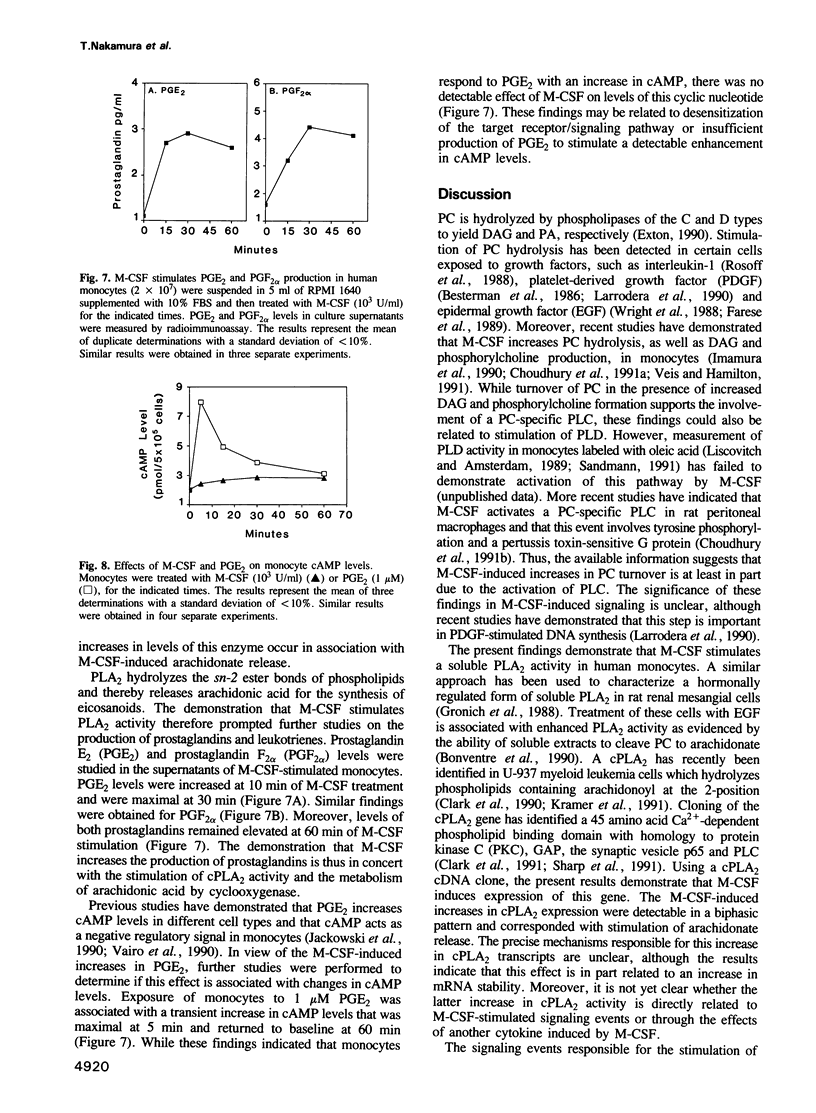
Abstract
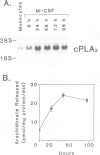
The macrophage colony stimulating factor (M-CSF) is required for the proliferation and differentiation of monocytes. Previous studies have demonstrated that M-CSF stimulation is associated with phosphatidylcholine (PC) hydrolysis and increased formation of both diacylglycerol (DAG) and phosphorylcholine. The present work extends those results by demonstrating that treatment of human monocytes with M-CSF is associated with increases in a cytoplasmic Ca(2+)-dependent activity which hydrolyzes 1-palmitoyl,2-arachidonoyl PC to arachidonic acid. The finding that this hydrolysis of PC is associated with increases in production of lysophosphatidylcholine indicates that M-CSF stimulates a cytoplasmic phospholipase A2 (cPLA2) activity. These results are supported by the demonstration that M-CSF induces cPLA2 gene expression. M-CSF-induced increases in cPLA2 mRNA levels were biphasic and corresponded with rapid (30-60 min) and delayed (24-72 h) increases in cPLA2 activity. The results demonstrate that this effect of M-CSF on cPLA2 expression is controlled at least in part by post-transcriptional stabilization of cPLA2 transcripts. The finding that M-CSF treatment is also associated with phosphorylation of the cPLA2 protein further suggests that expression of this enzyme is regulated at multiple levels. Finally, the stimulation of cPLA2 activity and arachidonate release is supported by increases in prostaglandin (PG) synthesis. In this regard, levels of both PGE2 and PGF2 alpha were increased in response to M-CSF. Taken together, these results indicate that M-CSF stimulates PC hydrolysis in human monocytes by inducing cPLA2 activity and thereby formation of eicosanoids.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besterman J. M., Duronio V., Cuatrecasas P. Rapid formation of diacylglycerol from phosphatidylcholine: a pathway for generation of a second messenger. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6785–6789. doi: 10.1073/pnas.83.18.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonventre J. V., Gronich J. H., Nemenoff R. A. Epidermal growth factor enhances glomerular mesangial cell soluble phospholipase A2 activity. J Biol Chem. 1990 Mar 25;265(9):4934–4938. [PubMed] [Google Scholar]
- Bonventre J. V., Swidler M. Calcium dependency of prostaglandin E2 production in rat glomerular mesangial cells. Evidence that protein kinase C modulates the Ca2+-dependent activation of phospholipase A2. J Clin Invest. 1988 Jul;82(1):168–176. doi: 10.1172/JCI113566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortner D. M., Ulivi M., Roussel M. F., Ostrowski M. C. The carboxy-terminal catalytic domain of the GTPase-activating protein inhibits nuclear signal transduction and morphological transformation mediated by the CSF-1 receptor. Genes Dev. 1991 Oct;5(10):1777–1785. doi: 10.1101/gad.5.10.1777. [DOI] [PubMed] [Google Scholar]
- Burch R. M., Axelrod J. Dissociation of bradykinin-induced prostaglandin formation from phosphatidylinositol turnover in Swiss 3T3 fibroblasts: evidence for G protein regulation of phospholipase A2. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6374–6378. doi: 10.1073/pnas.84.18.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R. M., Jelsema C., Axelrod J. Cholera toxin and pertussis toxin stimulate prostaglandin E2 synthesis in a murine macrophage cell line. J Pharmacol Exp Ther. 1988 Feb;244(2):765–773. [PubMed] [Google Scholar]
- Cheung D. L., Hamilton J. A. Regulation of human monocyte DNA synthesis by colony-stimulating factors, cytokines, and cyclic adenosine monophosphate. Blood. 1992 Apr 15;79(8):1972–1981. [PubMed] [Google Scholar]
- Choudhury G. G., Sylvia V. L., Sakaguchi A. Y. Activation of a phosphatidylcholine-specific phospholipase C by colony stimulating factor 1 receptor requires tyrosine phosphorylation and a guanine nucleotide-binding protein. J Biol Chem. 1991 Dec 5;266(34):23147–23151. [PubMed] [Google Scholar]
- Choudhury G. G., Sylvia V. L., Wang L. M., Pierce J., Sakaguchi A. Y. The kinase insert domain of colony stimulating factor-1 receptor is dispensable for CSF-1 induced phosphatidylcholine hydrolysis. FEBS Lett. 1991 May 6;282(2):351–354. doi: 10.1016/0014-5793(91)80511-z. [DOI] [PubMed] [Google Scholar]
- Clark J. D., Lin L. L., Kriz R. W., Ramesha C. S., Sultzman L. A., Lin A. Y., Milona N., Knopf J. L. A novel arachidonic acid-selective cytosolic PLA2 contains a Ca(2+)-dependent translocation domain with homology to PKC and GAP. Cell. 1991 Jun 14;65(6):1043–1051. doi: 10.1016/0092-8674(91)90556-e. [DOI] [PubMed] [Google Scholar]
- Clark J. D., Milona N., Knopf J. L. Purification of a 110-kilodalton cytosolic phospholipase A2 from the human monocytic cell line U937. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7708–7712. doi: 10.1073/pnas.87.19.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Dominguez I., Marshall M. S., Gibbs J. B., García de Herreros A., Cornet M. E., Graziani G., Diaz-Meco M. T., Johansen T., McCormick F., Moscat J. Role of GTPase activating protein in mitogenic signalling through phosphatidylcholine-hydrolysing phospholipase C. EMBO J. 1991 Nov;10(11):3215–3220. doi: 10.1002/j.1460-2075.1991.tb04884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing J. R., Rettenmier C. W., Sherr C. J. Ligand-induced tyrosine kinase activity of the colony-stimulating factor 1 receptor in a murine macrophage cell line. Mol Cell Biol. 1988 Apr;8(4):1795–1799. doi: 10.1128/mcb.8.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Farese R. V., Nair G. P., Sierra C. G., Standaert M. L., Pollet R. J., Cooper D. R. Insulin-like effects of epidermal growth factor and insulin-like growth factor-I on [3H]2-deoxyglucose uptake, diacylglycerol generation and protein kinase C activation in BC3H-1 myocytes. Biochem J. 1989 Aug 1;261(3):927–934. doi: 10.1042/bj2610927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronich J. H., Bonventre J. V., Nemenoff R. A. Identification and characterization of a hormonally regulated form of phospholipase A2 in rat renal mesangial cells. J Biol Chem. 1988 Nov 15;263(32):16645–16651. [PubMed] [Google Scholar]
- Guilbert L. J., Stanley E. R. The interaction of 125I-colony-stimulating factor-1 with bone marrow-derived macrophages. J Biol Chem. 1986 Mar 25;261(9):4024–4032. [PubMed] [Google Scholar]
- Gupta S. K., Diez E., Heasley L. E., Osawa S., Johnson G. L. A G protein mutant that inhibits thrombin and purinergic receptor activation of phospholipase A2. Science. 1990 Aug 10;249(4969):662–666. doi: 10.1126/science.2166341. [DOI] [PubMed] [Google Scholar]
- Halenda S. P., Banga H. S., Zavoico G. B., Lau L. F., Feinstein M. B. Synergistic release of arachidonic acid from platelets by activators of protein kinase C and Ca2+ ionophores. Evidence for the role of protein phosphorylation in the activation of phospholipase A2 and independence from the Na+/H+ exchanger. Biochemistry. 1989 Sep 5;28(18):7356–7363. doi: 10.1021/bi00444a031. [DOI] [PubMed] [Google Scholar]
- Hamilton J. A., Vairo G., Lingelbach S. R. Activation and proliferation signals in murine macrophages: stimulation of glucose uptake by hemopoietic growth factors and other agents. J Cell Physiol. 1988 Mar;134(3):405–412. doi: 10.1002/jcp.1041340311. [DOI] [PubMed] [Google Scholar]
- Han J. W., McCormick F., Macara I. G. Regulation of Ras-GAP and the neurofibromatosis-1 gene product by eicosanoids. Science. 1991 Apr 26;252(5005):576–579. doi: 10.1126/science.1902323. [DOI] [PubMed] [Google Scholar]
- Hartmann T., Seuwen K., Roussel M. F., Sherr C. J., Pouysségur J. Functional expression of the human receptor for colony-stimulating factor 1 (CSF-1) in hamster fibroblasts: CSF-1 stimulates Na+/H+ exchange and DNA-synthesis in the absence of phosphoinositide breakdown. Growth Factors. 1990;2(4):289–300. doi: 10.3109/08977199009167024. [DOI] [PubMed] [Google Scholar]
- He Y. X., Hewlett E., Temeles D., Quesenberry P. Inhibition of interleukin 3 and colony-stimulating factor 1-stimulated marrow cell proliferation by pertussis toxin. Blood. 1988 May;71(5):1187–1195. [PubMed] [Google Scholar]
- Imamura K., Dianoux A., Nakamura T., Kufe D. Colony-stimulating factor 1 activates protein kinase C in human monocytes. EMBO J. 1990 Aug;9(8):2423-8, 2389. doi: 10.1002/j.1460-2075.1990.tb07418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K., Kufe D. Colony-stimulating factor 1-induced Na+ influx into human monocytes involves activation of a pertussis toxin-sensitive GTP-binding protein. J Biol Chem. 1988 Oct 5;263(28):14093–14098. [PubMed] [Google Scholar]
- Jackowski S., Rettenmier C. W., Rock C. O. Prostaglandin E2 inhibition of growth in a colony-stimulating factor 1-dependent macrophage cell line. J Biol Chem. 1990 Apr 25;265(12):6611–6616. [PubMed] [Google Scholar]
- Kramer R. M., Roberts E. F., Manetta J., Putnam J. E. The Ca2(+)-sensitive cytosolic phospholipase A2 is a 100-kDa protein in human monoblast U937 cells. J Biol Chem. 1991 Mar 15;266(8):5268–5272. [PubMed] [Google Scholar]
- Larrodera P., Cornet M. E., Diaz-Meco M. T., Lopez-Barahona M., Diaz-Laviada I., Guddal P. H., Johansen T., Moscat J. Phospholipase C-mediated hydrolysis of phosphatidylcholine is an important step in PDGF-stimulated DNA synthesis. Cell. 1990 Jun 15;61(6):1113–1120. doi: 10.1016/0092-8674(90)90074-o. [DOI] [PubMed] [Google Scholar]
- Lin L. L., Lin A. Y., Knopf J. L. Cytosolic phospholipase A2 is coupled to hormonally regulated release of arachidonic acid. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6147–6151. doi: 10.1073/pnas.89.13.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscovitch M., Amsterdam A. Gonadotropin-releasing hormone activates phospholipase D in ovarian granulosa cells. Possible role in signal transduction. J Biol Chem. 1989 Jul 15;264(20):11762–11767. [PubMed] [Google Scholar]
- Lowndes J. M., Gupta S. K., Osawa S., Johnson G. L. GTPase-deficient G alpha i2 oncogene gip2 inhibits adenylylcyclase and attenuates receptor-stimulated phospholipase A2 activity. J Biol Chem. 1991 Aug 5;266(22):14193–14197. [PubMed] [Google Scholar]
- Margolis B. L., Holub B. J., Troyer D. A., Skorecki K. L. Epidermal growth factor stimulates phospholipase A2 in vasopressin-treated rat glomerular mesangial cells. Biochem J. 1988 Dec 1;256(2):469–474. doi: 10.1042/bj2560469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. K., Browning P. J., White M. F., Roberts T. M. Tyrosine phosphorylations in vivo associated with v-fms transformation. Mol Cell Biol. 1988 Jan;8(1):176–185. doi: 10.1128/mcb.8.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J., Daniel L. W., Waite M. Evidence of protein kinase C involvement in phorbol diester-stimulated arachidonic acid release and prostaglandin synthesis. J Biol Chem. 1987 Apr 15;262(11):5385–5393. [PubMed] [Google Scholar]
- Reedijk M., Liu X. Q., Pawson T. Interactions of phosphatidylinositol kinase, GTPase-activating protein (GAP), and GAP-associated proteins with the colony-stimulating factor 1 receptor. Mol Cell Biol. 1990 Nov;10(11):5601–5608. doi: 10.1128/mcb.10.11.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosoff P. M., Savage N., Dinarello C. A. Interleukin-1 stimulates diacylglycerol production in T lymphocytes by a novel mechanism. Cell. 1988 Jul 1;54(1):73–81. doi: 10.1016/0092-8674(88)90181-x. [DOI] [PubMed] [Google Scholar]
- Sandmann J., Peralta E. G., Wurtman R. J. Coupling of transfected muscarinic acetylcholine receptor subtypes to phospholipase D. J Biol Chem. 1991 Apr 5;266(10):6031–6034. [PubMed] [Google Scholar]
- Sengupta A., Liu W. K., Yeung Y. G., Yeung D. C., Frackelton A. R., Jr, Stanley E. R. Identification and subcellular localization of proteins that are rapidly phosphorylated in tyrosine in response to colony-stimulating factor 1. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8062–8066. doi: 10.1073/pnas.85.21.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp J. D., White D. L., Chiou X. G., Goodson T., Gamboa G. C., McClure D., Burgett S., Hoskins J., Skatrud P. L., Sportsman J. R. Molecular cloning and expression of human Ca(2+)-sensitive cytosolic phospholipase A2. J Biol Chem. 1991 Aug 15;266(23):14850–14853. [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Guilbert L. J., Tushinski R. J., Bartelmez S. H. CSF-1--a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21(2):151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- Tsai M. H., Yu C. L., Wei F. S., Stacey D. W. The effect of GTPase activating protein upon ras is inhibited by mitogenically responsive lipids. Science. 1989 Jan 27;243(4890):522–526. doi: 10.1126/science.2536192. [DOI] [PubMed] [Google Scholar]
- Tushinski R. J., Stanley E. R. The regulation of macrophage protein turnover by a colony stimulating factor (CSF-1). J Cell Physiol. 1983 Jul;116(1):67–75. doi: 10.1002/jcp.1041160111. [DOI] [PubMed] [Google Scholar]
- Vairo G., Argyriou S., Bordun A. M., Whitty G., Hamilton J. A. Inhibition of the signaling pathways for macrophage proliferation by cyclic AMP. Lack of effect on early responses to colony stimulating factor-1. J Biol Chem. 1990 Feb 15;265(5):2692–2701. [PubMed] [Google Scholar]
- Vairo G., Hamilton J. A. Activation and proliferation signals in murine macrophages: stimulation of Na+,K+-ATPase activity by hemopoietic growth factors and other agents. J Cell Physiol. 1988 Jan;134(1):13–24. doi: 10.1002/jcp.1041340103. [DOI] [PubMed] [Google Scholar]
- Varticovski L., Druker B., Morrison D., Cantley L., Roberts T. The colony stimulating factor-1 receptor associates with and activates phosphatidylinositol-3 kinase. Nature. 1989 Dec 7;342(6250):699–702. doi: 10.1038/342699a0. [DOI] [PubMed] [Google Scholar]
- Veis N., Hamilton J. A. Colony stimulating factor-1 stimulates diacylglycerol generation in murine bone marrow-derived macrophages, but not in resident peritoneal macrophages. J Cell Physiol. 1991 May;147(2):298–305. doi: 10.1002/jcp.1041470215. [DOI] [PubMed] [Google Scholar]
- Whetton A. D., Monk P. N., Consalvey S. D., Downes C. P. The haemopoietic growth factors interleukin 3 and colony stimulating factor-1 stimulate proliferation but do not induce inositol lipid breakdown in murine bone-marrow-derived macrophages. EMBO J. 1986 Dec 1;5(12):3281–3286. doi: 10.1002/j.1460-2075.1986.tb04640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright T. M., Rangan L. A., Shin H. S., Raben D. M. Kinetic analysis of 1,2-diacylglycerol mass levels in cultured fibroblasts. Comparison of stimulation by alpha-thrombin and epidermal growth factor. J Biol Chem. 1988 Jul 5;263(19):9374–9380. [PubMed] [Google Scholar]