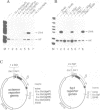
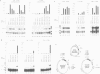
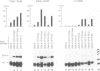
Abstract
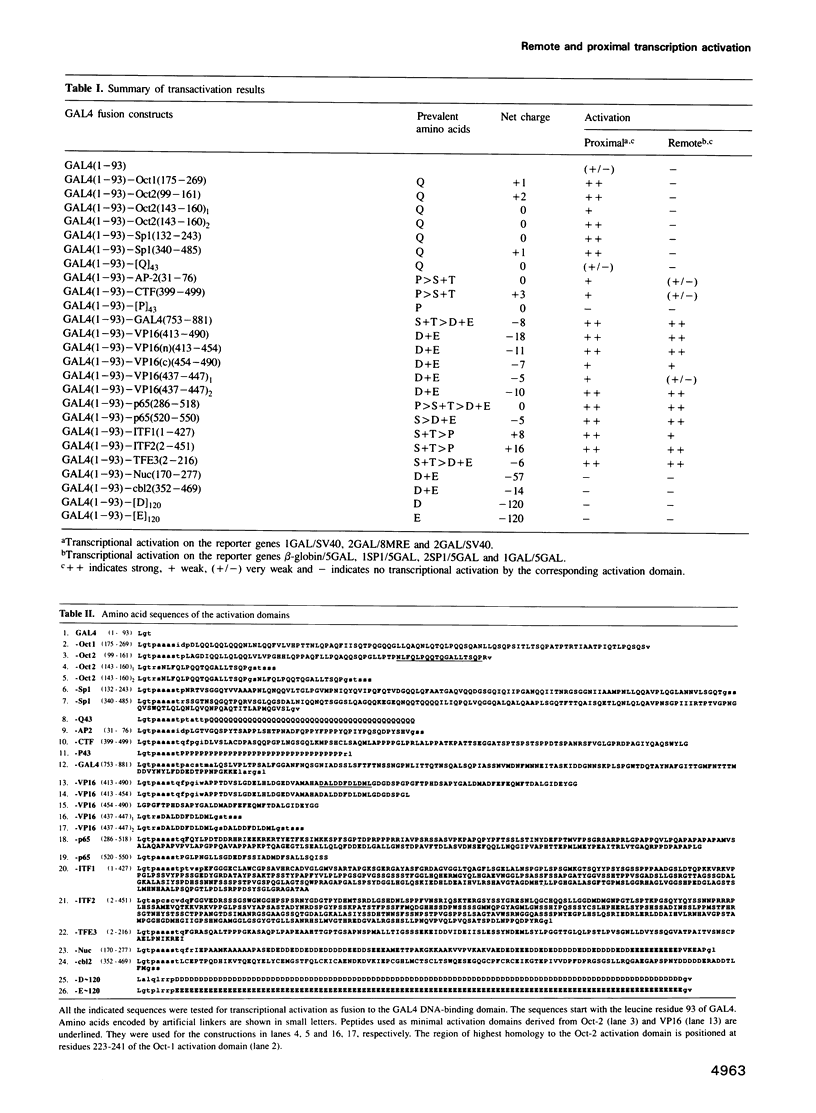
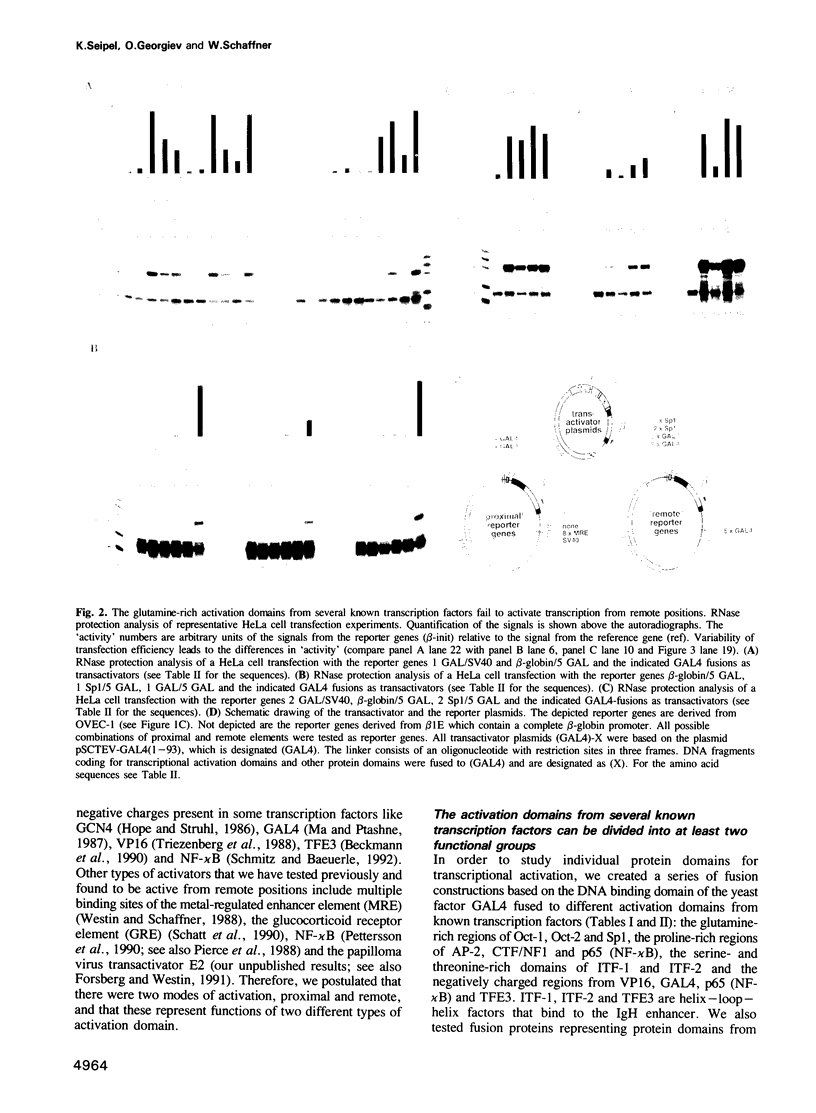
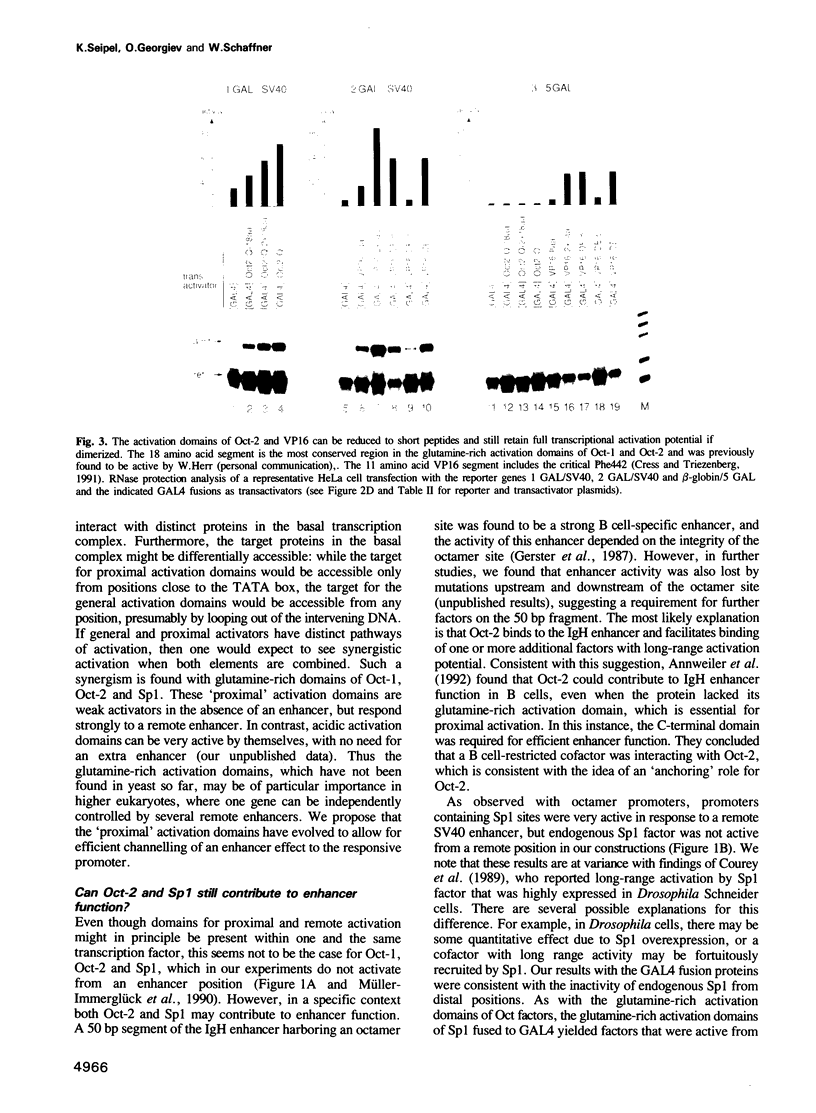
We reported previously that the lymphocyte-derived octamer transcription factor 2A (Oct-2A or OTF-2A) activated both natural immunoglobulin promoters and synthetic promoters which contain the 'octamer' site, but was unable by itself to stimulate transcription from a remote enhancer position. Here we examine a larger set of transcription factors with respect to their proximal versus remote activation. Since a transcription factor may contain more than one activation domain, we have chosen to study the potential of individual activation domains in the context of fusion proteins that contain the DNA binding domain of GALA. We have identified at least two distinct functional classes of transcriptional activation domains. 'Proximal' activation domains, exemplified by glutamine-rich domains of Oct-1, Oct-2A and Sp1, stimulate transcription only from a position close to the TATA box, usually in response to a remote enhancer. 'General' activation domains, derived from VP16, GAL4, p65 (NF-chi B), TFE3, ITF-1 and ITF-2, can activate transcription from remote as well as proximal positions. These domains contain many acidic amino acids and/or other features such as clusters of serine and threonine. The proline-rich activation domains of AP-2 and CTF/NF1 may represent a third class with considerable promoter activity and low but significant enhancer activity. Furthermore, activation domains of both the acidic and glutamine-rich types seem to have a modular structure, since duplicated subdomains can substitute for the entire domain.
Full text
PDF
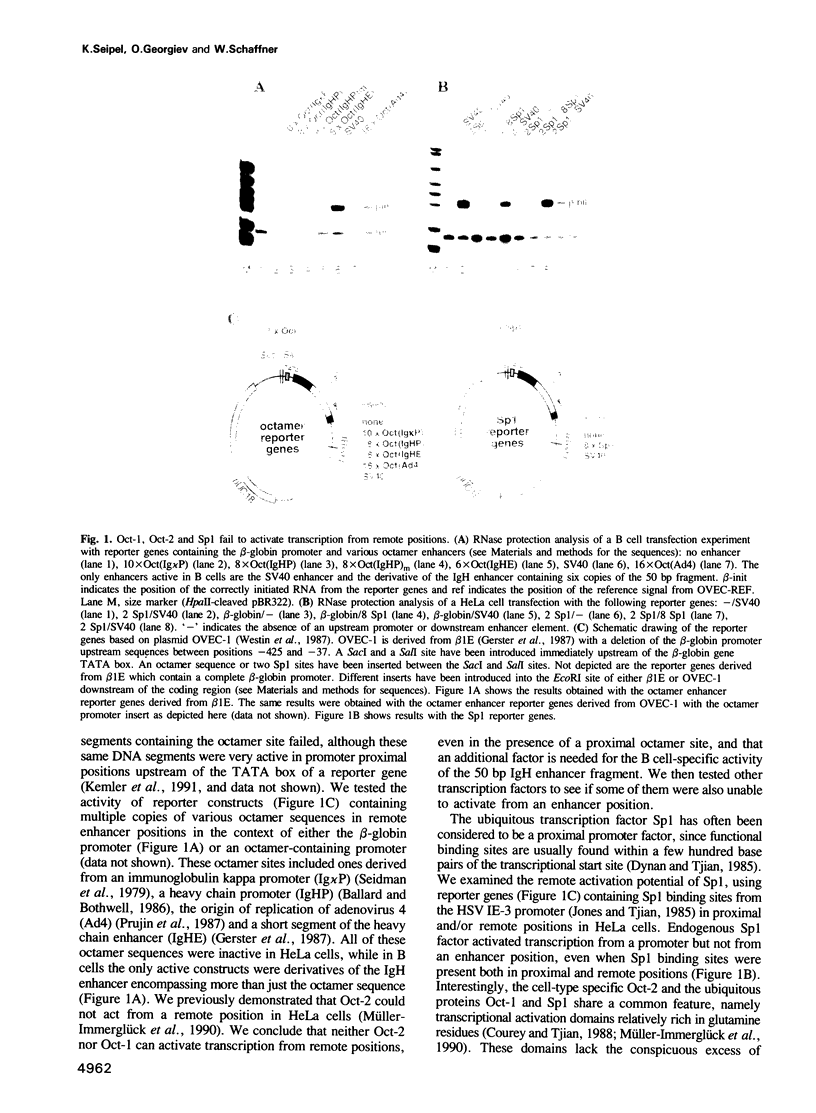



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annweiler A., Müller-Immerglück M., Wirth T. Oct2 transactivation from a remote enhancer position requires a B-cell-restricted activity. Mol Cell Biol. 1992 Jul;12(7):3107–3116. doi: 10.1128/mcb.12.7.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard D. W., Bothwell A. Mutational analysis of the immunoglobulin heavy chain promoter region. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9626–9630. doi: 10.1073/pnas.83.24.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji J., Rusconi S., Schaffner W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981 Dec;27(2 Pt 1):299–308. doi: 10.1016/0092-8674(81)90413-x. [DOI] [PubMed] [Google Scholar]
- Beckmann H., Su L. K., Kadesch T. TFE3: a helix-loop-helix protein that activates transcription through the immunoglobulin enhancer muE3 motif. Genes Dev. 1990 Feb;4(2):167–179. doi: 10.1101/gad.4.2.167. [DOI] [PubMed] [Google Scholar]
- Berger S. L., Piña B., Silverman N., Marcus G. A., Agapite J., Regier J. L., Triezenberg S. J., Guarente L. Genetic isolation of ADA2: a potential transcriptional adaptor required for function of certain acidic activation domains. Cell. 1992 Jul 24;70(2):251–265. doi: 10.1016/0092-8674(92)90100-q. [DOI] [PubMed] [Google Scholar]
- Brent R., Ptashne M. A eukaryotic transcriptional activator bearing the DNA specificity of a prokaryotic repressor. Cell. 1985 Dec;43(3 Pt 2):729–736. doi: 10.1016/0092-8674(85)90246-6. [DOI] [PubMed] [Google Scholar]
- Carey M., Kakidani H., Leatherwood J., Mostashari F., Ptashne M. An amino-terminal fragment of GAL4 binds DNA as a dimer. J Mol Biol. 1989 Oct 5;209(3):423–432. doi: 10.1016/0022-2836(89)90007-7. [DOI] [PubMed] [Google Scholar]
- Carey M., Leatherwood J., Ptashne M. A potent GAL4 derivative activates transcription at a distance in vitro. Science. 1990 Feb 9;247(4943):710–712. doi: 10.1126/science.2405489. [DOI] [PubMed] [Google Scholar]
- Cochran M. D., Weissmann C. Modular structure of the beta-globin and the TK promoters. EMBO J. 1984 Nov;3(11):2453–2459. doi: 10.1002/j.1460-2075.1984.tb02155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courey A. J., Holtzman D. A., Jackson S. P., Tjian R. Synergistic activation by the glutamine-rich domains of human transcription factor Sp1. Cell. 1989 Dec 1;59(5):827–836. doi: 10.1016/0092-8674(89)90606-5. [DOI] [PubMed] [Google Scholar]
- Courey A. J., Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell. 1988 Dec 2;55(5):887–898. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- Cress W. D., Triezenberg S. J. Critical structural elements of the VP16 transcriptional activation domain. Science. 1991 Jan 4;251(4989):87–90. doi: 10.1126/science.1846049. [DOI] [PubMed] [Google Scholar]
- Croston G. E., Kerrigan L. A., Lira L. M., Marshak D. R., Kadonaga J. T. Sequence-specific antirepression of histone H1-mediated inhibition of basal RNA polymerase II transcription. Science. 1991 Feb 8;251(4994):643–649. doi: 10.1126/science.1899487. [DOI] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Cochran M. D., Dobkin C., Reiser J., Weissmann C. Three regions upstream from the cap site are required for efficient and accurate transcription of the rabbit beta-globin gene in mouse 3T6 cells. Cell. 1983 Mar;32(3):695–706. doi: 10.1016/0092-8674(83)90055-7. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Forsberg M., Westin G. Enhancer activation by a single type of transcription factor shows cell type dependence. EMBO J. 1991 Sep;10(9):2543–2551. doi: 10.1002/j.1460-2075.1991.tb07794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel A. D., Kim P. S. Modular structure of transcription factors: implications for gene regulation. Cell. 1991 May 31;65(5):717–719. doi: 10.1016/0092-8674(91)90378-c. [DOI] [PubMed] [Google Scholar]
- Gerster T., Matthias P., Thali M., Jiricny J., Schaffner W. Cell type-specificity elements of the immunoglobulin heavy chain gene enhancer. EMBO J. 1987 May;6(5):1323–1330. doi: 10.1002/j.1460-2075.1987.tb02371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniger E., Ptashne M. Transcription in yeast activated by a putative amphipathic alpha helix linked to a DNA binding unit. Nature. 1987 Dec 17;330(6149):670–672. doi: 10.1038/330670a0. [DOI] [PubMed] [Google Scholar]
- Henthorn P., Kiledjian M., Kadesch T. Two distinct transcription factors that bind the immunoglobulin enhancer microE5/kappa 2 motif. Science. 1990 Jan 26;247(4941):467–470. doi: 10.1126/science.2105528. [DOI] [PubMed] [Google Scholar]
- Hope I. A., Struhl K. Functional dissection of a eukaryotic transcriptional activator protein, GCN4 of yeast. Cell. 1986 Sep 12;46(6):885–894. doi: 10.1016/0092-8674(86)90070-x. [DOI] [PubMed] [Google Scholar]
- Jones K. A., Tjian R. Sp1 binds to promoter sequences and activates herpes simplex virus 'immediate-early' gene transcription in vitro. Nature. 1985 Sep 12;317(6033):179–182. doi: 10.1038/317179a0. [DOI] [PubMed] [Google Scholar]
- Kemler I., Bucher E., Seipel K., Müller-Immerglück M. M., Schaffner W. Promoters with the octamer DNA motif (ATGCAAAT) can be ubiquitous or cell type-specific depending on binding affinity of the octamer site and Oct-factor concentration. Nucleic Acids Res. 1991 Jan 25;19(2):237–242. doi: 10.1093/nar/19.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laybourn P. J., Kadonaga J. T. Role of nucleosomal cores and histone H1 in regulation of transcription by RNA polymerase II. Science. 1991 Oct 11;254(5029):238–245. doi: 10.1126/science.254.5029.238. [DOI] [PubMed] [Google Scholar]
- Ma J., Ptashne M. Deletion analysis of GAL4 defines two transcriptional activating segments. Cell. 1987 Mar 13;48(5):847–853. doi: 10.1016/0092-8674(87)90081-x. [DOI] [PubMed] [Google Scholar]
- Martinez E., Dusserre Y., Wahli W., Mermod N. Synergistic transcriptional activation by CTF/NF-I and the estrogen receptor involves stabilized interactions with a limiting target factor. Mol Cell Biol. 1991 Jun;11(6):2937–2945. doi: 10.1128/mcb.11.6.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias P., Müller M. M., Schreiber E., Rusconi S., Schaffner W. Eukaryotic expression vectors for the analysis of mutant proteins. Nucleic Acids Res. 1989 Aug 11;17(15):6418–6418. doi: 10.1093/nar/17.15.6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Moreau P., Hen R., Wasylyk B., Everett R., Gaub M. P., Chambon P. The SV40 72 base repair repeat has a striking effect on gene expression both in SV40 and other chimeric recombinants. Nucleic Acids Res. 1981 Nov 25;9(22):6047–6068. doi: 10.1093/nar/9.22.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Immerglück M. M., Schaffner W., Matthias P. Transcription factor Oct-2A contains functionally redundant activating domains and works selectively from a promoter but not from a remote enhancer position in non-lymphoid (HeLa) cells. EMBO J. 1990 May;9(5):1625–1634. doi: 10.1002/j.1460-2075.1990.tb08282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M. M., Ruppert S., Schaffner W., Matthias P. A cloned octamer transcription factor stimulates transcription from lymphoid-specific promoters in non-B cells. Nature. 1988 Dec 8;336(6199):544–551. doi: 10.1038/336544a0. [DOI] [PubMed] [Google Scholar]
- Pettersson M., Schaffner W. Synergistic activation of transcription by multiple binding sites for NF-kappa B even in absence of co-operative factor binding to DNA. J Mol Biol. 1990 Jul 20;214(2):373–380. doi: 10.1016/0022-2836(90)90187-q. [DOI] [PubMed] [Google Scholar]
- Pierce J. W., Lenardo M., Baltimore D. Oligonucleotide that binds nuclear factor NF-kappa B acts as a lymphoid-specific and inducible enhancer element. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1482–1486. doi: 10.1073/pnas.85.5.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruijn G. J., van Driel W., van Miltenburg R. T., van der Vliet P. C. Promoter and enhancer elements containing a conserved sequence motif are recognized by nuclear factor III, a protein stimulating adenovirus DNA replication. EMBO J. 1987 Dec 1;6(12):3771–3778. doi: 10.1002/j.1460-2075.1987.tb02712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusconi S., Severne Y., Georgiev O., Galli I., Wieland S. A novel expression assay to study transcriptional activators. Gene. 1990 May 14;89(2):211–221. doi: 10.1016/0378-1119(90)90008-f. [DOI] [PubMed] [Google Scholar]
- Sadowski I., Ma J., Triezenberg S., Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988 Oct 6;335(6190):563–564. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- Sadowski I., Ptashne M. A vector for expressing GAL4(1-147) fusions in mammalian cells. Nucleic Acids Res. 1989 Sep 25;17(18):7539–7539. doi: 10.1093/nar/17.18.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatt M. D., Rusconi S., Schaffner W. A single DNA-binding transcription factor is sufficient for activation from a distant enhancer and/or from a promoter position. EMBO J. 1990 Feb;9(2):481–487. doi: 10.1002/j.1460-2075.1990.tb08134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirm S., Jiricny J., Schaffner W. The SV40 enhancer can be dissected into multiple segments, each with a different cell type specificity. Genes Dev. 1987 Mar;1(1):65–74. doi: 10.1101/gad.1.1.65. [DOI] [PubMed] [Google Scholar]
- Schmitz M. L., Baeuerle P. A. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO J. 1991 Dec;10(12):3805–3817. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman J. G., Max E. E., Leder P. A kappa-immunoglobulin gene is formed by site-specific recombination without further somatic mutation. Nature. 1979 Aug 2;280(5721):370–375. doi: 10.1038/280370a0. [DOI] [PubMed] [Google Scholar]
- Serfling E., Lübbe A., Dorsch-Häsler K., Schaffner W. Metal-dependent SV40 viruses containing inducible enhancers from the upstream region of metallothionein genes. EMBO J. 1985 Dec 30;4(13B):3851–3859. doi: 10.1002/j.1460-2075.1985.tb04157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava M., Fleming P. J., Pollard H. B., Burns A. L. Cloning and sequencing of the human nucleolin cDNA. FEBS Lett. 1989 Jun 19;250(1):99–105. doi: 10.1016/0014-5793(89)80692-1. [DOI] [PubMed] [Google Scholar]
- Staudt L. M., Lenardo M. J. Immunoglobulin gene transcription. Annu Rev Immunol. 1991;9:373–398. doi: 10.1146/annurev.iy.09.040191.002105. [DOI] [PubMed] [Google Scholar]
- Sturm R. A., Das G., Herr W. The ubiquitous octamer-binding protein Oct-1 contains a POU domain with a homeo box subdomain. Genes Dev. 1988 Dec;2(12A):1582–1599. doi: 10.1101/gad.2.12a.1582. [DOI] [PubMed] [Google Scholar]
- Tanese N., Pugh B. F., Tjian R. Coactivators for a proline-rich activator purified from the multisubunit human TFIID complex. Genes Dev. 1991 Dec;5(12A):2212–2224. doi: 10.1101/gad.5.12a.2212. [DOI] [PubMed] [Google Scholar]
- Tasset D., Tora L., Fromental C., Scheer E., Chambon P. Distinct classes of transcriptional activating domains function by different mechanisms. Cell. 1990 Sep 21;62(6):1177–1187. doi: 10.1016/0092-8674(90)90394-t. [DOI] [PubMed] [Google Scholar]
- Triezenberg S. J., Kingsbury R. C., McKnight S. L. Functional dissection of VP16, the trans-activator of herpes simplex virus immediate early gene expression. Genes Dev. 1988 Jun;2(6):718–729. doi: 10.1101/gad.2.6.718. [DOI] [PubMed] [Google Scholar]
- Westin G., Gerster T., Müller M. M., Schaffner G., Schaffner W. OVEC, a versatile system to study transcription in mammalian cells and cell-free extracts. Nucleic Acids Res. 1987 Sep 11;15(17):6787–6798. doi: 10.1093/nar/15.17.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin G., Schaffner W. A zinc-responsive factor interacts with a metal-regulated enhancer element (MRE) of the mouse metallothionein-I gene. EMBO J. 1988 Dec 1;7(12):3763–3770. doi: 10.1002/j.1460-2075.1988.tb03260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T., Admon A., Lüscher B., Tjian R. Cloning and expression of AP-2, a cell-type-specific transcription factor that activates inducible enhancer elements. Genes Dev. 1988 Dec;2(12A):1557–1569. doi: 10.1101/gad.2.12a.1557. [DOI] [PubMed] [Google Scholar]
- Workman J. L., Taylor I. C., Kingston R. E. Activation domains of stably bound GAL4 derivatives alleviate repression of promoters by nucleosomes. Cell. 1991 Feb 8;64(3):533–544. doi: 10.1016/0092-8674(91)90237-s. [DOI] [PubMed] [Google Scholar]