Abstract
Purpose
Nivolumab, a programmed death-1 (PD-1) immune checkpoint inhibitor antibody, has demonstrated improved survival over docetaxel in previously treated advanced non–small-cell lung cancer (NSCLC). First-line monotherapy with nivolumab for advanced NSCLC was evaluated in the phase I, multicohort, Checkmate 012 trial.
Methods
Fifty-two patients received nivolumab 3 mg/kg intravenously every 2 weeks until progression or unacceptable toxicity; postprogression treatment was permitted per protocol. The primary objective was to assess safety; secondary objectives included objective response rate (ORR) and 24-week progression-free survival (PFS) rate; overall survival (OS) was an exploratory end point.
Results
Any-grade treatment-related adverse events (AEs) occurred in 71% of patients, most commonly: fatigue (29%), rash (19%), nausea (14%), diarrhea (12%), pruritus (12%), and arthralgia (10%). Ten patients (19%) reported grade 3 to 4 treatment-related AEs; grade 3 rash was the only grade 3 to 4 event occurring in more than one patient (n = 2; 4%). Six patients (12%) discontinued because of a treatment-related AE. The confirmed ORR was 23% (12 of 52), including four ongoing complete responses. Nine of 12 responses (75%) occurred by first tumor assessment (week 11); eight (67%) were ongoing (range, 5.3+ to 25.8+ months) at the time of data lock. ORR was 28% (nine of 32) in patients with any degree of tumor PD–ligand 1 expression and 14% (two of 14) in patients with no PD–ligand 1 expression. Median PFS was 3.6 months, and the 24-week PFS rate was 41% (95% CI, 27 to 54). Median OS was 19.4 months, and the 1-year and 18-month OS rates were 73% (95% CI, 59 to 83) and 57% (95% CI, 42 to 70), respectively.
Conclusion
First-line nivolumab monotherapy demonstrated a tolerable safety profile and durable responses in first-line advanced NSCLC.
INTRODUCTION
Platinum-based doublet chemotherapy (PT-DC) is the current standard of care as first-line treatment of patients with advanced non–small-cell lung cancer (NSCLC) not driven by an epidermal growth factor receptor (EGFR) mutation or anaplastic lymphoma kinase (ALK) rearrangement, with objective response rates (ORRs) of 15% to 32%, median progression-free survival (PFS) and overall survival (OS) of 4.0 to 5.1 and 8.1 to 10.3 months, respectively, and 1- and 2-year OS rates of 30% to 44% and 10% to 18.9%, respectively.1-6 Addition of bevacizumab to first-line PT-DC modestly improves clinical outcome versus chemotherapy alone in patients with nonsquamous NSCLC (ORR, 33% to 35%; median OS, 12.3 to 13.4 months; and 1-year OS rate, 51% to 54.1%).3,7 In the small subset of patients with advanced NSCLC driven by EGFR or ALK genomic alterations, first-line therapy with EGFR or ALK tyrosine kinase inhibitors (TKIs), respectively, has consistently demonstrated higher ORRs (56% to 83%) and longer PFS (median, 9.2 to 13.1 months) with less toxicity than first-line PT-DC.8-13
Immune checkpoint inhibitors represent a distinct approach to treating malignancies, with durable antitumor activity and the potential for long-term survival demonstrated in multiple tumor types, including NSCLC.14-18 Nivolumab, a fully human IgG4 programmed death-1 (PD-1) immune checkpoint inhibitor antibody, binds with high affinity to PD-1 receptors expressed on T cells and disrupts negative signaling induced by PD-ligand 1 (PD-L1) and PD-ligand 2 to restore T-cell effector function.19,20 In heavily pretreated patients with advanced NSCLC, nivolumab monotherapy demonstrated an ORR of 17%, with 1-, 2-, and 3-year OS rates of 42%, 24%, and 18%, respectively, and a manageable safety profile.14 These initial signals of efficacy and tolerability prompted two phase III trials that demonstrated a survival benefit for salvage nivolumab over docetaxel in patients with advanced pretreated NSCLC,21,22 leading to its approval in the United States for treatment of patients with metastatic NSCLC whose disease has progressed on or after platinum-based chemotherapy and after an approved TKI therapy (if expressing EGFR or ALK genomic tumor aberrations).23 Also, nivolumab is approved in the European Union for locally advanced or metastatic NSCLC after prior chemotherapy.24
Given the safety and efficacy of nivolumab in the second- or later-line settings, CheckMate 012 (NCT01454102), a phase I, multicohort study, evaluated the potential benefit of nivolumab as monotherapy or combined with current standard therapies in first-line advanced NSCLC. Here, we report safety and efficacy from the full cohort of patients receiving first-line nivolumab monotherapy.
METHODS
Study Design and Treatment
This study was approved by local institutional review boards, and all patients or their legal representatives provided written informed consent before enrollment. Patients with stage IIIB to IV NSCLC who had no prior chemotherapy for advanced disease received nivolumab 3 mg/kg intravenous infusion on treatment day 1 and every 2 weeks thereafter until disease progression, discontinuation due to toxicity, withdrawal of consent, or loss to follow-up. Patients were permitted to continue study treatment beyond initial progressive disease, as defined by RECIST version 1.1,25 if they were considered by the investigator to be deriving clinical benefit (continuing symptom or disease control despite radiographic progression) and tolerating study treatment. Patients who continued study therapy beyond progression were required to discontinue if subsequent imaging demonstrated an additional 10% increase in tumor burden from the time of initial progression.
Follow-up visits after discontinuation of study therapy occurred 30 (± 14) and 100 (± 14) days after the last nivolumab dose. For patients who discontinued for reasons other than progressive disease, tumor assessments were performed every 3 months (± 14 days) until documented progression. Survival was evaluated every 12 weeks after the second follow-up visit. Patients were followed for treatment-related toxicities until they resolved, returned to baseline, or were deemed irreversible.
Patients
Eligible patients had histologically or cytologically confirmed stage IIIB to IV NSCLC (any histology),26 with radiographic proof of measurable disease according to RECIST V1.1.25 Patients had to be age 18 years or older with an Eastern Cooperative Oncology Group performance status of 0 or 1; have adequate hematologic, hepatic, and renal function; and have a life expectancy of at least 3 months. Patients had not received prior chemotherapy for advanced NSCLC. However, prior adjuvant or neoadjuvant chemotherapy was allowed. Prior radiotherapy and EGFR TKI therapy was permitted if completed at least 2 weeks before study drug administration. Collection of pretreatment excisional, incisional, or core needle tumor biopsies (fine-needle aspiration was insufficient) was required for biomarker evaluation but was not used to select patients. Patients could begin nivolumab treatment before confirming that tumor samples were sufficient for biomarker evaluation. Patients with history of brain metastases were eligible if they had completed radiotherapy, surgery, or radiosurgery at least 2 weeks before enrollment and did not require steroids for control of cerebral edema. Exclusion criteria included a history of active, known, or suspected autoimmune disease and evidence of active infection with hepatitis B or C or HIV.
Concomitant Treatments
Immunosuppressive agents, including immunosuppressive doses of systemic corticosteroids (eg, prednisone > 10 mg/d), and any use of concurrent hormonal therapy, immunotherapy, or standard or investigational agents for the treatment of NSCLC were prohibited during the study. However, a brief course of corticosteroids was permitted for prophylaxis (eg, contrast dye allergy) and corticosteroids or other immune-suppressive agents were allowed to manage symptomatic treatment-related immune toxicities.
Study Assessments
Safety Assessments.
The primary objective of the study was to assess the safety and tolerability of nivolumab monotherapy, as measured by the frequency of treatment-related adverse events (AEs) and through careful monitoring of laboratory abnormalities. Categories of select AEs (those with potential immunologic etiology that require more frequent monitoring or intervention) were based on a prespecified list of Medical Dictionary for Regulatory Activities terms. The causal relationship (related or not related) between study drug and AEs was determined by the investigator; severity of AEs was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.27
Efficacy Assessments.
The secondary study objective was antitumor activity of nivolumab monotherapy, as measured by ORR and PFS rate at 24 weeks using investigator-assessed tumor measurements, according to RECIST v1.1.25 Tumor response was assessed by the investigator at the beginning of weeks 11, 17, 23, and every 3 months thereafter until disease progression. For patients continuing nivolumab treatment beyond initial progression, tumor assessments were repeated 6 weeks after initial progression and every 12 weeks thereafter. Clinical activity also was assessed by histology, smoking history, EGFR and Kirsten rat sarcoma viral oncogene homolog (KRAS) mutation status, and tumor PD-L1 expression (Data Supplement). OS was included as an exploratory efficacy end point.
Statistical Analyses
Analyses of safety and efficacy, except for OS, are based on a March 2015 database lock; OS was updated based on an August 2015 database lock. Patient demographics, baseline characteristics, and frequency of AEs were summarized using descriptive statistics. All recorded AEs were coded according to Medical Dictionary for Regulatory Activities, version 17.0. ORR was defined as the proportion of all treated patients whose best overall response (BOR) was either a confirmed complete response or confirmed partial response, with corresponding two-sided 95% exact CIs calculated using the Clopper-Pearson method.28 Estimated time-to-event end points (PFS rate at 24 weeks, median duration of response [DOR], PFS, and OS) were calculated using the Kaplan-Meier method, with two-sided 95% exact CIs derived via log-log transformation.29
RESULTS
Patient Population and Disposition
Fifty-two patients with advanced NSCLC were treated with nivolumab monotherapy; 94% had stage IV disease, 75% (39 of 52) had tumors of nonsquamous histology, 15% (eight of 52) had EGFR-mutant tumors, and 79% (41 of 52) were former/current smokers (Table 1). Forty percent of patients had received prior radiotherapy, and 21% and 4% had received prior adjuvant and neoadjuvant systemic platinum-based therapy, respectively. Median follow-up for the overall population was 14.3 months (range, 0.2 to 30.1). At the time of analysis, 100% of patients (13 of 13) with squamous disease and 87% of patients with nonsquamous disease had discontinued nivolumab, most commonly because of disease progression (squamous, 77%; nonsquamous, 64%; Data Supplement).
Table 1.
Patient Characteristics and Summary of Prior Therapy
| Characteristic | Total (N = 52) |
|---|---|
| Median age (range), years | 67 (43-85) |
| Sex | |
| Male | 26 (50) |
| Female | 26 (50) |
| Disease stage | |
| Stage IIIB | 3 (6) |
| Stage IV | 49 (94) |
| Histology | |
| Nonsquamous* | 39 (75) |
| Squamous | 13 (25) |
| Tumor mutation status† | |
| EGFR mutation status | |
| Mutant‡ | 8 (15) |
| Exon 19 deletion | 3 (6) |
| L858R | 3 (6) |
| Unknown | 1 (2) |
| Other | 1 (2) |
| Wildtype | 31 (60) |
| Unknown | 13 (25) |
| KRAS mutation status | |
| Mutant | 9 (17) |
| Wildtype | 10 (19) |
| Unknown | 33 (64) |
| PD-L1 expression, % | |
| ≥ 1 | 32 (62) |
| ≥ 5 | 26 (50) |
| ≥ 10 | 20 (38) |
| ≥ 25 | 18 (35) |
| ≥ 50 | 12 (23) |
| Unknown§ | 6 (12) |
| Smoking status | |
| Never | 11 (21) |
| Current | 3 (6) |
| Former | 38 (73) |
| Prior surgery | 48 (92) |
| Prior radiotherapy | 21 (40) |
| Prior systemic therapy | 19 (37) |
| Regimen setting|| | ? |
| Adjuvant therapy | 11 (21) |
| Neoadjuvant therapy | 2 (4) |
| Metastatic disease¶ | 7 (13) |
NOTE. Data presented as No. (%) unless otherwise noted.
Abbreviations: ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; KRAS, Kirsten rat sarcoma viral oncogene homolog; TKI, tyrosine kinase inhibitor.
Includes three patients with histology type other.
There were no patients with known ALK rearrangements.
Prior TKI therapy was allowed (but not required) for patients with EGFR-mutant tumors.
Tumor programmed death–ligand 1 expression was not quantifiable in six patients, either because of suboptimal tissue amount or quality (eg, too few tumor cells or no cells, improper fixation, or sectioning artifacts; n = 5) or because tumor tissue was unavailable (n = 1).
More than one setting per patient may be reflected in the frequency.
All patients who received prior systemic therapy for metastatic disease were treated with erlotinib.
Safety
Treatment-related AEs of any grade were reported in 71% of patients (Table 2), most commonly (≥ 10% of patients) fatigue (29%), rash (19%), nausea (14%), diarrhea and pruritus (12% each), and arthralgia (10%). Most treatment-related AEs were of low severity. Grade 3 to 4 treatment-related AEs occurred in 10 patients (19%), including rash (two patients, 4%), increased amylase and lipase, increased ALT and AST, hyperglycemia, cardiac failure, dehydration and diarrhea, hyponatremia, lung infection, and pneumonitis (one patient each). Treatment-related AEs led to discontinuation in six patients (12%), including grade 4 increased ALT and grade 3 increased AST (one patient), and cardiac failure, hyperglycemia, increased lipase, diarrhea, and pneumonitis (all grade 3; one patient each). All but one patient (increased lipase) had resolution of these toxicities. Sixty-seven percent (four of six) of patients who discontinued because of treatment-related AEs had partial response as BOR; the remaining patients had stable disease (SD) as BOR.
Table 2.
Treatment-Related AEs Occurring in ≥ 5% of Patients With Advanced NSCLC Treated With Nivolumab Monotherapy
| Event | All Patients (N = 52) | |
|---|---|---|
| Any Grade* | Grade 3-4† | |
| Any event | 37 (71) | 10 (19) |
| Fatigue | 15 (29) | 0 |
| Rash | 10 (19) | 2 (4) |
| Nausea | 7 (14) | 0 |
| Diarrhea | 6 (12) | 1 (2) |
| Pruritus | 6 (12) | 0 |
| Arthralgia | 5 (10) | 0 |
| Constipation | 3 (6) | 0 |
| Hypothyroidism | 3 (6) | 0 |
| Pneumonitis | 3 (6) | 1 (2) |
| Vomiting | 3 (6) | 0 |
NOTE. Data presented as No. (%). Data are based on a March 2015 database lock. Includes events reported between first dose date and 100 days after the last dose of nivolumab. The causal relationship (related or not related) between study drug and AEs was determined by the investigator. Some patients had more than one AE.
Abbreviations: AE, adverse event; NSCLC, non–small-cell lung cancer.
No grade 5 events were reported.
Other grade 3 to 4 treatment-related AEs were increased ALT (grade 4, n = 1), increased amylase, increased AST, cardiac failure, dehydration, hyperglycemia, hyponatremia, increased lipase, and lung infection (all grade 3, n = 1 each).
The most common (≥ 10% of patients) categories of treatment-related select AEs (Data Supplement) were skin (any grade, 25%; grade 3 to 4, 4%), endocrine (any grade, 14%; grade 3 to 4, 0%), and gastrointestinal (any grade, 12%; grade 3 to 4, 2%). All treatment-related select pulmonary events were pneumonitis (any grade, 6%; grade 3 to 4, 2%).
At the time of analysis, 20 patients had died: 19 as a result of disease progression and one as a result of sepsis and lung infection (not related to study treatment). No treatment-related deaths were reported.
Response and Tumor Kinetics
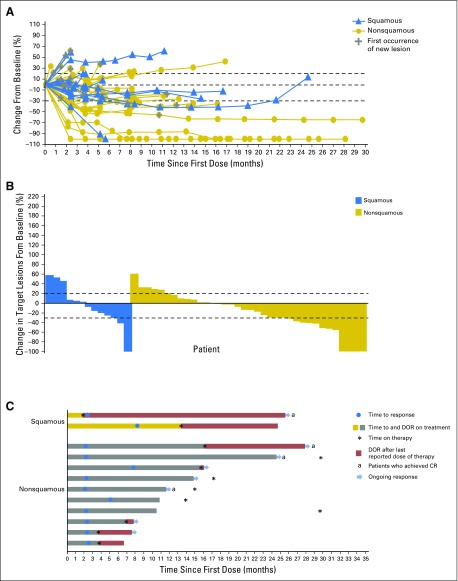
In the overall population, confirmed ORR was 23% (12 of 52), including four patients with ongoing complete responses (Table 3). An additional 27% of patients achieved SD for a disease control rate (DCR) of 50%; 19% (10 of 52) had SD lasting ≥ 21 weeks. Responses were durable (median DOR was not reached [NR]; range, 4.2 to 25.8+ months) and 75% (nine of 12) were achieved by the first tumor assessment (week 11). Reductions in tumor burden were observed regardless of NSCLC histology, and three patients had > 80% target lesion reduction by 18 weeks (Figs 1A and 1B). Among patients with ongoing responses (67%; 8 of 12), response durations ranged from 5.3+ to 25.8+ months (Fig 1C). Three additional patients had nonconventional immune-related responses, with 46%, 43%, and 35% maximum reductions in target lesions and simultaneous appearance of new lesions; OS for these patients was 12.8+, 14.5+, and 10.2 months, respectively. Fifty-eight percent of patients (seven of 12) had responses that continued after discontinuing nivolumab for reasons other than progressive disease (Fig 1C).
Table 3.
Tumor Response in Patients With Advanced NSCLC Treated With Nivolumab Monotherapya
| Response/Survival | Squamous (n = 13) | Nonsquamous (n = 39) | All Patients (N = 52) |
|---|---|---|---|
| Confirmed ORR,b No. (%) [95% CI] | 2 (15) [2 to 45] | 10 (26) [13 to 42] | 12 (23) [13 to 37] |
| Confirmed DCR,c No. (%) [95% CI] | 8 (62) [32 to 86] | 18 (46) [30 to 63] | 26 (50) [36 to 64] |
| Ongoing responders,d No. (%) | 1 (50) | 7 (70) | 8 (67) |
| BOR,e No. (%) | |||
| Confirmed CR | 1 (8) | 3 (8) | 4 (8) |
| Confirmed PR | 1 (8) | 7 (18) | 8 (15) |
| SD | 6 (46) | 8 (21) | 14 (27) |
| SD ≥ 21 weeksf | 3 (23) | 7 (18) | 10 (19) |
| Progressive disease | 5 (38) | 15 (38) | 20 (38) |
| Unable to determine | 0 | 6 (15)g | 6 (12) |
| Estimated DOR,h median (range), months | NR (16.5 to 23.3+) | NR (4.2 to 25.8+) | NR (4.2 to 25.8+) |
| PFS, median (range), months | 3.5 (1.4 to 25.6+) | 5.0 (< 0.1+ to 28.0+) | 3.6 (< 0.1+ to 28.0+) |
| PFS at 24 weeks,i % (95% CI) | 31 (9 to 55) | 45 (28 to 60) | 41 (27 to 54) |
| OS, median (range), months | 16.8 (3.1 to 32.5+) | NR (0.2 to 35.8+) | 19.4 (0.2 to 35.8+) |
| 1-year OS, % (95% CI) | 76 (43 to 92) | 72 (55 to 83) | 73 (59 to 83) |
| 18-month OS, % (95% CI) | 42 (16 to 67) | 63 (45 to 76) | 57 (42 to 70) |
NOTE. Not reached (NR) was due to a high percentage of ongoing response or insufficient number of events and/or follow up. Plus symbol (+) indicates a censored value.
Abbreviations: BOR, best overall response; CR, complete response; DCR, disease control rate; DOR, duration of response; NSCLC, non–small-cell lung cancer; ORR, objective response rate; OS, overall survival; PR, partial response; PFS, progression-free survival; SD, stable disease.
Data for response and PFS are based on a March 2015 database lock. Data for OS are based on an August 2015 database lock.
Includes patients with initial observations of CR and PR that were subsequently confirmed by repeat scans performed no earlier than 4 weeks after the original observation.
Includes patients with initial observations of CR and PR that were subsequently confirmed by repeat scans performed no earlier than 4 weeks after the original observation and patients with BOR of SD.
Includes patients with confirmed CR or PR who neither progressed nor died within 100 days of last nivolumab dose.
Tumor assessments up to initial disease progression or initiation of subsequent anticancer therapy, whichever occurred first, were considered for BOR assessment.
The 21-week time point was chosen based on the timing of tumor assessments.
Includes patients who discontinued trial therapy because of clinical progression of disease before first on-trial imaging assessment or patients only with on-treatment tumor assessments suggestive of, but that did not satisfy, the required minimum duration for SD.
Time from first response to documented progression, death within 100 days of last nivolumab dose, or last tumor assessment before subsequent anticancer therapy (for censored data).
PFS rate was defined as the probability of a patient remaining progression free and alive up to 24 weeks.
Fig 1.
Characteristics of response in patients with advanced non–small-cell lung cancer treated with nivolumab monotherapy. Data are based on a March 2015 database lock. (A) Percent change in target lesion tumor burden from baseline over time. Only includes patients with baseline target lesion and one or more postbaseline target lesion assessments with nonmissing value (n = 49). Horizontal lines denote 30% decrease, 20% increase, and no change. (B) Best percent change in target lesion tumor burden from baseline. Only includes patients with baseline target lesion and one or more postbaseline target lesion assessments with nonmissing value (n = 49). Maximum percent reductions in target lesion tumor burden from baseline across all tumor assessments before subsequent therapy are used. Positive change in tumor burden indicates tumor growth; negative change in tumor burden indicates tumor reduction. Horizontal lines denote 30% decrease and 20% increase. Not all reductions of ≥ 30% from baseline are partial responses (ie, decrease in target lesion tumor burden but new or progressive nontarget lesions). (C) Time to and duration of response. CR, complete response; DOR, duration of response.
Overall Survival and Progression-Free Survival
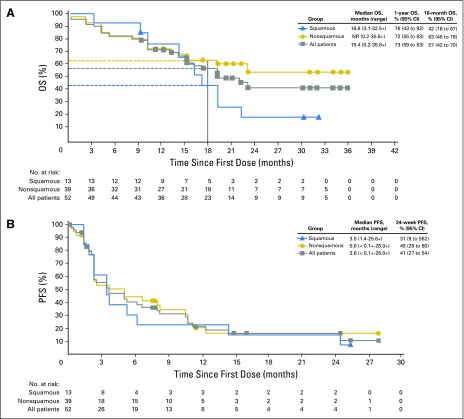
Median OS, an exploratory end point, was 19.4 months (range, 0.2 to 35.8+) for the overall population, and 16.8 months (range, 3.1 to 32.5+) and NR (range, 0.2 to 35.8+ months) for patients with squamous and nonsquamous histology, respectively (Table 3 and Fig 2A). The 12-month OS rate was 73% (95% CI, 59% to 83%) for the overall population, and 76% (95% CI, 43% to 92%) and 72% (95% CI, 55% to 83%) for patients with squamous and nonsquamous histology, respectively. The 18-month OS rate was 57% (95% CI, 42% to 70%) for the overall population and 42% (95% CI, 16% to 67%) and 63% (95% CI, 45% to 76%) for patients with squamous and nonsquamous histology, respectively. Median PFS was 3.6 months (range, < 0.1+ to 28.0+ months), and the 24-week PFS rate was 41% (95% CI, 27% to 54%; Table 3). Median PFS and 24-week PFS rate were 3.5 months (range, 1.4 to 25.6+ months) and 31% (95% CI, 9% to 55%) in patients with squamous NSCLC, and 5.0 months (range, < 0.1+ to 28.0+ months) and 45% (95% CI, 28% to 60%) in patients with nonsquamous NSCLC (Table 3 and Fig 2B).
Fig 2.
Kaplan-Meier curves of progression-free survival (PFS) and overall survival (OS) by histology in patients with advanced non–small-cell lung cancer (NSCLC) treated with nivolumab monotherapy. (A) OS by NSCLC histology. Data for OS are based on an August 2015 database lock. (B) PFS by NSCLC histology. Data for PFS are based on a March 2015 database lock. Symbols denote censored observations.
Efficacy by Tumor PD-L1 Expression
Tumor PD-L1 expression was not quantifiable in 12% of patients (six of 52), either because of suboptimal tissue amount or quality (eg, too few tumor cells or no cells, improper fixation, or sectioning artifacts; n = 5) or because tumor tissue was unavailable (n = 1). Of the 46 patients (88%) with tumor specimens evaluable for PD-L1 expression, 70% (32 of 46) and 30% (14 of 46) had ≥ 1% and < 1% PD-L1 expression, respectively; 57% (26 of 46) and 43% (20 of 46) had ≥ 5% and < 5% PD-L1 expression, respectively. Clinical activity was observed regardless of PD-L1 expression, with higher ORRs in patients whose tumors expressed PD-L1 versus patients with low tumor PD-L1 expression across all expression levels (Table 4). Confirmed ORR was 28% (nine of 32) and 14% (two of 14) in tumors with ≥ 1% and < 1% PD-L1 expression and 31% (eight of 26) and 15% (three of 20) in tumors with ≥ 5% and < 5% PD-L1 expression, respectively. Best percentage change in target lesion tumor burden from baseline by 1% PD-L1 expression is shown in the Data Supplement. There was no clear association between PFS or OS and baseline PD-L1 expression (Data Supplement).
Table 4.
Efficacy of Nivolumab Monotherapy by Baseline Tumor PD-L1 Expression*
| PD-L1 Expression, % | Confirmed ORR†, % (n/N) | Median DOR‡, Months (Range) | Ongoing Responders§, % | PFS at 24 Weeks||, % (95% CI) | Median PFS, Months (Range) | 1-Year OS, % (95% CI) | 18-Month OS, % (95% CI) |
|---|---|---|---|---|---|---|---|
| ≥ 50 | 50 (6/12) | NR (5.3+ to 25.8+) | 83 | 58 (27 to 80) | 8.3 (2.2 to 28.0+) | 83 (48 to 96) | 83 (48 to 96) |
| ≥ 25 | 44 (8/18) | NR (4.2 to 25.8+) | 75 | 50 (26 to 70) | 5.8 (0.2 to 28.0+) | 78 (51 to 91) | 71 (43 to 87) |
| ≥ 10 | 40 (8/20) | NR (4.2 to 25.8+) | 75 | 45 (23 to 65) | 5.2 (0.2 to 28.0+) | 80 (55 to 92) | 68 (41 to 84) |
| ≥ 5 | 31 (8/26) | NR (4.2 to 25.8+) | 75 | 40 (21 to 58) | 3.5 (< 0.1+ to 28.0+) | 73 (52 to 86) | 54 (32 to 71) |
| ≥ 1 | 28 (9/32) | NR (4.2 to 25.8+) | 78 | 39 (22 to 55) | 3.5 (< 0.1+ to 28.0+) | 69 (50 to 82) | 53 (34 to 70) |
| < 50 | 15 (5/34) | NR (4.2 to 12.6+) | 60 | 36 (20 to 52) | 2.4 (< 0.1+ to 16.0+) | 68 (49 to 81) | 48 (30 to 64) |
| < 25 | 11 (3/28) | NR (5.8 to 9.5+) | 67 | 36 (18 to 54) | 2.4 (< 0.1+ to 16.0+) | 68 (47 to 82) | 48 (29 to 66) |
| < 10 | 12 (3/26) | NR (5.8 to 9.5+) | 67 | 39 (20 to 58) | 3.5 (< 0.1+ to 16.0+) | 65 (44 to 80) | 49 (28 to 66) |
| < 5 | 15 (3/20) | NR (5.8 to 9.5+) | 67 | 45 (22 to 65) | 5.0 (< 0.1+ to 16.0+) | 70 (45 to 85) | 60 (36 to 78) |
| < 1 | 14 (2/14) | NR (5.8 to 9.5+) | 50 | 50 (21 to 74) | 6.6 (< 0.1+ to 12.4) | 79 (47 to 93) | 64 (34 to 83) |
| Unknown | 17 (1/6) | 16.5 (16.5 to 16.5) | 0 | NC | 3.7 (1.2+ to 24.7) | 83 (27 to 97) | NC |
NOTE. NR due to high percentage of ongoing response. Plus symbol (+) indicates a censored value.
Abbreviations: CR, complete response; DOR, duration of response; NC, not calculated; NR, not reached; ORR, objective response rate; OS, overall survival; PD-L1, programmed death–ligand 1; PFS, progression-free survival; PR, partial response.
Data for response and PFS are based on a March 2015 database lock. Data for OS are based on an August 2015 database lock.
Includes patients with initial observations of CR and PR that were subsequently confirmed by repeat scans performed no earlier than 4 weeks after the original observation.
Time from first response to documented progression, death within 100 days of last nivolumab dose, or last tumor assessment before subsequent anticancer therapy (for censored data).
Includes patients with confirmed CR or PR who neither progressed nor died within 100 days of last nivolumab dose.
PFS rate was defined as the probability of a patient remaining progression-free and alive up to 24 weeks.
Efficacy by Smoking History and by EGFR and KRAS Mutation Status
Confirmed ORRs and disease control rates were numerically higher among patients who had a history of smoking (current, 33% [one of three] and 67% [two of three]; former, 26% [10 of 38] and 53% [20 of 38]; and never, 9% [one of 11] and 36% [four of 11]; Data Supplement). Median PFS also seemed longer in current (10.5 months, [range, 2.2 to 14.5]) and former (3.7 months [range, < 0.1+ to 28.0+]) smokers compared with never smokers (2.0 months [range, < 0.1+ to 8.1]).
Among patients with nonsquamous NSCLC, responses occurred regardless of EGFR or KRAS mutation status (Data Supplement). ORR in patients with EGFR-mutant, EGFR-wildtype, KRAS-mutant, and KRAS-wildtype tumors was 14% (one of seven), 30% (nine of 30), 33% (three of nine), and 25% (two of eight), respectively. Median PFS was numerically shorter and the 24-week PFS rate numerically lower for patients with EGFR-mutant tumors (1.8 months [range, 0.2 to 7.6+ months] and 14% [95% CI, 1% to 46%]) versus patients with EGFR-wildtype tumors (6.6 months [range, < 0.1+ to 28.0+ months] and 51% [95% CI, 30% to 68%]). Conversely, median PFS was numerically longer and the 24-week PFS rate numerically higher for patients with KRAS-mutant tumors (11.8 months [range, < 0.1+ to 28.0+ months] and 88% [95% CI, 39% to 98%]) versus patients with KRAS-wildtype tumors (2.3 months [range, 1.2+ to 11.6+ months] and 29% [95% CI, 4% to 61%]).
DISCUSSION
Current treatment algorithms for first-line advanced non–EGFR/ALK-driven NSCLC include PT-DC with or without bevacizumab, with modest response rates and survival, and risk for significant toxicity.1-7 Alternative strategies are clearly needed to improve survival with better tolerance. Here, we show robust activity of nivolumab in the first-line setting, with good tolerance relative to standard first-line chemotherapy. Although results are limited by a highly selected population without randomization to standard chemotherapy, DOR (NR, range 4.2 to 25.8+ months) and survival (median OS, 19.4 months; 1 year and 18-month OS, 73% and 57%, respectively) are encouraging, far exceeding expectations with chemotherapy alone (median DOR, 4.5 to 9.4 months; median OS, 8.1 to 10.3 months; 1-year OS rates, 30% to 44%).1-6 Furthermore, durable, complete clinical responses in four patients would be unexpected with chemotherapy and speaks to the potential of immunotherapy.
As first-line therapy, nivolumab was well tolerated, with 19% of patients reporting grade 3 to 4 treatment-related AEs and no treatment-related deaths. In contrast to typical toxicities of PT-DC,1,4-6 no cases of neutropenia, thrombocytopenia, or anemia were observed with nivolumab monotherapy. Consistent with prior nivolumab studies,14,21,22,30 treatment-related select AEs affecting the skin, endocrine, gastrointestinal, and pulmonary organ classes were generally of low grade (grade 3 to 4, 0% to 4% across categories) and manageable with drug interruption or discontinuation, immune-suppressive agents (primarily corticosteroids), and/or hormone replacement per established guidelines. Six percent of patients (three of 52) developed pneumonitis, with one high-grade event (grade 3) treated successfully with corticosteroids.
As with any systemic anticancer therapy, patient selection on the basis of clinical and/or molecular features promises to spare patients from potentially toxic therapies with low likelihood of benefit, allowing timely treatment with other therapies. Currently, first-line therapy for patients with advanced non–EGFR/ALK-driven lung cancer is based on histology, without established predictive molecular markers.2 The value of tumor PD-L1 expression as a predictive biomarker for benefit with PD-1–axis inhibitors like nivolumab has not been fully established. Although pembrolizumab, another anti–PD-1 antibody, is currently approved for use only in PD-L1–positive, previously treated advanced NSCLC (on the basis of phase I data),31 nivolumab use in this setting does not require tumor PD-L1 expression. Phase III trials leading to the approval of nivolumab as salvage therapy in advanced NSCLC did consider the predictive value of PD-L1 expression as a secondary end point,21,22 suggesting a higher magnitude of benefit with nivolumab in patients with PD-L1–expressing nonsquamous NSCLC.22 However, the absence of PD-L1 expression did not preclude response to or compromise survival with nivolumab in patients with squamous or nonsquamous NSCLC.21,22 In the first-line setting, where chemotherapy has a higher response rate and greater survival advantage than in the second-line setting,2 tumor PD-L1 expression may have a more important role in selecting a PD-1–axis inhibitor over standard chemotherapy. In the current study, responses were noted regardless of tumor PD-L1 expression; however, numerically higher ORRs were observed in patients whose tumors expressed PD-L1, with a trend toward greater response as PD-L1 expression level increased. Despite the limitations of quantifying PD-L1 expression—a continuous variable—using arbitrary cutoffs, results from this exploratory analysis, and the higher prevalence of PD-L1 expression observed in the first-line versus second- or later-line settings,21,22 may support the use of nivolumab as first-line therapy for advanced PD-L1–expressing NSCLC. Two phase III trials are evaluating the predictive role of PD-L1 for nivolumab efficacy in the first-line setting. CheckMate 026 (NCT02041533) is evaluating nivolumab versus standard PT-DC in patients with stage IV or recurrent PD-L1–positive NSCLC who had no prior chemotherapy for advanced disease and has completed accrual. The other trial, CheckMate 227 (NCT02477826), is evaluating nivolumab or nivolumab combined with ipilimumab versus standard PT-DC with or without nivolumab.
As observed in other trials evaluating nivolumab as salvage therapy for advanced NSCLC, smoking history seemed to influence nivolumab activity, although the small number of patients limits conclusions. One potential explanation for lower activity in never-smokers has been lower tumor mutational load and associated neoantigens expected in these populations, with less-immunogenic tumors. Indeed, preliminary data suggest increased sensitivity to immune-checkpoint inhibitors in patients with tumors bearing a high mutational load (eg, smoking-associated lung cancer).32,33
In conclusion, nivolumab monotherapy as first-line therapy for patients with advanced NSCLC was generally well tolerated, showing promising activity with a manageable safety profile. Nivolumab is currently being evaluated in phase III trials versus standard first-line therapies for patients with PD-L1–positive advanced NSCLC.
Acknowledgment
We thank the patients and their families, as well as the investigators and study teams, for making this trial possible; the staff at Dako North America for collaborative development of the automated PD-L1 immunohistochemistry assay; and Suresh Alaparthy for serving as protocol manager. Medical writing and editorial assistance were provided by Elyse Smith and Anne Cooper, of StemScientific, with funding from Bristol-Myers Squibb.
Footnotes
Supported by Bristol-Myers Squibb.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT01454102.
Presented in part at the European Cancer Congress Annual Meeting, Vienna, Austria, September 25-29, 2015; and the American Society of Clinical Oncology Annual Meeting, Chicago, IL, May 29-June 2, 2015.
See accompanying editorial on page 2953
AUTHOR CONTRIBUTIONS
Conception and design: Scott Gettinger, Julie Brahmer, Frances A. Shepherd, Scott Antonia, Yun Shen, Christopher T. Harbison, Matthew D. Hellmann
Provision of study materials or patients: Laura Q. Chow, Hossein Borghaei, Julie Brahmer, Frances A. Shepherd, Rosalyn A. Juergens, Scott A. Laurie, Matthew D. Hellmann
Collection and assembly of data: Scott Gettinger, Naiyer A. Rizvi, Laura Q. Chow, Hossein Borghaei, Julie Brahmer, Neal Ready, David E. Gerber, Scott Antonia, Jonathan W. Goldman, Faith E. Nathan, Matthew D. Hellmann
Data analysis and interpretation: Scott Gettinger, Laura Q. Chow, Hossein Borghaei, Julie Brahmer, Neal Ready, David E. Gerber, Scott Antonia, Jonathan W. Goldman, Rosalyn A. Juergens, Scott A. Laurie, Faith E. Nathan, Yun Shen, Christopher T. Harbison, Matthew D. Hellmann
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Nivolumab Monotherapy for First-Line Treatment of Advanced Non–Small-Cell Lung Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Scott Gettinger
Consulting or Advisory Role: Janssen Pharmaceuticals, Bristol-Myers Squibb, ARIAD Pharmaceuticals
Research Funding: Bristol-Myers Squibb (Inst), Genentech (Inst), ARIAD Pharmaceuticals (Inst), AstraZeneca/MedImmune (Inst), Boehringer Ingelheim (Inst), Incyte (Inst), Pfizer (Inst)
Naiyer A. Rizvi
Stock or Other Ownership: Gritstone Oncology
Honoraria: Merck Sharp & Dohme
Consulting or Advisory Role: AstraZeneca/MedImmune, Genentech, Novartis, Merck Sharp & Dohme, Bristol-Myers Squibb
Laura Q. Chow
Honoraria: Amgen, Astellas Pharma
Consulting or Advisory Role: Merck, Novartis, Emergent BioSolutions, Amgen, Bristol-Myers Squibb, Seattle Genetics
Research Funding: Genentech (Inst), Pfizer (Inst), Merck (Inst), Novartis (Inst), Bristol-Myers Squibb (Inst), VentiRx Pharmaceuticals (Inst), AstraZeneca/MedImmune (Inst), Eli Lilly (Inst), OSI Pharmaceuticals (Inst), GlaxoSmithKline/NCCN (Inst), Incyte (Inst)
Travel, Accommodations, Expenses: Merck, Novartis
Hossein Borghaei
Honoraria: Bristol-Myers Squibb, Celgene
Consulting or Advisory Role: Bristol-Myers Squibb, Eli Lilly, Celgene, Clovis Oncology, Genentech
Research Funding: Millennium Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Eli Lilly, Clovis Oncology, Celgene, Genentech
Julie Brahmer
Consulting or Advisory Role: Merck KGaA, Bristol-Myers Squibb, Eli Lilly
Research Funding: Bristol-Myers Squibb (Inst), Merck (Inst), AstraZeneca (Inst), Celgene (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Merck
Other Relationship: Bristol-Myers Squibb
Neal Ready
Honoraria: Bristol-Myers Squibb, Celgene, Heat Biologics
Travel, Accommodations, Expenses: AstraZeneca
David E. Gerber
Stock or Other Ownership: Gilead Sciences
Research Funding: ImmunoGen (Inst), ArQule (Inst), Synta Pharmaceuticals (Inst), Genentech (Inst), Celgene (Inst), ImClone Systems (Inst), BerGenBio (Inst)
Patents, Royalties, Other Intellectual Property: Royalties from Oxford University Press from two books; royalties from Decision Support in Medicine from the Clinical Decision Support–Oncology on-line program
Travel, Accommodations, Expenses: Eli Lilly, ArQule, Eli Lilly
Frances A. Shepherd
Stock or Other Ownership: Eli Lilly, AstraZeneca
Honoraria: Eli Lilly, AstraZeneca, Bristol-Myers Squibb, Merck Serono, Genentech, Merck/Schering Plough, Boehringer Ingelheim
Consulting or Advisory Role: Eli Lilly, AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Recombio, Synta Pharmaceuticals, Bristol-Myers Squibb
Research Funding: Boehringer Ingelheim (Inst)
Scott Antonia
Stock or Other Ownership: Cellular Biomedicine Group
Honoraria: AstraZeneca, Bristol-Myers Squibb, Merck, Boehringer Ingelheim
Consulting or Advisory Role: Bristol-Myers Squibb, AstraZeneca, Merck, Boehringer Ingelheim
Travel, Accommodations, Expenses: Bristol-Myers Squibb, AstraZeneca, Merck, Boehringer Ingelheim
Jonathan W. Goldman
Honoraria: Clovis Oncology, Genentech, Bristol-Myers Squibb
Research Funding: Lilly, Genentech/Roche, Astex Pharmaceuticals, Synta Pharmaceuticals, Clovis Oncology, Bristol-Myers Squibb, AstraZeneca/MedImmune, Threshold Pharmaceuticals
Rosalyn A. Juergens
Honoraria: Bayer AG, Bristol-Myers Squibb, Boehringer Ingelheim, Beta Pharma, AstraZeneca, AstraZeneca/MedImmune, Roche Canada, Merck Sharp & Dohme
Consulting or Advisory Role: AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche Canada
Research Funding: AstraZeneca/MedImmune (Inst), Bristol-Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Novartis (Inst)
Scott A. Laurie
Honoraria: Pfizer, Bayer AG, Novartis, Boehringer Ingelheim
Faith E. Nathan
Employment: Bristol-Myers Squibb
Stock or Other Ownership: Bristol-Myers Squibb, AstraZeneca, Johnson & Johnson, Eli Lilly
Yun Shen
Employment: Bristol-Myers Squibb, Bristol-Myers Squibb (I)
Stock or Other Ownership: Bristol-Myers Squibb, Bristol-Myers Squibb (I)
Christopher T. Harbison
Employment: Bristol-Myers Squibb
Stock or Other Ownership: Bristol-Myers Squibb
Matthew D. Hellmann
Consulting or Advisory Role: Third Rock Ventures, Bristol-Myers Squibb, Merck, Genentech, Alexion Pharmaceuticals, Inovio Biomedical, AstraZeneca/MedImmune
Research Funding: Bristol-Myers Squibb
REFERENCES
- 1.Kelly K Crowley J Bunn PA Jr, etal: Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non--small-cell lung cancer: A Southwest Oncology Group trial J Clin Oncol 19:3210–3218,2001 [DOI] [PubMed] [Google Scholar]
- 2. National Comprehensive Cancer Network: NSCLC Guidelines v7. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#nscl.
- 3.Sandler A Gray R Perry MC, etal: Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer N Engl J Med 355:2542–2550,2006 [DOI] [PubMed] [Google Scholar]
- 4.Scagliotti GV De Marinis F Rinaldi M, etal: Phase III randomized trial comparing three platinum-based doublets in advanced non-small-cell lung cancer J Clin Oncol 20:4285–4291,2002 [DOI] [PubMed] [Google Scholar]
- 5.Scagliotti GV Parikh P von Pawel J, etal: Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer J Clin Oncol 26:3543–3551,2008 [DOI] [PubMed] [Google Scholar]
- 6.Schiller JH Harrington D Belani CP, etal: Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer N Engl J Med 346:92–98,2002 [DOI] [PubMed] [Google Scholar]
- 7.Patel JD Socinski MA Garon EB, etal: PointBreak: A randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer J Clin Oncol 31:4349–4357,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haaland B Tan PS de Castro G Jr, etal: Meta-analysis of first-line therapies in advanced non-small-cell lung cancer harboring EGFR-activating mutations J Thorac Oncol 9:805–811,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuykendall A, Chiappori A: Advanced EGFR mutation-positive non-small-cell lung cancer: Case report, literature review, and treatment recommendations Cancer Contr 21:67–73,2014 [DOI] [PubMed] [Google Scholar]
- 10.Rosell R Carcereny E Gervais R, etal: Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial Lancet Oncol 13:239–246,2012 [DOI] [PubMed] [Google Scholar]
- 11.Sequist LV Yang JC Yamamoto N, etal: Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations J Clin Oncol 31:3327–3334,2013 [DOI] [PubMed] [Google Scholar]
- 12.Solomon BJ Mok T Kim DW, etal: First-line crizotinib versus chemotherapy in ALK-positive lung cancer N Engl J Med 371:2167–2177,2014 [DOI] [PubMed] [Google Scholar]
- 13.Steuer CE, Khuri FR, Ramalingam SS: The next generation of epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of lung cancer Cancer 121:E1–E6,2015 [DOI] [PubMed] [Google Scholar]
- 14.Gettinger SN Horn L Gandhi L, etal: Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer J Clin Oncol 33:2004–2012,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larkin J Lao CD Urba WJ, etal: Efficacy and safety of nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: A pooled analysis of 4 clinical trials JAMA Oncol 1:433–440,2015 [DOI] [PubMed] [Google Scholar]
- 16.Pardoll DM: The blockade of immune checkpoints in cancer immunotherapy Nat Rev Cancer 12:252–264,2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schadendorf D Hodi FS Robert C, etal: Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma J Clin Oncol 33:1889–1894,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topalian SL Sznol M McDermott DF, etal: Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab J Clin Oncol 32:1020–1030,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brahmer JR Drake CG Wollner I, etal: Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates J Clin Oncol 28:3167–3175,2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C Thudium KB Han M, etal: In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates Cancer Immunol Res 2:846–856,2014 [DOI] [PubMed] [Google Scholar]
- 21.Brahmer J Reckamp KL Baas P, etal: Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer N Engl J Med 373:123–135,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borghaei H Paz-Ares L Horn L, etal: Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer N Engl J Med 373:1627–1639,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. OPDIVO (nivolumab) [package insert]. Princeton, NJ, Bristol-Myers Squibb Company, October 2015.
- 24. European public assessment report (EPAR) for nivolumab BMS product information: Summary of product characteristics. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003985/WC500189765.pdf.
- 25.Eisenhauer EA Therasse P Bogaerts J, etal: New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer 45:228–247,2009 [DOI] [PubMed] [Google Scholar]
- 26. Goldstraw P (ed): International Association for the Study of Lung Cancer: Staging Manual in Thoracic Oncology. Orange Park, FL, Editorial Rx Press, 2009. [Google Scholar]
- 27. National Cancer Institute: Common Terminology Criteria for Adverse Events v4.0. NIH publication 09-7473. May 29, 2009 (v.4.03: June 14, 2010). http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf.
- 28.Clopper CJ, Pearson ES: The use of confidence or fiducial limits illustrated in the case of the binomial Biometrika 26:404–413,1934 [Google Scholar]
- 29. Greenwood M.: The natural duration of cancer. Reports on Public Health and Medical Subjects. 33:1-26. London, United Kingdom, HM Stationery Office, 1926. [Google Scholar]
- 30.Rizvi NA Mazières J Planchard D, etal: Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial Lancet Oncol 16:257–265,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. KEYTRUDA (pembrolizumab) [package insert]. Whitehouse Station, NJ, Merck Sharp & Dohme, a subsidiary of Merck, October 2015.
- 32.Champiat S Ferté C Lebel-Binay S, etal: Exomics and immunogenics: Bridging mutational load and immune checkpoints efficacy OncoImmunology 3:e27817,2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizvi NA Hellmann MD Snyder A, etal: Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer Science 348:124–128,2015 [DOI] [PMC free article] [PubMed] [Google Scholar]