Abstract
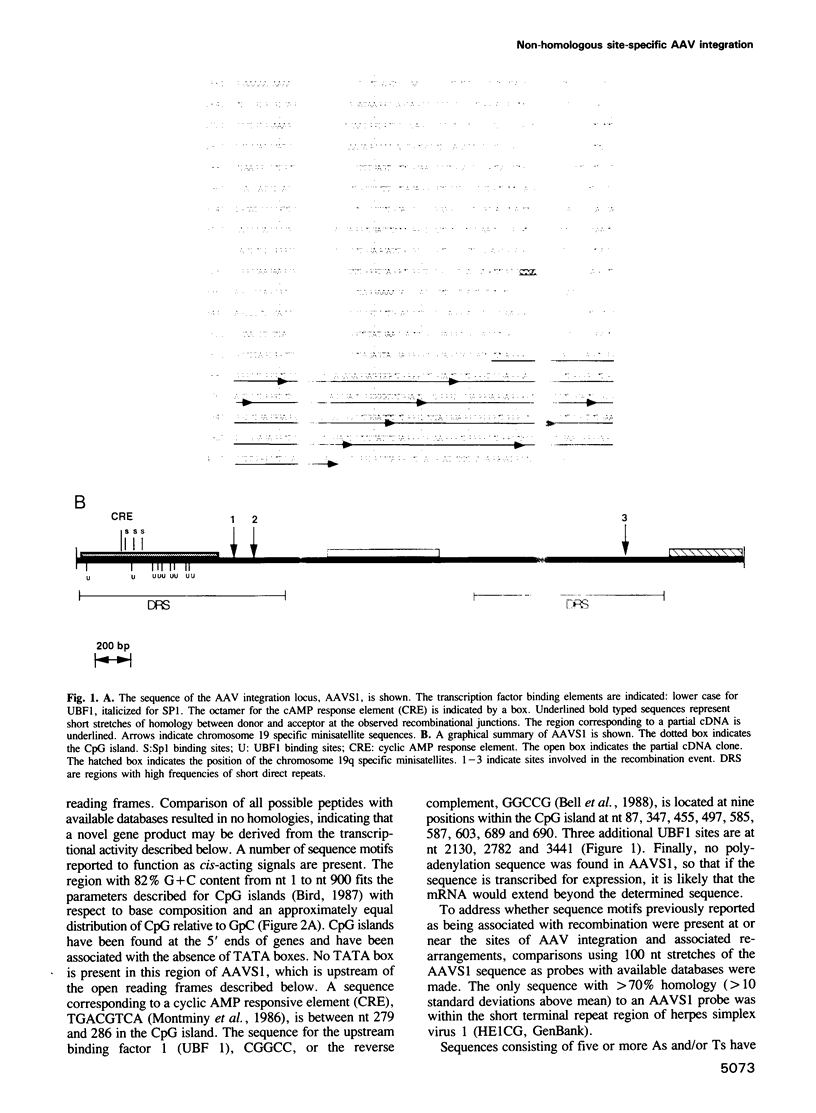
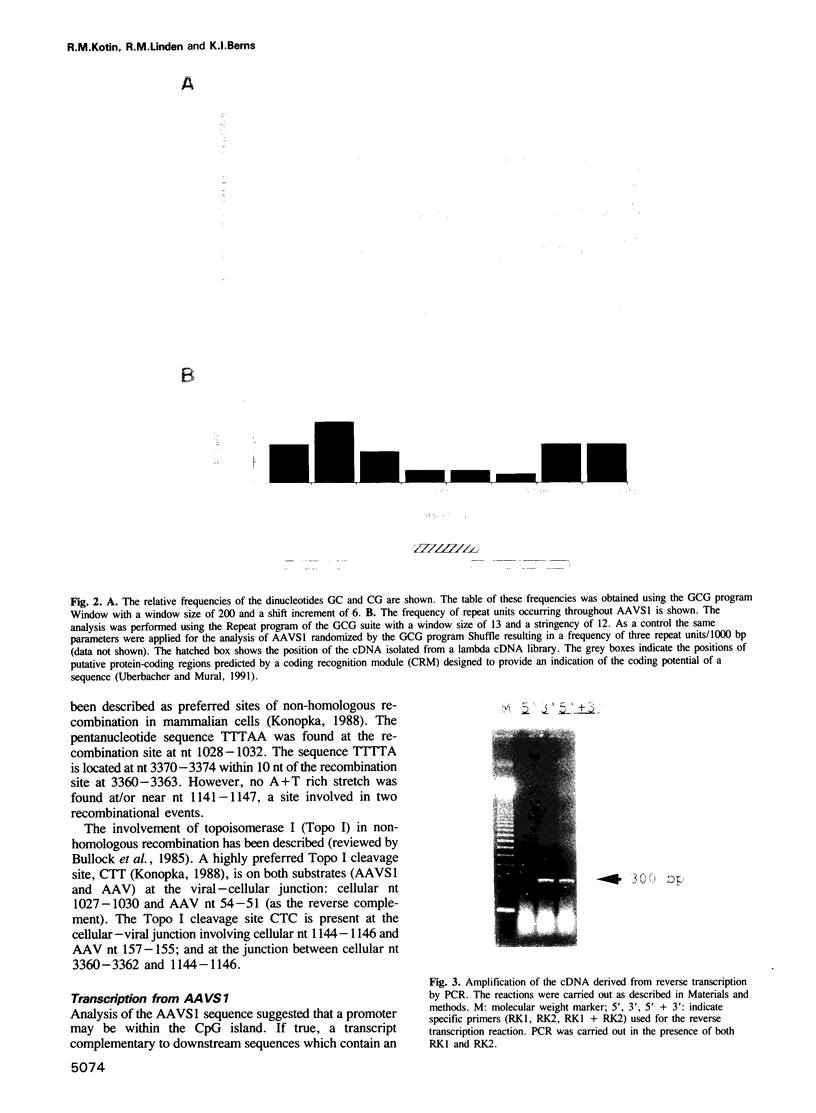
The human parvovirus, adeno-associated virus (AAV), has been shown to integrate preferentially into human chromosome 19 q13.3-qter. The human target sequence for AAV integration (AAVS1) was cloned and sequenced. By analysis of the proviral junctions it was determined that integration of the AAV DNA occurred via a non-homologous recombination pathway although there were either four or five identical nucleotides at the junctions. Integration was a multistep, concerted process that resulted in cellular sequence rearrangements. The sequence of the integration locus was analyzed for possible recombination signals. Direct repeats at a much greater than random occurrence were found distributed non-uniformly throughout the AAVS1 sequence. A CpG island containing transcription factor binding site elements is suggestive of a TATA-less promoter. Evidence for transcriptional activity was provided by PCR amplification of reverse transcribed RNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Okazaki K., Sakano H. Two pairs of recombination signals are sufficient to cause immunoglobulin V-(D)-J joining. Science. 1987 Nov 20;238(4830):1134–1138. doi: 10.1126/science.3120312. [DOI] [PubMed] [Google Scholar]
- Aslanidis C., Jansen G., Amemiya C., Shutler G., Mahadevan M., Tsilfidis C., Chen C., Alleman J., Wormskamp N. G., Vooijs M. Cloning of the essential myotonic dystrophy region and mapping of the putative defect. Nature. 1992 Feb 6;355(6360):548–551. doi: 10.1038/355548a0. [DOI] [PubMed] [Google Scholar]
- Bantel-Schaal U., Stöhr M. Influence of adeno-associated virus on adherence and growth properties of normal cells. J Virol. 1992 Feb;66(2):773–779. doi: 10.1128/jvi.66.2.773-779.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell S. P., Learned R. M., Jantzen H. M., Tjian R. Functional cooperativity between transcription factors UBF1 and SL1 mediates human ribosomal RNA synthesis. Science. 1988 Sep 2;241(4870):1192–1197. doi: 10.1126/science.3413483. [DOI] [PubMed] [Google Scholar]
- Berns K. I. Parvovirus replication. Microbiol Rev. 1990 Sep;54(3):316–329. doi: 10.1128/mr.54.3.316-329.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohenzky R. A., LeFebvre R. B., Berns K. I. Sequence and symmetry requirements within the internal palindromic sequences of the adeno-associated virus terminal repeat. Virology. 1988 Oct;166(2):316–327. doi: 10.1016/0042-6822(88)90502-8. [DOI] [PubMed] [Google Scholar]
- Bullock P., Champoux J. J., Botchan M. Association of crossover points with topoisomerase I cleavage sites: a model for nonhomologous recombination. Science. 1985 Nov 22;230(4728):954–958. doi: 10.1126/science.2997924. [DOI] [PubMed] [Google Scholar]
- Cheung A. K., Hoggan M. D., Hauswirth W. W., Berns K. I. Integration of the adeno-associated virus genome into cellular DNA in latently infected human Detroit 6 cells. J Virol. 1980 Feb;33(2):739–748. doi: 10.1128/jvi.33.2.739-748.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collick A., Dunn M. G., Jeffreys A. J. Minisatellite binding protein Msbp-1 is a sequence-specific single-stranded DNA-binding protein. Nucleic Acids Res. 1991 Dec 11;19(23):6399–6404. doi: 10.1093/nar/19.23.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das H. K., Jackson C. L., Miller D. A., Leff T., Breslow J. L. The human apolipoprotein C-II gene sequence contains a novel chromosome 19-specific minisatellite in its third intron. J Biol Chem. 1987 Apr 5;262(10):4787–4793. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feichtinger W., Schmid M. Increased frequencies of sister chromatid exchanges at common fragile sites (1)(q42) and (19)(q13). Hum Genet. 1989 Sep;83(2):145–147. doi: 10.1007/BF00286707. [DOI] [PubMed] [Google Scholar]
- Grossman Z., Berns K. I., Winocour E. Structure of simian virus 40-adeno-associated virus recombinant genomes. J Virol. 1985 Nov;56(2):457–465. doi: 10.1128/jvi.56.2.457-465.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman Z., Winocour E., Berns K. I. Recombination between simian virus 40 and adeno-associated virus: virion coinfection compared to DNA cotransfection. Virology. 1984 Apr 15;134(1):125–137. doi: 10.1016/0042-6822(84)90278-2. [DOI] [PubMed] [Google Scholar]
- Hermonat P. L., Labow M. A., Wright R., Berns K. I., Muzyczka N. Genetics of adeno-associated virus: isolation and preliminary characterization of adeno-associated virus type 2 mutants. J Virol. 1984 Aug;51(2):329–339. doi: 10.1128/jvi.51.2.329-339.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im D. S., Muzyczka N. Factors that bind to adeno-associated virus terminal repeats. J Virol. 1989 Jul;63(7):3095–3104. doi: 10.1128/jvi.63.7.3095-3104.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im D. S., Muzyczka N. The AAV origin binding protein Rep68 is an ATP-dependent site-specific endonuclease with DNA helicase activity. Cell. 1990 May 4;61(3):447–457. doi: 10.1016/0092-8674(90)90526-k. [DOI] [PubMed] [Google Scholar]
- Konopka A. K. Compilation of DNA strand exchange sites for non-homologous recombination in somatic cells. Nucleic Acids Res. 1988 Mar 11;16(5):1739–1758. doi: 10.1093/nar/16.5.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneluk R. G., MacLeod H. L., McKeithan T. W., Brooks J. D., MacKenzie A. E. A chromosome 19 clone from a translocation breakpoint shows close linkage and linkage disequilibrium with myotonic dystrophy. Genomics. 1989 Feb;4(2):146–151. doi: 10.1016/0888-7543(89)90293-0. [DOI] [PubMed] [Google Scholar]
- Kotin R. M., Berns K. I. Organization of adeno-associated virus DNA in latently infected Detroit 6 cells. Virology. 1989 Jun;170(2):460–467. doi: 10.1016/0042-6822(89)90437-6. [DOI] [PubMed] [Google Scholar]
- Kotin R. M., Menninger J. C., Ward D. C., Berns K. I. Mapping and direct visualization of a region-specific viral DNA integration site on chromosome 19q13-qter. Genomics. 1991 Jul;10(3):831–834. doi: 10.1016/0888-7543(91)90470-y. [DOI] [PubMed] [Google Scholar]
- Kotin R. M., Siniscalco M., Samulski R. J., Zhu X. D., Hunter L., Laughlin C. A., McLaughlin S., Muzyczka N., Rocchi M., Berns K. I. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin C. A., Cardellichio C. B., Coon H. C. Latent infection of KB cells with adeno-associated virus type 2. J Virol. 1986 Nov;60(2):515–524. doi: 10.1128/jvi.60.2.515-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre R. B., Riva S., Berns K. I. Conformation takes precedence over sequence in adeno-associated virus DNA replication. Mol Cell Biol. 1984 Jul;4(7):1416–1419. doi: 10.1128/mcb.4.7.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberg U., Zock C., Doerfler W. Insertion of adenovirus type 12 DNA in the vicinity of an intracisternal A particle genome in Syrian hamster tumor cells. J Virol. 1987 Sep;61(9):2719–2726. doi: 10.1128/jvi.61.9.2719-2726.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeithan T. W., Rowley J. D., Shows T. B., Diaz M. O. Cloning of the chromosome translocation breakpoint junction of the t(14;19) in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9257–9260. doi: 10.1073/pnas.84.24.9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani T., Horigome K., Nomura N., Kondo T., Ohtsuka H., Noguchi H., Honjo T. Deletion of human JK segments by site-specific recombination recognizing the conserved nonamer and heptamer sequences. Nucleic Acids Res. 1990 Sep 25;18(18):5529–5532. doi: 10.1093/nar/18.18.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettinger M. A., Schatz D. G., Gorka C., Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990 Jun 22;248(4962):1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Owens R. A., Trempe J. P., Chejanovsky N., Carter B. J. Adeno-associated virus rep proteins produced in insect and mammalian expression systems: wild-type and dominant-negative mutant proteins bind to the viral replication origin. Virology. 1991 Sep;184(1):14–22. doi: 10.1016/0042-6822(91)90817-u. [DOI] [PubMed] [Google Scholar]
- Rynditch A., Kadi F., Geryk J., Zoubak S., Svoboda J., Bernardi G. The isopycnic, compartmentalized integration of Rous sarcoma virus sequences. Gene. 1991 Oct 15;106(2):165–172. doi: 10.1016/0378-1119(91)90196-i. [DOI] [PubMed] [Google Scholar]
- Samulski R. J., Berns K. I., Tan M., Muzyczka N. Cloning of adeno-associated virus into pBR322: rescue of intact virus from the recombinant plasmid in human cells. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2077–2081. doi: 10.1073/pnas.79.6.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski R. J., Zhu X., Xiao X., Brook J. D., Housman D. E., Epstein N., Hunter L. A. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 1991 Dec;10(12):3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz D. G., Oettinger M. A., Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989 Dec 22;59(6):1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- Schulz M., Freisem-Rabien U., Jessberger R., Doerfler W. Transcriptional activities of mammalian genomes at sites of recombination with foreign DNA. J Virol. 1987 Feb;61(2):344–353. doi: 10.1128/jvi.61.2.344-353.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen D., Gilbert W. Novel DNA superstructures formed by telomere-like oligomers. Biochemistry. 1992 Jan 14;31(1):65–70. doi: 10.1021/bi00116a011. [DOI] [PubMed] [Google Scholar]
- Terleth C., Waters R., Brouwer J., van de Putte P. Differential repair of UV damage in Saccharomyces cerevisiae is cell cycle dependent. EMBO J. 1992 Mar;11(3):1228–1228. doi: 10.1002/j.1460-2075.1992.tb05163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tratschin J. D., Miller I. L., Carter B. J. Genetic analysis of adeno-associated virus: properties of deletion mutants constructed in vitro and evidence for an adeno-associated virus replication function. J Virol. 1984 Sep;51(3):611–619. doi: 10.1128/jvi.51.3.611-619.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uberbacher E. C., Mural R. J. Locating protein-coding regions in human DNA sequences by a multiple sensor-neural network approach. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11261–11265. doi: 10.1073/pnas.88.24.11261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahls W. P., Wallace L. J., Moore P. D. Hypervariable minisatellite DNA is a hotspot for homologous recombination in human cells. Cell. 1990 Jan 12;60(1):95–103. doi: 10.1016/0092-8674(90)90719-u. [DOI] [PubMed] [Google Scholar]
- Walz C., Schlehofer J. R. Modification of some biological properties of HeLa cells containing adeno-associated virus DNA integrated into chromosome 17. J Virol. 1992 May;66(5):2990–3002. doi: 10.1128/jvi.66.5.2990-3002.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocour E., Puzis L., Etkin S., Koch T., Danovitch B., Mendelson E., Shaulian E., Karby S., Lavi S. Modulation of the cellular phenotype by integrated adeno-associated virus. Virology. 1992 Sep;190(1):316–329. doi: 10.1016/0042-6822(92)91218-j. [DOI] [PubMed] [Google Scholar]