Abstract
Harnessing the immune system to attack cancer cells has represented a holy grail for greater than 100 years. While prospects of tumor-selective durable immune based therapies have provided small clinical signals for many decades, recent years have demonstrated a virtual explosion in progress. Melanoma has led the field of cancers in which immunotherapy has produced major clinical inroads. The most significant and impactful immunotherapies for melanoma utilize immune checkpoint inhibition to stimulate T cell mediated tumor killing. The major targets of checkpoint blockade have thus far been CTLA4 and PD1, two key receptors for central and peripheral immune tolerance. This review discusses current understanding of how these checkpoint blockade therapeutics have led to major clinical responses in patients with advanced melanoma. It is likely that we are poised to see significantly greater anti-cancer immunotherapy efficacy, both in improving response rates and durability for melanoma, and for other less immunogenic malignancies.
Keywords: melanoma, immunotherapy, T cell, checkpoint, vitiligo
The patient who was diagnosed with metastatic melanoma ten years ago, in general, could look forward to treatment with a single traditional chemotherapeutic (dacarbazine – FDA approved in 1975) and a 5-year overall survival rate of less than 10%. Roughly 90,000 new cases of melanoma and 9,000 deaths from the disease were seen in the United States in recent years, making it the deadliest skin cancer, with an incidence that is still expected to rise in coming years [1]. Yet in recent years the treatment of melanoma has been fully revolutionized by the development of effective, well-tolerated and widely-available immunotherapy. At the same time, the historical experience with immunotherapy in melanoma has triggered new key questions about the basic biology of cancer, the role of the immune system in cancer biology, and even the structure of clinical units which will be required to deploy new immunotherapies in the future.
In this review, we will focus on recent history and advances in melanoma immunotherapy, paying special attention to the class of drugs which block negative regulators (checkpoints) of the immune response. The most well-known of these therapies, targeted against cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1), have within the last years become frontline for the treatment of metastatic melanoma in many clinical scenarios. We first discuss earlier attempts at immunotherapy in melanoma, the biology of the immune response with an eye toward checkpoint inhibition, and the clinical experience with these drugs singly and in combination. Finally, we turn towards pressing open questions in melanoma immunotherapy, including understanding the determinants of response to these drugs, their most effective clinical uses, and new targets on the horizon.
The immune response and immune evasion in cancer
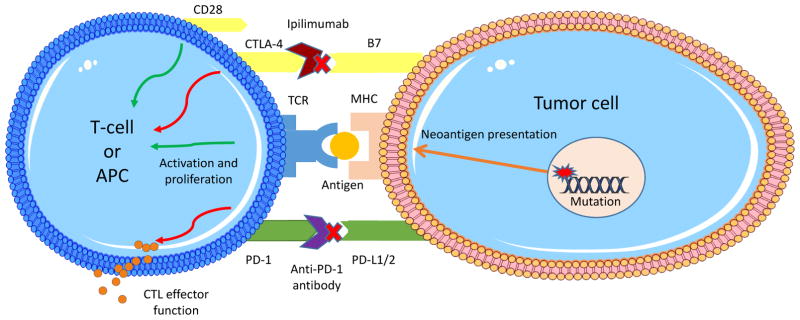
While a full discussion of the immune response to cancer would be well beyond the scope of this review and has been better dealt with elsewhere [2–4], a basic model and terminology deserves discussion (Figure 1).
Figure 1.
Mechanisms of action of PD-1 and CTLA-4 pathway inhibitors. Immune checkpoints operate at multiple points in the activation of specific anti-tumor responses to downregulate the immune response. Shown here for illustration are PD-1 and CTLA-4. PD-1 is important both in lymph organs and in the tumor environment for inhibiting the effector response to tumor cells, whereas CTLA-4, expressed on the surface of APCs, inhibits the activation of T-cell responses. In reality, immune checkpoints operate at multiple levels and it is likely a balance of inhibitory and activating signals that modulates the strength of the immune response.
Mutational events in cancer can be categorized as “driver” mutations which functionally contribute to malignant transformation, or “passenger” mutations which are less likely to be recurrent and do not measurably contribute to the cell-autonomous change in malignant behavior of the cancer cell. The genome of cutaneous melanomas typically contains a particularly high burden of UV signature mutations, characterized by pyrimidine dimers, and distinguishing melanoma from virtually all non-cutaneous human malignancies. Those mutations which produce altered protein epitopes that are capable of being successfully presented as antigens, produce a tumor specific repertoire of “neoantigens” which can stimulate an immune response including cytotoxic T-lymphocytes (CTLs) specifically designed to attack the tumor cell clone. Such a cancer must therefore evade natural host immune responses to these neoantigens in order to proliferate, which it can do by either (1) disabling some component of the normal immune response or (2) co-opting (increasing) down-regulatory stimuli in the immune system. These evasion mechanisms may be further thought of as either (1) intrinsic, i.e., those alterations which are found in the tumor cells themselves, or (2) extrinsic, usually represented by inflammatory cells and cytokine-mediated suppression of the anti-tumor responses in the local tumor microenvironment. Any given cancer may operate with some or all of these mechanisms at play at once. Intrinsic disabling of the normal immune response, for instance, may include mutation or downregulation of antigen-presenting MHC molecules in tumor cells [5, 6]. Likewise, mouse models suggest cancers may lose expression of antigens that are immunogenic, especially in the face of active immune responses, sometimes called “immune-editing” [7]. Immune checkpoints, which are a variety of systems that dampen immune responses in a normally function immune system, are extensively employed by cancers and will be discussed in detail below. Extrinsic mechanisms of immune evasion include tumor infiltration by immature myeloid cells [8] and dendritic cells which suppress specific T-cell responses [9], T-regulatory cells, and cytokines which shift the immune response towards one favorable to the tumor. The important function of regulatory T-cells (T-regs) in tumor immunology is fully dealt with elsewhere [10].
Early attempts at immunotherapy
That these evasive mechanisms can be clinically targeted and reversed in some form is the animating hypothesis of immunotherapy for cancer. One of the earliest demonstrations of immune-mediated tumor regressions was described by William Coley, who in 1893, suspected a connection between streptococcal infection and spontaneous tumor regressions. He demonstrated that purposeful inoculation of patients harboring unresectable tumors with bacteria were capable of producing some remissions [11]. Over the following century, numerous strategies were vigorously tested, to replicate or enhance such tumor immune responses—typically producing rare, though occasionally dramatic treatment responses. Melanoma played a central role in these early studies, as well as the more recent dramatic improvements of recent decades. Spontaneous regressions of melanomas, thought to be immune-mediated, are among the likely immune-mediated clinical features of seen occasionally in melanoma patients [12]. Likewise, a modest increase in frequency of melanomas in immunosuppressed patients [13], suggested an immunosurveillance mechanism—which is now recognized to be significantly more functionally profound in the case of cutaneous squamous cell carcinomas.
One of the first successful immunotherapies in melanoma was high-dose interleukin 2 (IL-2). A cytokine required for the in vitro culture and in vivo activation of T-cells, IL-2 became the first FDA-approved immunotherapy for cancer when it was approved for melanoma in 1998. Response rates are modest, on the order of chemotherapies, at 10–20% [14, 15]. But the remarkable benefit of IL-2 is seen in a small set of responses that appear truly durable, lasting from 5 to over 12 years, approaching the definition of “cure” for metastatic disease [16, 17]. Such responses are far rarer, almost vanishingly so, with traditional chemotherapy—indeed in such are cases, one wonders whether an immune mechanism might become activated following chemotherapy responses. Despite its success in melanoma, IL-2 met with little other success outside of renal cell cancer, failing in other trials in solid tumors, and leading to reinforcement, perhaps erroneously, of the hypothesis that melanoma is uniquely sensitive to immune attack [18, 19]. Unfortunately, due to its adverse effects, which can include multi-organ damage and a sepsis-like inflammatory response, the use of IL-2 has been limited to specialized centers and selected patient populations – usually the young and otherwise healthy. Early trials had therapy-related fatalities, though as supportive care became more sophisticated, these declined. Until being supplanted in recent years, IL-2 was frequently offered as a frontline therapy in these patients due to the chance, albeit small, of potential cure.
In the late 80s and early 90s, van der Bruggen and colleagues screened a library of CTLs against a genomic library derived from melanoma cells, thereby identifying the MAGE gene family, the first cancer neoantigen(s) to which T-cells could be shown to respond [20]. This observation and others led to attempts at vaccinating patients with known antigens to try and establish an anti-tumor response. The MAGE family and gp100/PMEL, a glycoprotein expressed primarily on the surface of melanocytes, have been the focus of most vaccine trials in melanoma [21]. A variety of delivery strategies – including peptides, dendritic cell conjugates, killed cell extracts, viruses, and heat shock proteins, along with adjuvants and generic immune stimulatory cytokines – have been used. Strategies, basic rationale, and a summary of trials in this area have been reviewed elsewhere [22–24]. Phase I/II trials have shown the development of circulating T-cells able to recognize peptide vaccines, but there has been limited clinical benefit [25–27]. While localized regressions of isolated treated lesions have been observed, vaccines have not typically produced systemic tumor responses. Several Phase III trials are underway for vaccines, and vaccines have been combined in multi-arm trials with the checkpoint inhibitors, as we will discuss below.
Another important early effort in immunotherapy in melanoma was adoptive T-cell transfer, pioneered by S. Rosenberg and colleagues, and which has been reviewed in detail elsewhere [28, 29]. Briefly, this technique involves the isolation of tumor-infiltrating lymphocytes (TILs) from a resected tumor sample, isolation and culture of lymphocytes, sometimes the introduction of recombinant T cell receptors known to target melanocytic antigens, and reinfusion of of the expanded TIL population in large numbers along with systemic IL-2 [30]. An abundance of TILs in most metastases made melanoma a natural fit for this technique. Since the first trial, in 1988, multiple additional trials have proved the theoretical feasibility of obtaining regressions with this technique, which has sometimes been combined with chemotherapy to total body irradiation to bias the immune repertoire towards the infused TILs [31, 32]. Adoptive T-cell transfer has never been trialed in very large wide-scale studies, and culture of TILs from tumors other than melanoma can be a challenge. This has led to interest in other methods of selecting or creating an anti-tumor T-cell population, such as T chimeric antigen receptors (TCARs), reviewed fully elsewhere [33]. Nonetheless, experiments showing that regressions could be achieved solely by harnessing the T-cell response to cancer was important in directing immunotherapy to the widely available treatments of today.
Immune checkpoints in the T cell response to cancer
In the normally functioning immune system, barriers are in place to prevent auto-immune tissue destruction [34]. These include negative selection against autoreactive T-cells during development [35], as well as a series of molecules that limit T-cell activation, proliferation, and cell killing. Even in the face of a good antigen and cognate T cell receptor (TCR) fit, a T-cell requires a “second signal” (or probably more correctly, a positive balance of inhibitory and stimulatory signals) to fully activate and mature. One such second signal comes in the form of CD28, a costimulatory receptor, binding to APC ligands B7-1 and B7-2 [36, 37]. CTLA-4, a molecule which is found on the T-cell surface, can bind to B7-1 and B7-2 with much higher affinity than CD28, and, when bound, inhibits the T-cell response, acting as a balancing inhibitory signal [38, 39]. CTLA-4 expression is rapidly induced by T-cell activation, but has generally been thought to exert its effect primarily in secondary lymphoid organs, and to involve modulation of CD4+ and Treg responses more than CD8+ ones [40]. Ctla-4 knockout mice display a fatal combination of immune hyperactivation and lymphoid proliferation consistent with widespread systemic autoimmunity [41, 42].
Another of the immune checkpoints is PD-1. PD-1 is expressed on the surface of T-cells and, when engaged by one of its two ligands, PD-L1 and PD-L2, inhibits the signaling pathways that normally lead to the CTL effector response [43, 44]. Unlike CTLA-4, which is primarily involved in immune cell activation and generation of de novo responses, the PD-1 system is important in immune escape at the point of CTL-mediated cell-kill, and appears primarily designed to limit over-response to infection and prevent autoimmunity in the periphery [44–47]. Chronic antigen exposure from viral antigens has been observed to induce PD-1 expression and create a state of anergy, or immune non-responsiveness in antigen-specific T-cells [48]. A similar upregulation of PD-L1/2 is observed on tumor cells [49] and other cells in the local tumor environment [46, 50]. PD-1 knockout mice do not have widespread lymphoid proliferation as do CTLA-4 deficient mice, but they do develop end-organ damage from T-cell activation [51, 52].
Although CTLA-4 and PD-1 are the most well-known checkpoints and the most common active drug targets currently, multiple other costimulatory and inhibitory ligands and receptors have been identified on CTLs, Tregs, and other immune cells in the tumor microenvironment. Lymphocyte activation gene 3 (LAG3), which is widely expressed on T-regs, downregulates T-cell responses similarly to PD-1, though its only known ligand is MHC Class II [53]. Dual knockout in mice or inhibition in vivo along with PD-1 can lead to robust anti-tumor as well as auto-immune responses more powerful than inhibiting either alone [54]. TIM3, BTLA, A2aR, OX40, and others are all inhibitory receptors which play a role in turning off CTL activation or killing, and have been reviewed before [2]. A systems-level understanding of these redundant pathways has yet to be achieved, but each provides remarkable opportunities for therapeutic intervention.
Clinical Experience with checkpoint inhibition
CTLA-4 blocking antibodies were the first immune checkpoint inhibitors to enter clinical trials. Despite the fatal phenotype in knockout mice, studies showed that partial CTLA-4 blockade with antibody produced a therapeutic window [55]. Skepticism over targeting CTLA-4 centered on the fact that it was not overexpressed in most tumors, and instead seemed to be a generic immune system break. Nonetheless, two anti-CTLA4 antibodies, tremelimumab and ipilimumab, entered clinical development around 2000. Early pilot trials were performed mostly in melanoma (often in large cohorts with a smattering of other advanced solid tumors). Tremelimumab, despite showing activity and displaying some long-term responses, did not significantly improve overall survival in a phase III study, and did not gain FDA approval.
Ipilimumab, however, did significantly improve overall survival in two large phase III trials [56, 57]. In one of the trials, ipilimumab was trialed in patients with stage IIIc or IV melanoma in two arms of a three-arm trial: ipilimumab with, or without, gp100 vaccine, versus vaccinated control. While the vaccine had no effect on survival, both ipilimumab groups had a statistically significant survival advantage. In both studies, overall response rates were on the order of 10–15%. More impressively, in what has become a common theme of trials of checkpoint inhibitors, the proportion of patients alive at 2 years or even 5 years was more than doubled in the ipilimumab group. In a recently published analysis of all phase II and phase III trial data which was available, the survival curve for melanoma patients treated with ipilimumab plateaued at about year 3, at slightly greater than 20% of patients [58]. Other earlier studies came to similar conclusions [59, 60]. Compared to historical cohorts of melanoma patients, this appears to be an absolute increase of 10% in survival over what would be expected after 3 years [61].
Anti-PD-1 therapy in melanoma soon followed CTLA-4 into the clinic. Multiple PD-1 inhibitors have entered clinical trials, alongside PD-L1 inhibitors, which are listed in Table 1. As noted above, and in contrast to CTLA-4, PD-1 expression is often upregulated on tumor cells as well as tumor-associated Tregs; inhibition of the PD-1 system has therefore been thought to represent a mechanism of specific reinduction of the anti-tumor immune response. In seminal phase III studies, nivolumab and pembrolizumab, two anti-PD-1 antibodies, were shown to have response rates in the range of 20–30% and have equally robust survival data at 2 and 3 year endpoints as ipilimumab [62, 63]. Remarkably, nivolumab and pembrolizumab moved from trials in the last-line setting to become frontline therapy for metastatic melanoma very rapidly, with higher response rates, a more tolerable side-effect profile, and fewer grade III or IV adverse events than ipilimumab. Targeted therapies, which only a few years ago gained approval in melanoma with great success, are now often held in reserve as a second-line even when available, though further studies are needed to find optimal sequencing [64, 65].
Table 1.
Major checkpoint inhibitors in clinical development for metastatic melanoma
| Drug | Other Names | Target | Antibody Class | Producer | Clinical Stage Completed in Melanoma |
|---|---|---|---|---|---|
| Ipilimumab | MDX010 | CTLA-4 | IgG1 human | Bristol-Myers Squibb | FDA approved 2011 |
| Nivolumab | MDX-1106, BMS-936558, ONO-4538 | PD-1 | IgG4 fully human | Bristol-Myers Squibb | FDA approved 2014 |
| Pembrolizumab | MK-3475, lambrolizumab | PD-1 | IgG4 humanized | Merck | FDA approved 2014 |
| Pidilizumab | CT-011 | PD-1 | IgG1 humanized | Curetech | Phase II |
| Atezolizumab | MPDL3280A | PD-L1 | IgG1 engineered fully human | Roche | Phase II |
| BMS-935559 | MDX-1105 | PD-L1 | IgG4 fully human | Bristol-Myers Squibb | Phase I |
| Durvalamab | MEDI4736 | PD-L1 | IgG1 engineered fully human | MedImmune/AstraZeneca | Phase I/II |
| IMP321 | None | LAG3 | LAG3-Ig fusion | Prima | Phase 1 ongoing |
As noted above, CTLA-4 and PD-1 operate on different parts of the immune activation cascade. Consequently, it was not long before combined inhibition of both systems was attempted, first in mouse model experiments, then in phase I tolerability trials, and, finally, in fully randomized phase III trials. The results of these studies are remarkable, with nearly 50–60% of patients achieving some objective response, significantly higher than the rate of either agent alone [66, 67]. Almost twice the rate of serious adverse events occur with combined inhibition (about 50%) compared to either ipilimumab monotherapy (20–30%) or anti-PD-1 therapy (10–20%). In patients who are able to tolerate combination therapy, however, it offers the most remarkable response rate achieved by immunotherapy to date – nearly a ten-fold increase in response rate to chemotherapy administered as frontline only years earlier – with a safer side effect profile and better survival data. In addition to immunotherapy combinations, PD-1 and CTLA-4 blockade is undergoing trials in combination with chemotherapy, radiation therapy, surgical resection, and targeted therapy. Little definitive data have emerged thus far, but are expected to do so in the near future.
Checkpoint inhibition response kinetics and adverse events
Two points about the clinical experience with immune checkpoint inhibitors in melanoma deserve mention for their illumination of the peculiar mechanism of immunotherapy: the kinetics of the response and adverse event types.
The criteria used by clinicians to judge responses to chemotherapy and targeted agents may be insufficient for judging response to checkpoint inhibition, or to immune-mediated therapies in general. Stable disease or partial responses, usually transient with traditional chemotherapy, can be observed for months with checkpoint inhibition. Time to complete response can be measured in years, if it is achieved [59, 60]. It is not unusual for some metastases to shrink and others to apparently transiently expand on sequential radiologic imaging. Such radiologic “progression” is now recognized to potentially harbor delayed clinical responses, which can occur only months later—thereby encouraging clinicians and patients to avoid early discontinuation of immune checkpoint blockade therapy. Patients with otherwise full regressions may have persistent pockets of disease which can be stable for months or years, likely representing necrotic tissue with surrounding immune infiltrates. In this context, new modified response criteria were developed to assess responses [68]. About 10% of melanoma patients will have responses under these criteria which are not considered responses by older criteria, and clinicians continue to refine their understanding of immune response kinetics as new immune checkpoint inhibitors add to the body of clinical experience.
Adverse events that occur with treatment with immunotherapies, generally termed immune-related adverse events (irAEs), may also provide novel insights into the biology of the anti-tumor response in melanoma. Clinically, irAEs are unlike the adverse events seen in traditional chemotherapeutics and they are managed with an entirely different paradigm in mind, in which the oncologist has had to become facile with immunosuppressive therapy [69]. While many adverse events are non-specific – nausea, diarrhea, rash – a significant portion are caused by immune attack on a host organ. These can commonly include colitis as well as hypophysitis, other endocrinopathies, pneumonitis, hepatitis, dermatitis, vitiligo, and, very rarely, nephritis, meningitis, pericarditis, and more [70]. In many cases, these can be managed with interruption of therapy, replacement of the lost function if needed (such as thyroid or adrenal hormone replacement) [71], or, for more serious reactions, immunosuppressive therapy such as steroids or TNF-alpha inhibitors. Ipilimumab seems to have a higher rate of adverse events, and a more idiosyncratic profile of effected organs, than PD-1 pathway inhibitors. Clinically, the experience gained in supportive therapy from earlier immunotherapies – such as high-dose IL-2 or bone marrow transplant – has proved invaluable. Scientifically and mechanistically, predicting adverse events and correlations with tumor responses is an area of significant interest.
Who responds to checkpoint inhibitors – and why
Despite their paradigm-shifting success in melanoma therapy, the majority of patients still do not respond (or respond durably) to checkpoint inhibitors. A more complete understanding of the determinants of response, either from clinical or basic studies, could lead to more rationally targeted immunotherapies – as well as novel ones. Why do some patients respond to CTLA-4 or PD-1 inhibition, while others do not? Speculation about this question is at the frontier of immunotherapy and immunobiology.
As noted above, PD-1 is expressed on the surface of many tumors. Consequently, both retrospective analyses and pre-specified trial stratification have attempted to find correlations between PD-1 expression and response to anti-PD-1 therapy. There are increases in response rates in some trials and studies with increasing expression of PD-L1 on the surface of tumor cells or TILs [63, 72–74]. MHC class II expression has been correlated with response [75], as has proximity of PD-1 and PD-L1 expression in relation to the T-cell clonal repertoire [76]. Ultimately, great variation still exists in the immunohistochemistry for PD pathway staining, responses can be seen in patients without any PD-L1 or PD-1 expression on tumor cells, and PD-1 expression has no apparent bearing on response to CTLA-4 inhibitors. Consequently, at the current moment, PD-1 expression analyses are of unclear actual clinical utility, and no assays have yet been FDA-approved as companion diagnostics.
Not every cancer seems to respond equally well to checkpoint inhibition. In addition to melanoma, PD-1 inhibitors have been approved for non-small cell lung cancer (NSCLC), and has shown promise in renal cell carcinoma, bladder cancer, and some breast cancers. Many solid cancers, however, do not exhibit as robust and predictable a response rate; ipilimumab itself has had less success in lung cancer than in melanoma [77, 78]. In early trials, only 1 of 33 patients with colon cancer responded to PD-1 blockers [79, 80], but in a recent small phase 2 trial, patients with mismatch repair-deficient colorectal cancers were compared to patients with mismatch repair-proficient colorectal cancers and mismatch-repair deficient tumors of any other type. Remarkably, 70–90% of patients with mismatch repair-deficient tumors had disease control, compared to 11% of patients with colorectal cancer with intact mismatch repair. Similarly, early trials of PD-1 blockade for patients with relapsed-refractory Hodgkin’s lymphoma who have undergone at least three prior lines of therapy have shown remarkable rates of objective response [72]. At least one of those lines of therapy almost always includes highly mutagenic anthracycline and/or alkylating agents.
It is therefore reasonable to speculate that mutational load, in some form, allows for more neoantigens and therefore more likelihood of response. Indeed, mutation burden alone has been correlated with the response of melanoma to ipilimumab [81–83]. Other circumstantial evidence from smokers with lung cancer treated with PD-1 blockade supports this possibility [84].
Mutations that lead to neoantigens, however, are only half the picture: such neoantigens must be presented on MHC molecules and find a receptive cognate TCR. Several computational and experimental approaches have attempted to predict optimal load of antigens on MHC molecules, and from there to determine whether responses can be retroactively or prospectively explained [81, 82, 85]. Indeed, much excitement now centers around the combination of immunotherapy with personalized medicine, in which an individual’s tumor might be sequenced for neoepitopes, and optimal antigens then presented to the immune system either by vaccination or by direct modification of T cells [86].
One further clinical observation from checkpoint blockade in melanoma deserves mention. Melanoma patients treated with PD-1 inhibitors may sometimes develop vitiligo, which is not seen with PD-1 blockade in other cancers. Remarkably, recent studies have shown that patients who develop vitiligo are significantly more likely to respond to therapy [87, 88]. This raises the intriguing possibility that vitiligo, which is caused by immune-mediated destruction of normal pigmented cells, is an adverse event caused by the education of T-cells in the local tumor environment and then cross-reacting against normal melanocyte antigens, rather than neoantigens alone. So-called “epitope spreading” [89] could offer a new way to induce an anti-tumor T-cell repertoire. Local physical or chemical destruction of a tumor to induce surface expression of epitopes, and creation of an optimal local environment for wildtype tissue-restricted epitope recognition, may be able to induce more robust responses to checkpoint blockade.
Conclusion
The history of cancer therapy in the 20th century is one in which diseases once thought completely untreatable become significantly more curable. Progress of that kind, however, has been out of reach of most patients afflicted with the most common solid cancers, including melanoma – until recently. Immunotherapy for melanoma has gone from an option for only the healthiest patients to a widely tolerable, often efficacious, and sometimes apparently curative therapeutic modality. Many questions remain about how best to select patients who will benefit from checkpoint inhibitors, how to expand their reach, and how to optimally combine different complementary immunotherapy approaches with each other and with traditional cancer treatments. Key unknowns include understanding the mechanisms responsible for efficacy of immune checkpoint blockade responses, mechanisms promoting delayed as well as acute resistance to these therapies, mechanisms which limit efficacies in cancers outside of melanoma, identification of biomarkers that predict responsiveness, testing of new immune checkpoint targets, and combinatorial approaches aimed at optimizing this important therapeutic modality. Nonetheless, the progress in only a few short years has been truly remarkable, and the frontiers of melanoma immunotherapy are likely to expand our understanding of cancer, biology, and human immunology altogether.
Footnotes
Conflict of Interest:
No conflicts of interest to report
References
- 1.Society, A.C; A.C. Society, editor. Cancer Facts & Figures 2016. Atlanta: 2016. [Google Scholar]
- 2.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol. 2014;11(1):24–37. doi: 10.1038/nrclinonc.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6(1):24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 5.Natali PG, et al. Selective changes in expression of HLA class I polymorphic determinants in human solid tumors. Proc Natl Acad Sci U S A. 1989;86(17):6719–23. doi: 10.1073/pnas.86.17.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanchet O, et al. Altered binding of regulatory factors to HLA class I enhancer sequence in human tumor cell lines lacking class I antigen expression. Proc Natl Acad Sci U S A. 1992;89(8):3488–92. doi: 10.1073/pnas.89.8.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urban JL, Kripke ML, Schreiber H. Stepwise immunologic selection of antigenic variants during tumor growth. J Immunol. 1986;137(9):3036–41. [PubMed] [Google Scholar]
- 8.Kusmartsev S, Gabrilovich DI. Effect of tumor-derived cytokines and growth factors on differentiation and immune suppressive features of myeloid cells in cancer. Cancer Metastasis Rev. 2006;25(3):323–31. doi: 10.1007/s10555-006-9002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munn DH, et al. Potential regulatory function of human dendritic cells expressing indoleamine 2,3-dioxygenase. Science. 2002;297(5588):1867–70. doi: 10.1126/science.1073514. [DOI] [PubMed] [Google Scholar]
- 10.Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108(3):804–11. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 11.Coley W. The treatment of malignant tumors by repeated inoculations of erysipelas with a report of ten original cases. Am J Med Sci. 1893;105:487–511. [PubMed] [Google Scholar]
- 12.Cole WH, Everson TC. Spontaneous regression of cancer: preliminary report. Ann Surg. 1956;144(3):366–83. doi: 10.1097/00000658-195609000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greene MH, Young TI, Clark WH., Jr Malignant melanoma in renal-transplant recipients. Lancet. 1981;1(8231):1196–9. doi: 10.1016/s0140-6736(81)92359-x. [DOI] [PubMed] [Google Scholar]
- 14.Atkins MB, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17(7):2105–16. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 15.Fyfe G, et al. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13(3):688–96. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 16.Fisher RI, Rosenberg SA, Fyfe G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am. 2000;6(Suppl 1):S55–7. [PubMed] [Google Scholar]
- 17.Atkins MB, et al. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6(Suppl 1):S11–4. [PubMed] [Google Scholar]
- 18.Jansen RL, et al. Interleukin-2 and interferon-alpha in the treatment of patients with advanced non-small-cell lung cancer. J Immunother (1991) 1992;12(1):70–3. doi: 10.1097/00002371-199207000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Schiller JH, Morgan-Ihrig C, Levitt ML. Concomitant administration of interleukin-2 plus tumor necrosis factor in advanced non-small cell lung cancer. Am J Clin Oncol. 1995;18(1):47–51. doi: 10.1097/00000421-199502000-00010. [DOI] [PubMed] [Google Scholar]
- 20.van der Bruggen P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254(5038):1643–7. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 21.Chianese-Bullock KA, et al. MAGE-A1-, MAGE-A10-, and gp100-derived peptides are immunogenic when combined with granulocyte-macrophage colony-stimulating factor and montanide ISA-51 adjuvant and administered as part of a multipeptide vaccine for melanoma. J Immunol. 2005;174(5):3080–6. doi: 10.4049/jimmunol.174.5.3080. [DOI] [PubMed] [Google Scholar]
- 22.Butterfield LH. Cancer vaccines. BMJ. 2015;350:h988. doi: 10.1136/bmj.h988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribas A, et al. Current developments in cancer vaccines and cellular immunotherapy. J Clin Oncol. 2003;21(12):2415–32. doi: 10.1200/JCO.2003.06.041. [DOI] [PubMed] [Google Scholar]
- 24.Melero I, et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat Rev Clin Oncol. 2014;11(9):509–24. doi: 10.1038/nrclinonc.2014.111. [DOI] [PubMed] [Google Scholar]
- 25.Vujanovic L, Butterfield LH. Melanoma cancer vaccines and anti-tumor T cell responses. J Cell Biochem. 2007;102(2):301–10. doi: 10.1002/jcb.21473. [DOI] [PubMed] [Google Scholar]
- 26.Hoon DS, et al. Melanoma patients immunized with melanoma cell vaccine induce antibody responses to recombinant MAGE-1 antigen. J Immunol. 1995;154(2):730–7. [PubMed] [Google Scholar]
- 27.Kruit WH, et al. Phase 1/2 study of subcutaneous and intradermal immunization with a recombinant MAGE-3 protein in patients with detectable metastatic melanoma. Int J Cancer. 2005;117(4):596–604. doi: 10.1002/ijc.21264. [DOI] [PubMed] [Google Scholar]
- 28.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–81. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenberg SA, et al. Gene transfer into humans--immunotherapy of patients with advanced melanoma, using tumor-infiltrating lymphocytes modified by retroviral gene transduction. N Engl J Med. 1990;323(9):570–8. doi: 10.1056/NEJM199008303230904. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg SA, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319(25):1676–80. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 31.Dudley ME, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenberg SA, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer. 2013;13(8):525–41. doi: 10.1038/nrc3565. [DOI] [PubMed] [Google Scholar]
- 34.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–82. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 35.Surh CD, Sprent J. T-cell apoptosis detected in situ during positive and negative selection in the thymus. Nature. 1994;372(6501):100–3. doi: 10.1038/372100a0. [DOI] [PubMed] [Google Scholar]
- 36.Brunet JF, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328(6127):267–70. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 37.Linsley PS, et al. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174(3):561–9. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walunas TL, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1(5):405–13. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 39.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183(6):2541–50. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–61. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tivol EA, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–7. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 42.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270(5238):985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 43.Blank C, et al. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64(3):1140–5. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- 44.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishida Y, et al. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–95. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keir ME, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spranger S, et al. Mechanism of tumor rejection with doublets of CTLA-4, PD-1/PD-L1, or IDO blockade involves restored IL-2 production and proliferation of CD8(+) T cells directly within the tumor microenvironment. J Immunother Cancer. 2014;2:3. doi: 10.1186/2051-1426-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 49.Dong H, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 50.Ahmadzadeh M, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishimura H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–51. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 52.Nishimura H, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291(5502):319–22. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 53.Huang CT, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21(4):503–13. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 54.Woo SR, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 2012;72(4):917–27. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–6. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 56.Robert C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 57.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schadendorf D, et al. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol. 2015;33(17):1889–94. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Prieto PA, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18(7):2039–47. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wolchok JD, et al. Four-year survival rates for patients with metastatic melanoma who received ipilimumab in phase II clinical trials. Ann Oncol. 2013;24(8):2174–80. doi: 10.1093/annonc/mdt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ribas A, Flaherty KT. Gauging the Long-Term Benefits of Ipilimumab in Melanoma. J Clin Oncol. 2015;33(17):1865–6. doi: 10.1200/JCO.2014.59.5041. [DOI] [PubMed] [Google Scholar]
- 62.Robert C, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372(26):2521–32. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 63.Robert C, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–30. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 64.Ackerman A, et al. Outcomes of patients with metastatic melanoma treated with immunotherapy prior to or after BRAF inhibitors. Cancer. 2014;120(11):1695–701. doi: 10.1002/cncr.28620. [DOI] [PubMed] [Google Scholar]
- 65.Bhatia S, Thompson JA. PD-1 Blockade in Melanoma: A Promising Start, but a Long Way to Go. JAMA. 2016;315(15):1573–5. doi: 10.1001/jama.2016.4012. [DOI] [PubMed] [Google Scholar]
- 66.Wolchok JD, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(13):1270–1. doi: 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]
- 68.Wolchok JD, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 69.Gangadhar TC, Vonderheide RH. Mitigating the toxic effects of anticancer immunotherapy. Nat Rev Clin Oncol. 2014;11(2):91–9. doi: 10.1038/nrclinonc.2013.245. [DOI] [PubMed] [Google Scholar]
- 70.Weber JS, et al. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119(9):1675–82. doi: 10.1002/cncr.27969. [DOI] [PubMed] [Google Scholar]
- 71.Dillard T, et al. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary. 2010;13(1):29–38. doi: 10.1007/s11102-009-0193-z. [DOI] [PubMed] [Google Scholar]
- 72.Ansell SM, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311–9. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Herbst RS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taube JM, et al. Differential Expression of Immune-Regulatory Genes Associated with PD-L1 Display in Melanoma: Implications for PD-1 Pathway Blockade. Clin Cancer Res. 2015;21(17):3969–76. doi: 10.1158/1078-0432.CCR-15-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson DB, et al. Melanoma-specific MHC-II expression represents a tumour-autonomous phenotype and predicts response to anti-PD-1/PD-L1 therapy. Nat Commun. 2016;7:10582. doi: 10.1038/ncomms10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tumeh PC, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lynch TJ, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30(17):2046–54. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 78.Reck M, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line therapy in extensive-disease-small-cell lung cancer: results from a randomized, double-blind, multicenter phase 2 trial. Ann Oncol. 2013;24(1):75–83. doi: 10.1093/annonc/mds213. [DOI] [PubMed] [Google Scholar]
- 79.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brahmer JR, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Snyder A, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371(23):2189–99. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rizvi NA, et al. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science. 2015 doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van Allen EM, et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350(6257):207–11. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garon EB, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372(21):2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 85.Yadav M, et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature. 2014;515(7528):572–6. doi: 10.1038/nature14001. [DOI] [PubMed] [Google Scholar]
- 86.Jones B. Clinical genetics. Sequencing for tailored melanoma immunotherapy. Nat Rev Genet. 2015;16(5):259. doi: 10.1038/nrg3945. [DOI] [PubMed] [Google Scholar]
- 87.Hua C, et al. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol. 2016;152(1):45–51. doi: 10.1001/jamadermatol.2015.2707. [DOI] [PubMed] [Google Scholar]
- 88.Sanlorenzo M, et al. Pembrolizumab Cutaneous Adverse Events and Their Association With Disease Progression. JAMA Dermatol. 2015;151(11):1206–12. doi: 10.1001/jamadermatol.2015.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002;2(2):85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]