0.9 | −0.4 | −0.7 |
| SD | 1.1 | 1.3 | 1.1 | 1.1 | 1 | 1.3 |
| 95% CI | −1.9 to −0.7 | −1.7 to −0.1 | −1.2 to 0 | −1.5 to −0.3 | −1 to 0.2 | −1.4 to 0 |
| Motor | ||||||
| GPT-D | ||||||
| Mean z-score* | −1.7 | −1.6 | −1.1 | −0.9 | −1.3 | −1.5 |
| SD | 1.1 | 1.1 | 1.1 | 0.8 | 0.9 | 1.1 |
| 95% CI | −2.3 to −1.1 | −2.3 to −0.9 | −1.7 to −0.5 | −1.4 to −0.4 | −1.8 to −0.8 | −2.1 to −0.9 |
| GPT-ND | ||||||
| Mean z-score* | −1.3 | −1.5 | −0.8 | −1 | −1.1 | −1.7 |
| SD | 1.0 | 1.0 | 1.2 | 1 | 1 | 1.2 |
| 95% CI | −1.9 to −0.7 | −2.1 to −0.9 | −1.5 to −0.1 | −1.6 to −0.4 | −1.7 to −0.5 | −2.4 to −1 |
| Mood/quality-of-life scales | ||||||
| BDI | ||||||
| Mean raw score | 8.2 | 8.2 | 6.1 | 5.8 | 6.6 | 5.1 |
| SD | 4.8 | 6 | 7.7 | 4.9 | 6 | 4.9 |
| 95% CI | 5.5 to 10.9 | 4.7 to 11.7 | 1.7 to 10.5 | 3 to 8.6 | 3.2 to 10 | 2.3 to 7.9 |
| FACT-BR | ||||||
| Mean raw score | 129 | 142 | 157 | 154 | 157 | 156 |
| SD | 22 | 26 | 24 | 15 | 21 | 18 |
| 95% CI | 116 to 142 | 127 to 158 | 144 to 171 | 145 to 163 | 145 to 169 | 146 to 166 |
Abbreviations: BDI, Beck Depression Inventory; BTA, Brief Test of Attention; FACT-BR, Functional Assessment of Cancer Therapy–Brain Cancer; GPT-D, Grooved Pegboard Test–Dominant Hand; GPT-ND, Grooved Pegboard Test–Non-Dominant Hand; HVLT-R-DEL, Hopkins Verbal Learning Test–Revised–Delayed Recall; HVLT-R-DI, Hopkins Verbal Learning Test–Revised–Discrimination Index; HVLT-R-TL, Hopkins Verbal Learning Test–Revised–Total Learning; R-MPV, rituximab, methotrexate, procarbazine, and vincristine; SD, standard deviation; TMTA, Trail Making Test Part A; TMTB, Trail Making Test Part B.
z-scores ≤ 1.5 SD below the mean represent impairment.
Analysis of white matter changes in patients with available scans (Table 2) showed that, at baseline, five of 12 patients had grade ≥ 2 white matter disease, which decreased to one of 12 after chemotherapy. Throughout follow-up, the proportion of patients with white matter changes increased; at the 4-year evaluation, five patients displayed grade 2 and two patients grade 3 white matter changes. No patient developed Fazekas scores of 4 or 5 throughout the evaluated period.
Table 2.
White Matter Changes in Patients Undergoing Neuropsychological Evaluation After Receiving Reduced-Dose Whole-Brain Radiotherapy (Fazekas Scale; n = 12)20
| Grade | Baseline | Post R-MPV (prior to rdWBRT) | 1 Year | 2 Years | 3 Years | 4 Years |
|---|---|---|---|---|---|---|
| 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| 1 | 5 | 11 | 8 | 8 | 5 | 5 |
| 2 | 3 | 1 | 3 | 3 | 5 | 5 |
| 3 | 2 | 0 | 1 | 1 | 2 | 2 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 | 0 | 0 |
NOTE. Grades are defined as follows: grade 0, no white matter change; grade 1, minimal patchy white matter foci; grade 2, start of confluence of white matter disease; grade 3, large confluent areas; grade 4, confluence of white matter changes with cortical and subcortical involvement; grade 5, leukoencephalopathy.
Abbreviations: rdWBRT, reduced-dose whole-brain radiotherapy; R-MPV, rituximab, methotrexate, procarbazine, and vincristine.
A total of 28 patients had DWI scans available for review and correlation with efficacy end points. There was no association between higher baseline ADC and CR (Table 3 and Appendix Table A1, online only).24 Similarly, there were no differences in PFS (P = .27) or OS (P = .33) by baseline ADC. The 2-year PFS for patients below and above the median ADC was 86% (95% CI, 67% to 100%) and 57% (95% CI, 31% to 83%), respectively (P = .27).
Table 3.
Correlation of Baseline Apparent Diffusion Coefficients and Response (n = 28)
| Variable | Overall (n = 28) | Response After 5 Cycles of R-MPV |
P* | |
|---|---|---|---|---|
| CR (n = 14) | No CR (n = 14) | |||
| Overall mean of ADC means | 897 × 10−6 mm2/s | 675 × 10−6 mm2/s | 1,120 × 10−6 mm2/s | .15 |
| Overall mean of ADC minimums | 200 × 10−6 mm2/s | 162 × 10−6 mm2/s | 238 × 10−6 mm2/s | .69 |
| Overall mean of ADC 25th percentiles | 691 × 10−6 mm2/s | 531 × 10−6 mm2/s | 851 × 10−6 mm2/s | .43 |
Abbreviations: ADC, apparent diffusion coefficients; CR, complete response; R-MPV, rituximab, methotrexate, procarbazine, and vincristine.
Comparison is for CR versus no CR by Wilcoxon test.
DISCUSSION
In this prospective phase II study, the R-MPV induction chemotherapy followed by consolidation rdWBRT and cytarabine was found to be feasible and effective. Response rates were high (79% CR), allowing a large proportion of patients to receive rdWBRT. Those patients achieved durable disease control (2-year PFS, 77%), associated with favorable neurocognitive outcomes. Although comparison across different trials is challenging, the ORR (95%), intent-to-treat PFS (median, 3.3 years) and most notably intent-to-treat OS (median, 6.6 years) observed in this trial are among the highest reported in any study of PCNSL to date (Table 4), surpassing the survival estimates of our preliminary report16 and comparing favorably to our previous study of MPV-A (without rituximab) using standard doses of WBRT (45 Gy), which achieved a CR rate of 56% and median OS of 4.2 years.9 The current regimen performance in elderly patients (≥ 60 years), a traditionally poor prognosis group (OS, 1 to 1.5 years),39–42 was particularly striking, with an intent-to-treat median OS of 5.5 years.
Table 4.
Selected High-Dose Methotrexate Trials in Primary CNS Lymphoma
| Reference | Regimen | No. of Patients | Median PFS (months) | Median OS (months) |
|---|---|---|---|---|
| Regimens with WBRT | ||||
| DeAngelis (1992)27 | MTX 1 g/m2, IT MTX, cytarabine, dexamethasone, WBRT | 31 | 41 | 43 |
| Glass (1994)28 | MTX 3.5 g/m2, WBRT | 25 | 32* | 33 |
| Abrey (2000)9 | MTX 3.5 g/m2, pcb, vinc, IT MTX, cytarabine, WBRT | 52 | 129* | 51 |
| DeAngelis (2002)29 | MTX 2.5 g/m2, pcb, vinc, IT MTX, WBRT | 98 | 24 | 37 |
| Bessell (2002)30 | MTX 1.5 g/m2, cyclophos, dox, vinc, dexamethasone, BCNU, cytarabine, WBRT | 57 | NA | 40 |
| Poortmans (2003)31 | MTX 3 g/m2, teniposide, BCNU, methylpred, IT MTX, cytarabine, hydrocortisone, WBRT | 52 | NA | 46 |
| Omuro (2005)32 | MTX 1 g/m2, pcb, thiotepa, IT MTX, WBRT | 17 | 18 | 32 |
| Korfel (2005)33 | MTX 1.5 g/m2, BCNU, pcb, dexamethasone, IT MTX ± idarubicin/ifos or cytarabine ± WBRT | 56 | 10† | 12 |
| Ferreri (2006)34 | MTX 3.5 g/m2, cytarabine, idarubicin, thiotepa, WBRT | 41 | 13‡ | 15 |
| Ferreri (2009)35 | MTX 3.5 g/m2, WBRT | 40 | 5‡ | 10 |
| or | ||||
| MTX 3.5 g/m2, cytarabine, WBRT | 39 | 9‡ | 31 | |
| Regimens without WBRT | ||||
| Herrlinger (2002)36 | MTX 8 g/m2 | 37 | 10 | 25 |
| Pels (2003),8 Pels (2009)37 | MTX 5 g/m2, vinc, ifos, cyclophos, vindesine, dexamethasone, IT MTX, cytarabine, prednisolone | 65 | 21† | 54 |
| or | ||||
| MTX 5 g/m2, vinc, ifos, cyclophos, vindesine, dexamethasone | 17 | 9† | 30 | |
| Batchelor (2005)7 | MTX 8 g/m2 | 25 | 13 | 55 |
| Wieduwilt (2012)38 | MTX 8 g/m2, tmz, ritux, etop, cytarabine | 31 | 24 | 66 |
| Randomized trial of MTX ± WBRT | ||||
| Thiel (2010)5 | MTX 4 g/m2 ± ifos + WBRT | 154 | 18 | 32 |
| or | ||||
| MTX 4 g/m2 ± ifos | 164 | 12 | 37 | |
| Reduced-dose WBRT | ||||
| Morris (2013; current study) | MTX 3.5 g/m2, ritux, vinc, pcb, cytarabine, reduced-dose WBRT | 52 | 40 | 79 |
Abbreviations: BCNU, carmustine; cyclophos, cyclophosphamide; dox, doxorubicin; EFS, event-free survival; etop, etoposide; Ifos, ifosfamide; IT, intrathecal; methylpred, methylprednisolone; MTX, methotrexate; NA, not available; OS, overall survival; pcb, procarbazine; PFS, progression-free survival; ritux, rituximab; tmz, temozolomide; TTP, time to progression; vinc, vincristine; WBRT, whole-brain radiotherapy.
Time to progression in responding patients.
Time to progression.
Failure-free survival.
The effectiveness of the induction chemotherapy was an important factor contributing to these favorable results. In comparison with previous MPV regimen studies, a few modifications were incorporated, including increased number of methotrexate cycles and addition of rituximab. Increasing the number of cycles has been a recent trend in PCNSL,7,38 which not only optimizes cytoreduction in preparation for consolidation treatments, but also exposes more aggressive disease, resulting in improved selection of patients for response-adapted strategies. The incorporation of rituximab into standard treatment regimens for PCNSL is an ongoing focus of investigation. As demonstrated in our patients,16 the CSF penetration of this drug is poor, but responses have been reported in recurrent CNS lymphoma with the use of single-agent intravenous rituximab.43 Other studies have since incorporated rituximab into PCNSL chemotherapy regimens,38,42 but the individual contribution of this drug to efficacy or toxicity cannot be determined; ongoing randomized trials will address this question.
The mechanisms underlying radiation-related neurotoxicity are poorly understood and may involve tissue oxidative stress, vasculopathy, demyelination, and depletion of progenitor oligodendroglial cells and neural stem cells.44 Such effects are dose-dependent and cumulative over time, making long-term survivors most vulnerable.10 Given such risks, there has been an understandable shift toward omission of WBRT from initial therapy. However, chemotherapy-only regimens tend to achieve shorter PFS (9 to 22 months), in comparison with regimens using WBRT (PFS, 18 to 41 months), although differences in survival are not apparent (Table 4). This trend was also observed in a randomized trial5 investigating chemotherapy (methotrexate with or without ifosfamide) alone or in combination with WBRT (45 Gy). The median PFS was 18 months in the WBRT arm and 12 months in the chemotherapy-only arm (P = .14); the median OS was 32 months (95% CI, 26 to 39 months) versus 37 months (95% CI, 28 to 47 months), respectively (P = .71). Unfortunately, neuropsychological evaluations are rarely included in PCNSL trials. Baseline and post–R-MPV neuropsychological evaluation in our patients suggest that PCNSL itself has deleterious effects on cognition, which improves with chemotherapy, but not to premorbid functioning levels. Relapsing PCNSL may therefore cause further cognitive decline, which can be aggravated by salvage treatments; developing treatments that improve PFS remains of high interest. Our regimen provides a treatment alternative that prolongs PFS and OS, without compromising neurocognitive function.
This study has a number of limitations. R-MPV has not been tested without WBRT, and it is unknown whether this regimen could achieve similar efficacy alone. The aggregate results of the neurocognitive and quality-of-life assessments are reassuring and far superior to historical controls using full-dose WBRT.22 However, a few long-term survivors developed new white matter changes on MRI, suggesting there may be some long-term effects from rdWBRT; studies to identify the patients at greatest risk for neurotoxicity are clearly needed. Although our intent-to-treat population was relatively large, cognitive testing is inherently restricted to the smaller number of patients who are progression-free and achieve long-term survival; results must therefore be confirmed in larger studies. Finally, our evaluation of imaging biomarkers to predict outcome was performed post hoc. Although we analyzed a larger number of patients than reported in two previous studies,24,38 we were unable to confirm a prognostic role for diffusion MRI parameters. This could reflect the low number of failures in our trial, rather than a lack of ADC prognostic implications per se.
In conclusion, therapeutic options for PCNSL have been expanding over the years; patients are surviving longer, and there is reason for optimism. Our study adds to the existing therapeutic arsenal for newly diagnosed patients, providing an alternative treatment option that could potentially benefit patients of all ages. Several other promising strategies are currently being tested, including the use of higher methotrexate doses as well as high-dose chemotherapy with stem-cell transplantation in young patients.45,46 To confirm and build on our results, the Radiation Therapy Oncology Group (RTOG) is conducting a randomized study (RTOG 1114) comparing the R-MPV regimen with or without reduced-dose WBRT. That study will include prospective neuropsychological evaluation, collection of neuroimaging, and evaluation of other potential biomarkers predicting efficacy and neurotoxicity, which could ultimately guide the individualization of treatment choices to achieve optimal outcomes.
Glossary Terms
- Rituximab:
A monoclonal antibody therapy that is indicated for relapsed or refractory low-grade or follicular, CD20+, B-cell non-Hodgkin's lymphoma.
Appendix
Table A1.
Logistic Regression Analysis of ADC Values as a Predictor of Best Response After Five Chemotherapy Cycles
| Variable | Overall (n = 28) |
P | |
|---|---|---|---|
| OR* | 95% CI | ||
| Overall mean of ADC means† | 0.2 | 0.0 to 1.3 | .09 |
| Overall mean of ADC mins‡ | 0.2 | 0.0 to 6.3 | .40 |
| Overall mean of ADC 25th percentiles§ | 0.3 | 0.1 to 1.7 | .18 |
Abbreviations: ADC, apparent diffusion coefficients; CR, complete response; mins, minimums; OR, odds ratio.
Logistic model outcome models CR.
OR is per 1,000 units of overall mean of ADC means.
OR is per 1,000 units of overall of ADC mins.
OR is per 1,000 units of overall mean of ADC 25th percentiles.
Footnotes
Supported by the Memorial Sloan-Kettering Cancer Center Department of Neurology Research and Development funds. Genentech provided rituximab and partial financial support.
Presented at the 45th Annual Meeting of the American Society of Clinical Oncology, May 29-June 2, 2009, Orlando, FL; and the 48th Annual Meeting of the American Society of Clinical Oncology, June 1-5, 2012, Chicago, IL.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00594815.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Gaurav Shah, Novartis (C); Lauren E. Abrey, Roche (C) Consultant or Advisory Role: Denise D. Correa, Genentech (C); David Schiff, Genentech (C), Roche (C); Antonio Omuro, Roche (C) Stock Ownership: Lauren E. Abrey, Roche Honoraria: Patrick G. Morris, OncolologySTAT, Nordic Group Research Funding: None Expert Testimony: None Patents: None Other Remuneration: Patrick G. Morris, Novartis
AUTHOR CONTRIBUTIONS
Conception and design: Patrick G. Morris, Joachim Yahalom, Sasan Karimi, Lauren E. Abrey, Lisa M. DeAngelis, Antonio Omuro
Financial support: Lisa M. DeAngelis
Administrative support: Lisa M. DeAngelis
Provision of study materials or patients: Jeffrey J. Raizer, David Schiff, Barbara Grant, Sean Grimm, Rose K. Lai, Lauren E. Abrey, Lisa M. DeAngelis, Antonio Omuro
Collection and assembly of data: Patrick G. Morris, Denise D. Correa, Jeffrey J. Raizer, David Schiff, Barbara Grant, Sean Grimm, Rose K. Lai, Sasan Karimi, Gaurav Shah, Antonio Omuro
Data analysis and interpretation: Patrick G. Morris, Denise D. Correa, Anne S. Reiner, Kathy Panageas, Sasan Karimi, Richard Curry, Lauren E. Abrey, Lisa M. DeAngelis, Antonio Omuro
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Morris PG, Abrey LE: Therapeutic challenges in primary CNS lymphoma Lancet Neurol 8:581–592,2009 [DOI] [PubMed] [Google Scholar]
- 2.Ferreri AJ DeAngelis L Illerhaus G, etal: Whole-brain radiotherapy in primary CNS lymphoma Lancet Oncol 12:118–119,2011 [DOI] [PubMed] [Google Scholar]
- 3.Korfel A Thiel E Martus P, etal: Whole-brain radiotherapy in primary CNS lymphoma (authors' reply) Lancet Oncol 12:119–120,2011 [DOI] [PubMed] [Google Scholar]
- 4.Graber JJ, Omuro A: Primary central nervous system lymphoma: Is there still a role for radiotherapy? Curr Opin Neurol 24:633–640,2011 [DOI] [PubMed] [Google Scholar]
- 5.Thiel E Korfel A Martus P, etal: High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): A phase 3, randomised, non-inferiority trial Lancet Oncol 11:1036–1047,2010 [DOI] [PubMed] [Google Scholar]
- 6.Omuro A Taillandier L Chinot O, etal: Primary CNS lymphoma in patients younger than 60: Can whole-brain radiotherapy be deferred? J Neurooncol 104:323–330,2011 [DOI] [PubMed] [Google Scholar]
- 7.Batchelor T Carson K O'Neill A, etal: Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: A report of NABTT 96-07 J Clin Oncol 21:1044–1049,2003 [DOI] [PubMed] [Google Scholar]
- 8.Pels H Schmidt-Wolf IG Glasmacher A, etal: Primary central nervous system lymphoma: Results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy J Clin Oncol 21:4489–4495,2003 [DOI] [PubMed] [Google Scholar]
- 9.Abrey LE, Yahalom J, DeAngelis LM: Treatment for primary CNS lymphoma: The next step J Clin Oncol 18:3144–3150,2000 [DOI] [PubMed] [Google Scholar]
- 10.Abrey LE, DeAngelis LM, Yahalom J: Long-term survival in primary CNS lymphoma J Clin Oncol 16:859–863,1998 [DOI] [PubMed] [Google Scholar]
- 11.Omuro AM Ben-Porat LS Panageas KS, etal: Delayed neurotoxicity in primary central nervous system lymphoma Arch Neurol 62:1595–1600,2005 [DOI] [PubMed] [Google Scholar]
- 12.Ferreri AJ Reni M Pasini F, etal: A multicenter study of treatment of primary CNS lymphoma Neurology 58:1513–1520,2002 [DOI] [PubMed] [Google Scholar]
- 13.Ekenel M Iwamoto FM Ben-Porat LS, etal: Primary central nervous system lymphoma: The role of consolidation treatment after a complete response to high-dose methotrexate-based chemotherapy Cancer 113:1025–1031,2008 [DOI] [PubMed] [Google Scholar]
- 14.Gavrilovic IT Hormigo A Yahalom J, etal: Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma J Clin Oncol 24:4570–4574,2006 [DOI] [PubMed] [Google Scholar]
- 15.Coiffier B Lepage E Briere J, etal: CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma N Engl J Med 346:235–242,2002 [DOI] [PubMed] [Google Scholar]
- 16.Shah GD Yahalom J Correa DD, etal: Combined immunochemotherapy with reduced whole-brain radiotherapy for newly diagnosed primary CNS lymphoma J Clin Oncol 25:4730–4735,2007 [DOI] [PubMed] [Google Scholar]
- 17.Abrey LE Batchelor TT Ferreri AJ, etal: Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma J Clin Oncol 23:5034–5043,2005 [DOI] [PubMed] [Google Scholar]
- 18.Correa DD DeAngelis LM Shi W, etal: Cognitive functions in survivors of primary central nervous system lymphoma Neurology 62:548–555,2004 [DOI] [PubMed] [Google Scholar]
- 19.Correa DD Maron L Harder H, etal: Cognitive functions in primary central nervous system lymphoma: Literature review and assessment guidelines Ann Oncol 18:1145–1151,2007 [DOI] [PubMed] [Google Scholar]
- 20.Correa DD Rocco-Donovan M Deangelis LM, etal: Prospective cognitive follow-up in primary CNS lymphoma patients treated with chemotherapy and reduced-dose radiotherapy J Neurooncol 91:315–321,2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fazekas F Chawluk JB Alavi A, etal: MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging AJR Am J Roentgenol 149:351–356,1987 [DOI] [PubMed] [Google Scholar]
- 22.Correa DD Shi W Abrey LE, etal: Cognitive functions in primary CNS lymphoma after single or combined modality regimens Neuro Oncol 14:101–108,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corn BW Yousem DM Scott CB, etal: White matter changes are correlated significantly with radiation dose: Observations from a randomized dose-escalation trial for malignant glioma (Radiation Therapy Oncology Group 83-02) Cancer 74:2828–2835,1994 [DOI] [PubMed] [Google Scholar]
- 24.Barajas RF Jr Rubenstein JL Chang JS, etal: Diffusion-weighted MR imaging derived apparent diffusion coefficient is predictive of clinical outcome in primary central nervous system lymphoma AJNR Am J Neuroradiol 31:60–66,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abrey LE Ben-Porat L Panageas KS, etal: Primary central nervous system lymphoma: The Memorial Sloan-Kettering Cancer Center prognostic model J Clin Oncol 24:5711–5715,2006 [DOI] [PubMed] [Google Scholar]
- 26.Morris PG Abrey LE Reiner AS, etal: Methotrexate area under the curve as a prognostic factor in primary central nervous system lymphoma treated with immunochemoradiotherapy Leuk Lymphoma 52:1891–1897,2011 [DOI] [PubMed] [Google Scholar]
- 27.DeAngelis LM Yahalom J Thaler HT, etal: Combined modality therapy for primary CNS lymphoma J Clin Oncol 10:635–643,1992 [DOI] [PubMed] [Google Scholar]
- 28.Glass J Gruber ML Cher L, etal: Preirradiation methotrexate chemotherapy of primary central nervous system lymphoma: Long-term outcome J Neurosurg 81:188–195,1994 [DOI] [PubMed] [Google Scholar]
- 29.DeAngelis LM Seiferheld W Schold SC, etal: Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: Radiation Therapy Oncology Group Study 93-10 J Clin Oncol 20:4643–4648,2002 [DOI] [PubMed] [Google Scholar]
- 30.Bessell EM López-Guillermo A Villá S, etal: Importance of radiotherapy in the outcome of patients with primary CNS lymphoma: An analysis of the CHOD/BVAM regimen followed by two different radiotherapy treatments J Clin Oncol 20:231–236,2002 [DOI] [PubMed] [Google Scholar]
- 31.Poortmans PM Kluin-Nelemans HC Haaxma-Reiche H, etal: High-dose methotrexate-based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: European Organization for Research and Treatment of Cancer Lymphoma Group Phase II Trial 20962 J Clin Oncol 21:4483–4488,2003 [DOI] [PubMed] [Google Scholar]
- 32.Omuro AM DeAngelis LM Yahalom J, etal: Chemoradiotherapy for primary CNS lymphoma: An intent-to-treat analysis with complete follow-up Neurology 64:69–74,2005 [DOI] [PubMed] [Google Scholar]
- 33.Korfel A Martus P Nowrousian MR, etal: Response to chemotherapy and treating institution predict survival in primary central nervous system lymphoma Br J Haematol 128:177–183,2005 [DOI] [PubMed] [Google Scholar]
- 34.Ferreri AJ Dell'Oro S Foppoli M, etal: MATILDE regimen followed by radiotherapy is an active strategy against primary CNS lymphomas Neurology 66:1435–1438,2006 [DOI] [PubMed] [Google Scholar]
- 35.Ferreri AJ Reni M Foppoli M, etal: High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: A randomised phase 2 trial Lancet 374:1512–1520,2009 [DOI] [PubMed] [Google Scholar]
- 36.Herrlinger U Schabet M Brugger W, etal: German Cancer Society Neuro-Oncology Working Group NOA-03 multicenter trial of single-agent high-dose methotrexate for primary central nervous system lymphoma Ann Neurol 51:247–252,2002 [DOI] [PubMed] [Google Scholar]
- 37.Pels H Juergens A Glasmacher A, etal: Early relapses in primary CNS lymphoma after response to polychemotherapy without intraventricular treatment: Results of a phase II study J Neurooncol 91:299–305,2009 [DOI] [PubMed] [Google Scholar]
- 38.Wieduwilt MJ Valles F Issa S, etal: Immunochemotherapy with intensive consolidation for primary CNS lymphoma: A pilot study and prognostic assessment by diffusion-weighted MRI Clin Cancer Res 18:1146–1155,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoang-Xuan K Taillandier L Chinot O, etal: Chemotherapy alone as initial treatment for primary CNS lymphoma in patients older than 60 years: A multicenter phase II study (26952) of the European Organization for Research and Treatment of Cancer Brain Tumor Group J Clin Oncol 21:2726–2731,2003 [DOI] [PubMed] [Google Scholar]
- 40.Omuro AM Taillandier L Chinot O, etal: Temozolomide and methotrexate for primary central nervous system lymphoma in the elderly J Neurooncol 85:207–211,2007 [DOI] [PubMed] [Google Scholar]
- 41.Gerstner ER Zhu JJ Engler DA, etal: High dose methotrexate for elderly patients with primary central nervous system lymphoma Neuro Oncol 11:211–215,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fritsch K Kasenda B Hader C, etal: Immunochemotherapy with rituximab, methotrexate, procarbazine, and lomustine for primary CNS lymphoma (PCNSL) in the elderly Ann Oncol 22:2080–2085,2011 [DOI] [PubMed] [Google Scholar]
- 43.Batchelor TT Grossman SA Mikkelsen T, etal: Rituximab monotherapy for patients with recurrent primary CNS lymphoma Neurology 76:929–930,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Omuro AM, Martin-Duverneuil N, Delattre JY: Complications of radiotherapy to the central nervous system Handb Clin Neurol 105:887–901,2012 [DOI] [PubMed] [Google Scholar]
- 45.Soussain C Hoang-Xuan K Taillandier L, etal: Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Societe Francaise de Greffe de Moelle Osseuse-Therapie Cellulaire J Clin Oncol 26:2512–2518,2008 [DOI] [PubMed] [Google Scholar]
- 46.Illerhaus G Marks R Ihorst G, etal: High-dose chemotherapy with autologous stem-cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma J Clin Oncol 24:3865–3870,2006 [DOI] [PubMed] [Google Scholar]