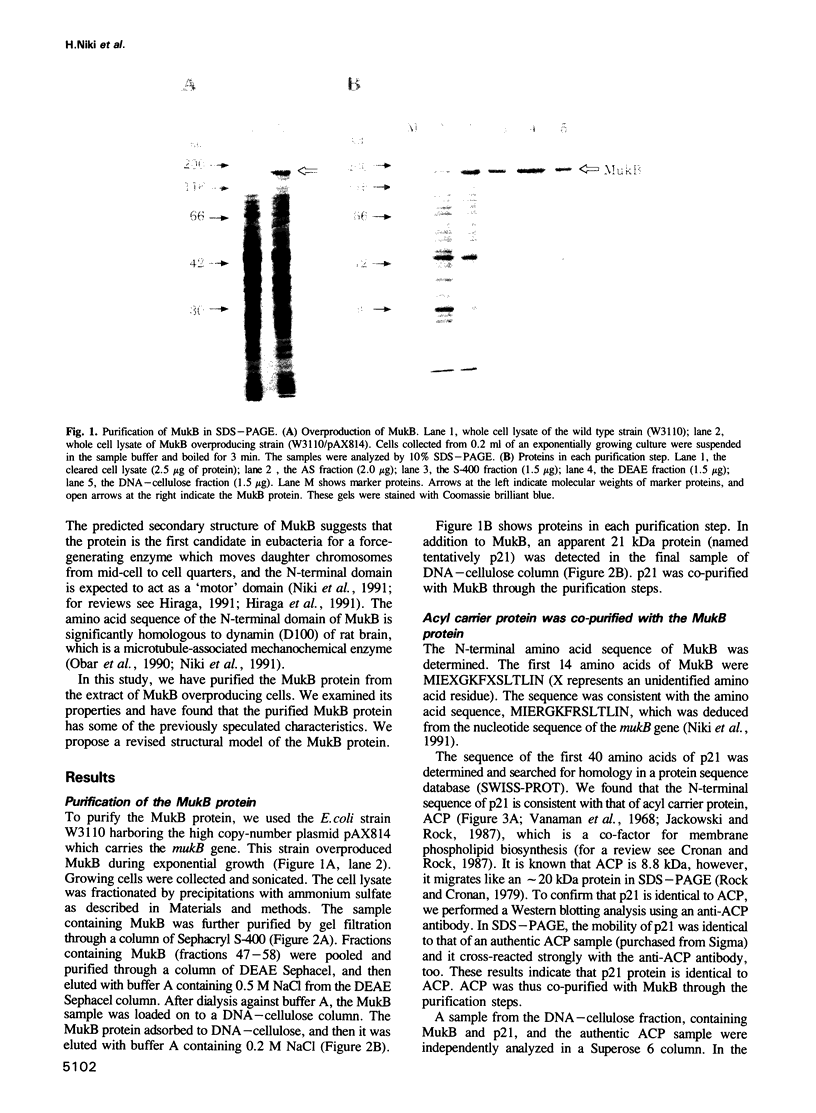
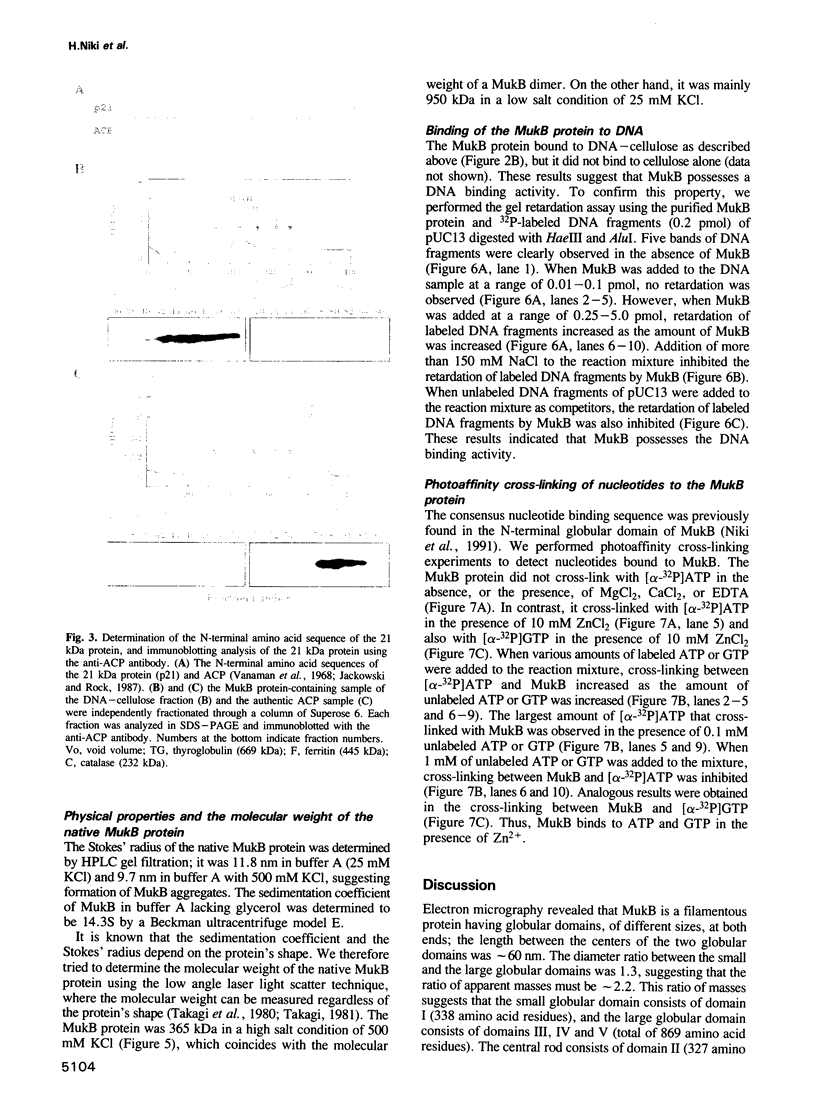
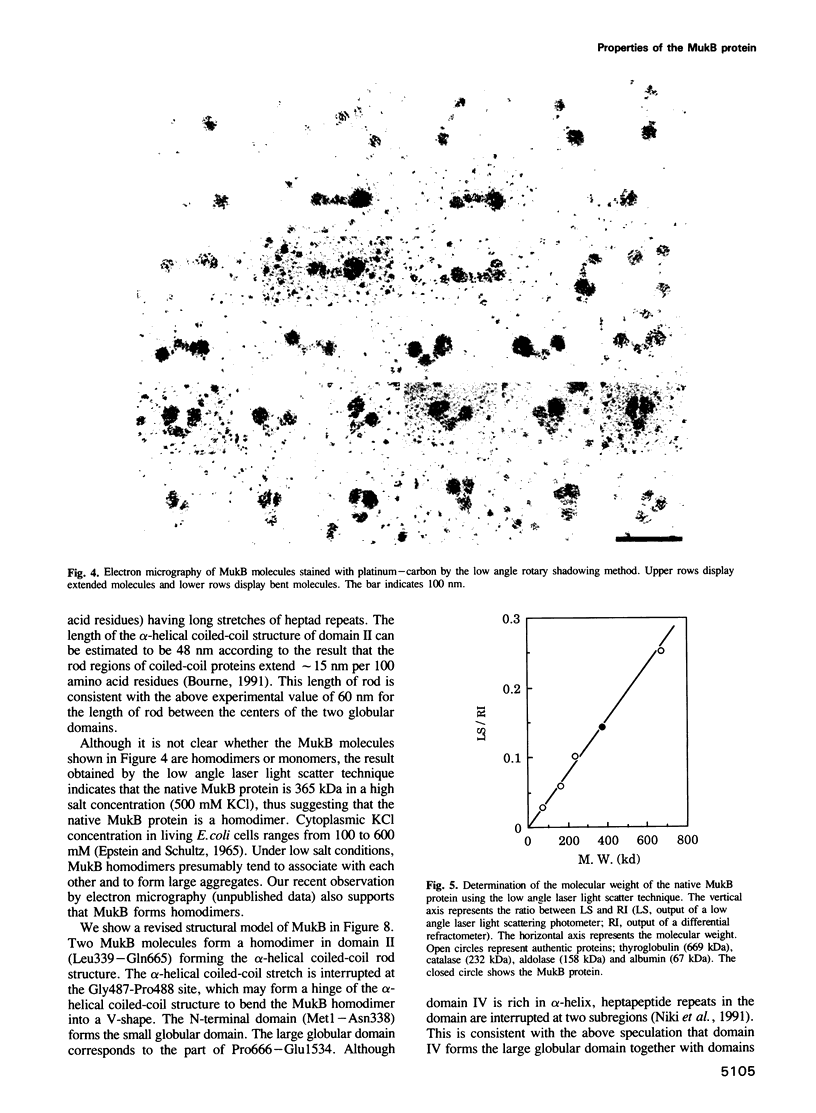
Abstract
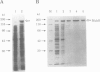
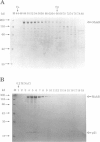
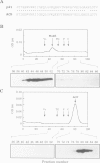
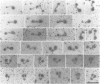
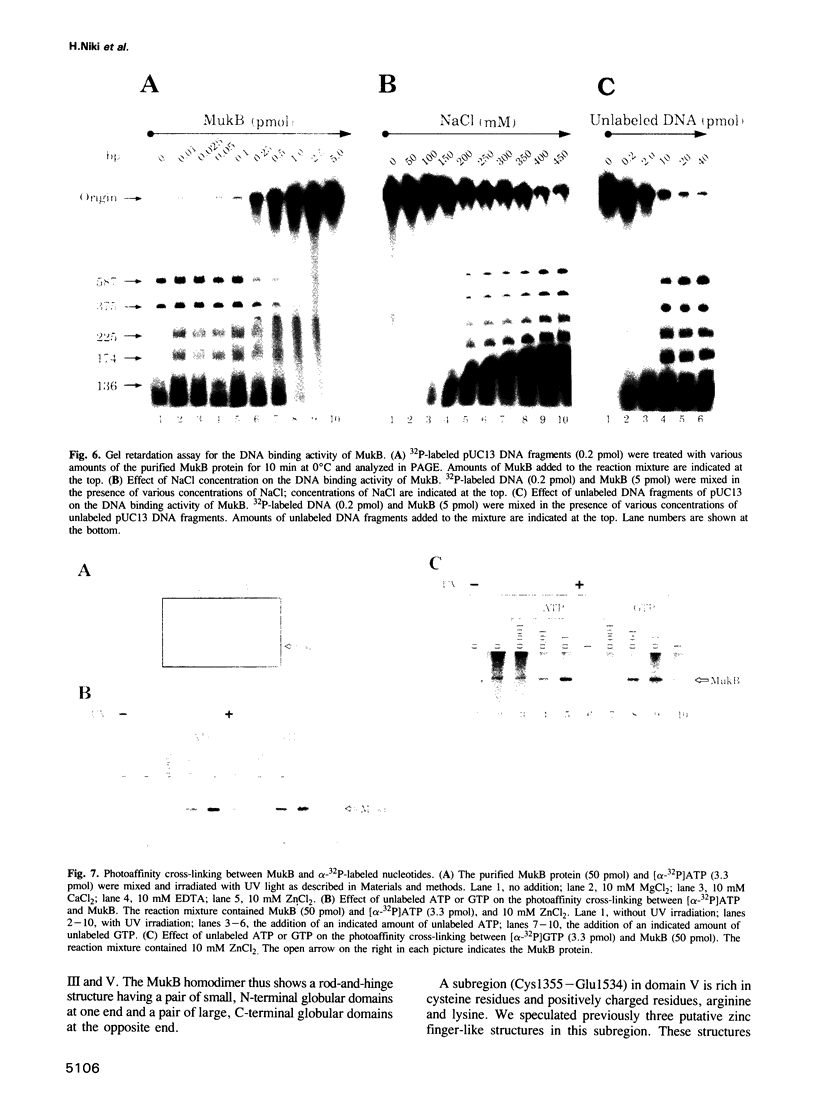
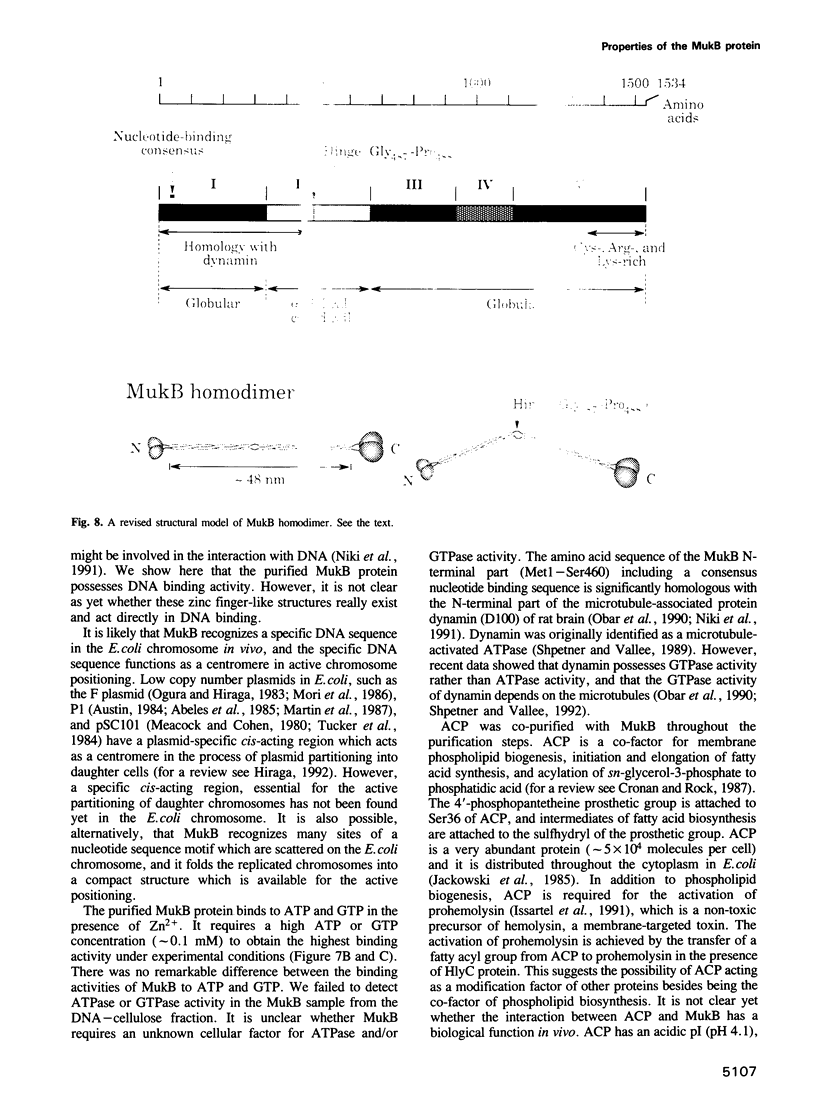
mukB mutants of Escherichia coli are defective in the correct partitioning of replicated chromosomes. This results in the appearance of normal-sized anucleate (chromosome-less) cells during cell proliferation. Based on the nucleotide sequence of the mukB gene, the MukB protein of 177 kDa was predicted to be a filamentous protein with globular domains at the ends, and also having DNA binding and nucleotide binding abilities. Here we present evidence that the purified MukB protein possesses these characteristics. MukB forms a homodimer with a rod-and-hinge structure having a pair of large, C-terminal globular domains at one end and a pair of small, N-terminal globular domains at the opposite end; it tends to bend at a middle hinge site of the rod section. Chromatography in a DNA-cellulose column and the gel retardation assay revealed that MukB possesses DNA binding activity. Photoaffinity cross-linking experiments showed that MukB binds to ATP and GTP in the presence of Zn2+. Throughout the purification steps, acyl carrier protein was co-purified with MukB.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles A. L., Friedman S. A., Austin S. J. Partition of unit-copy miniplasmids to daughter cells. III. The DNA sequence and functional organization of the P1 partition region. J Mol Biol. 1985 Sep 20;185(2):261–272. doi: 10.1016/0022-2836(85)90402-4. [DOI] [PubMed] [Google Scholar]
- Austin S. J. Bacterial plasmids that carry two functional centromere analogs are stable and are partitioned faithfully. J Bacteriol. 1984 May;158(2):742–745. doi: 10.1128/jb.158.2.742-745.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg K. J., Donachie W. D. Experiments on chromosome separation and positioning in Escherichia coli. New Biol. 1991 May;3(5):475–486. [PubMed] [Google Scholar]
- Bourne H. R. Colon cancer. Consider the coiled coil.... Nature. 1991 May 16;351(6323):188–190. doi: 10.1038/351188a0. [DOI] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J. Chromosome partition in Escherichia coli requires postreplication protein synthesis. J Bacteriol. 1989 Oct;171(10):5405–5409. doi: 10.1128/jb.171.10.5405-5409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson W. G., Kusano T., Yamaki H., Balakrishnan R., King M., Murchie J., Schaechter M. Binding of the origin of replication of Escherichia coli to the outer membrane. Cell. 1982 Oct;30(3):915–923. doi: 10.1016/0092-8674(82)90296-3. [DOI] [PubMed] [Google Scholar]
- Hiraga S. Chromosome and plasmid partition in Escherichia coli. Annu Rev Biochem. 1992;61:283–306. doi: 10.1146/annurev.bi.61.070192.001435. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Niki H., Imamura R., Ogura T., Yamanaka K., Feng J., Ezaki B., Jaffé A. Mutants defective in chromosome partitioning in E. coli. Res Microbiol. 1991 Feb-Apr;142(2-3):189–194. doi: 10.1016/0923-2508(91)90029-a. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Niki H., Ogura T., Ichinose C., Mori H., Ezaki B., Jaffé A. Chromosome partitioning in Escherichia coli: novel mutants producing anucleate cells. J Bacteriol. 1989 Mar;171(3):1496–1505. doi: 10.1128/jb.171.3.1496-1505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S., Ogura T., Niki H., Ichinose C., Mori H. Positioning of replicated chromosomes in Escherichia coli. J Bacteriol. 1990 Jan;172(1):31–39. doi: 10.1128/jb.172.1.31-39.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain K., Begg K. J., Salmond G. P., Donachie W. D. ParD: a new gene coding for a protein required for chromosome partitioning and septum localization in Escherichia coli. Mol Microbiol. 1987 Jul;1(1):73–81. doi: 10.1111/j.1365-2958.1987.tb00529.x. [DOI] [PubMed] [Google Scholar]
- Hussain K., Elliott E. J., Salmond G. P. The parD- mutant of Escherichia coli also carries a gyrAam mutation. The complete sequence of gyrA. Mol Microbiol. 1987 Nov;1(3):259–273. doi: 10.1111/j.1365-2958.1987.tb01932.x. [DOI] [PubMed] [Google Scholar]
- Issartel J. P., Koronakis V., Hughes C. Activation of Escherichia coli prohaemolysin to the mature toxin by acyl carrier protein-dependent fatty acylation. Nature. 1991 Jun 27;351(6329):759–761. doi: 10.1038/351759a0. [DOI] [PubMed] [Google Scholar]
- Jackowski S., Edwards H. H., Davis D., Rock C. O. Localization of acyl carrier protein in Escherichia coli. J Bacteriol. 1985 Apr;162(1):5–8. doi: 10.1128/jb.162.1.5-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski S., Rock C. O. Altered molecular form of acyl carrier protein associated with beta-ketoacyl-acyl carrier protein synthase II (fabF) mutants. J Bacteriol. 1987 Apr;169(4):1469–1473. doi: 10.1128/jb.169.4.1469-1473.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J., Nishimura Y., Imamura R., Niki H., Hiraga S., Suzuki H. New topoisomerase essential for chromosome segregation in E. coli. Cell. 1990 Oct 19;63(2):393–404. doi: 10.1016/0092-8674(90)90172-b. [DOI] [PubMed] [Google Scholar]
- Kato J., Nishimura Y., Suzuki H. Escherichia coli parA is an allele of the gyrB gene. Mol Gen Genet. 1989 May;217(1):178–181. doi: 10.1007/BF00330959. [DOI] [PubMed] [Google Scholar]
- Kato J., Nishimura Y., Yamada M., Suzuki H., Hirota Y. Gene organization in the region containing a new gene involved in chromosome partition in Escherichia coli. J Bacteriol. 1988 Sep;170(9):3967–3977. doi: 10.1128/jb.170.9.3967-3977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Martin K. A., Friedman S. A., Austin S. J. Partition site of the P1 plasmid. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8544–8547. doi: 10.1073/pnas.84.23.8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Kimura E., Mizushima S. Complementation of two overlapping fragments of SecA, a protein translocation ATPase of Escherichia coli, allows ATP binding to its amino-terminal region. J Biol Chem. 1990 May 25;265(15):8760–8765. [PubMed] [Google Scholar]
- Meacock P. A., Cohen S. N. Partitioning of bacterial plasmids during cell division: a cis-acting locus that accomplishes stable plasmid inheritance. Cell. 1980 Jun;20(2):529–542. doi: 10.1016/0092-8674(80)90639-x. [DOI] [PubMed] [Google Scholar]
- Mori H., Kondo A., Ohshima A., Ogura T., Hiraga S. Structure and function of the F plasmid genes essential for partitioning. J Mol Biol. 1986 Nov 5;192(1):1–15. doi: 10.1016/0022-2836(86)90459-6. [DOI] [PubMed] [Google Scholar]
- Morona R., Manning P. A., Reeves P. Identification and characterization of the TolC protein, an outer membrane protein from Escherichia coli. J Bacteriol. 1983 Feb;153(2):693–699. doi: 10.1128/jb.153.2.693-699.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki H., Imamura R., Ogura T., Hiraga S. Nucleotide sequence of the tolC gene of Escherichia coli. Nucleic Acids Res. 1990 Sep 25;18(18):5547–5547. doi: 10.1093/nar/18.18.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niki H., Jaffé A., Imamura R., Ogura T., Hiraga S. The new gene mukB codes for a 177 kd protein with coiled-coil domains involved in chromosome partitioning of E. coli. EMBO J. 1991 Jan;10(1):183–193. doi: 10.1002/j.1460-2075.1991.tb07935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obar R. A., Collins C. A., Hammarback J. A., Shpetner H. S., Vallee R. B. Molecular cloning of the microtubule-associated mechanochemical enzyme dynamin reveals homology with a new family of GTP-binding proteins. Nature. 1990 Sep 20;347(6290):256–261. doi: 10.1038/347256a0. [DOI] [PubMed] [Google Scholar]
- Ogden G. B., Pratt M. J., Schaechter M. The replicative origin of the E. coli chromosome binds to cell membranes only when hemimethylated. Cell. 1988 Jul 1;54(1):127–135. doi: 10.1016/0092-8674(88)90186-9. [DOI] [PubMed] [Google Scholar]
- Ogura T., Hiraga S. Partition mechanism of F plasmid: two plasmid gene-encoded products and a cis-acting region are involved in partition. Cell. 1983 Feb;32(2):351–360. doi: 10.1016/0092-8674(83)90454-3. [DOI] [PubMed] [Google Scholar]
- Rock C. O., Cronan J. E., Jr Re-evaluation of the solution structure of acyl carrier protein. J Biol Chem. 1979 Oct 10;254(19):9778–9785. [PubMed] [Google Scholar]
- Sato K., Nishina Y., Shiga K. Anion-induced conformational change of apo-electron-transferring flavoprotein. J Biochem. 1992 Mar;111(3):359–365. doi: 10.1093/oxfordjournals.jbchem.a123762. [DOI] [PubMed] [Google Scholar]
- Shpetner H. S., Vallee R. B. Dynamin is a GTPase stimulated to high levels of activity by microtubules. Nature. 1992 Feb 20;355(6362):733–735. doi: 10.1038/355733a0. [DOI] [PubMed] [Google Scholar]
- Shpetner H. S., Vallee R. B. Identification of dynamin, a novel mechanochemical enzyme that mediates interactions between microtubules. Cell. 1989 Nov 3;59(3):421–432. doi: 10.1016/0092-8674(89)90027-5. [DOI] [PubMed] [Google Scholar]
- Steck T. R., Drlica K. Bacterial chromosome segregation: evidence for DNA gyrase involvement in decatenation. Cell. 1984 Apr;36(4):1081–1088. doi: 10.1016/0092-8674(84)90058-8. [DOI] [PubMed] [Google Scholar]
- Takagi T. Confirmation of molecular weight of Aspergillus oryzae alpha-amylase using the low angle laser light scattering technique in combination with high pressure silica gel chromatography. J Biochem. 1981 Feb;89(2):363–368. doi: 10.1093/oxfordjournals.jbchem.a133210. [DOI] [PubMed] [Google Scholar]
- Takagi T., Miyake J., Nashima T. Assessment study on the use of the low angle laser light scattering technique for the estimation of molecular weight of the polypeptide forming a complex with sodium dodecyl sulfate. Biochim Biophys Acta. 1980 Nov 20;626(1):5–14. doi: 10.1016/0005-2795(80)90191-9. [DOI] [PubMed] [Google Scholar]
- Tucker W. T., Miller C. A., Cohen S. N. Structural and functional analysis of the par region of the pSC 10 1 plasmid. Cell. 1984 Aug;38(1):191–201. doi: 10.1016/0092-8674(84)90540-3. [DOI] [PubMed] [Google Scholar]
- Tyler J. M., Branton D. Rotary shadowing of extended molecules dried from glycerol. J Ultrastruct Res. 1980 May;71(2):95–102. doi: 10.1016/s0022-5320(80)90098-2. [DOI] [PubMed] [Google Scholar]
- Vanaman T. C., Wakil S. J., Hill R. L. The complete amino acid sequence of the acyl carrier protein of Escherichia coli. J Biol Chem. 1968 Dec 25;243(24):6420–6431. [PubMed] [Google Scholar]
- Vinella D., Jaffé A., D'Ari R., Kohiyama M., Hughes P. Chromosome partitioning in Escherichia coli in the absence of dam-directed methylation. J Bacteriol. 1992 Apr;174(7):2388–2390. doi: 10.1128/jb.174.7.2388-2390.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandersman C., Delepelaire P. TolC, an Escherichia coli outer membrane protein required for hemolysin secretion. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4776–4780. doi: 10.1073/pnas.87.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K., Ishihama A., Nagata K. Reconstitution of influenza virus RNA-nucleoprotein complexes structurally resembling native viral ribonucleoprotein cores. J Biol Chem. 1990 Jul 5;265(19):11151–11155. [PubMed] [Google Scholar]
- Yoshimoto M., Kambe-Honjoh H., Nagai K., Tamura G. Early replicative intermediates of Escherichia coli chromosome isolated from a membrane complex. EMBO J. 1986 Apr;5(4):787–791. doi: 10.1002/j.1460-2075.1986.tb04282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]