Abstract
Many organisms contain “head-to-head” isoprenoid synthases and here, we investigate three types of such enzymes from the pathogens Neisseria meningitidis, N. gonorrhoeae, and Enterococcus hirae. The E. hirae enzyme was found to produce dehydrosqualene and we solved an inhibitor-bound structure which revealed a fold similar to that of CrtM from Staphylococcus aureus. In contrast, the homologous proteins from Neisseria spp. carried out only the “first half” reaction, yielding presqualene diphosphate (PSPP). Based on product analyses, bioinformatics, and mutagenesis we conclude that the Neisseria proteins are HpnDs (PSPP synthases). The differences in chemical reactivity to CrtM are due at least in part to the presence of a PSPP-stablizing arginine in the HpnDs, decreasing the rate of dehydrosqualene biosynthesis. These results show that not only S. aureus but also other bacterial pathogens contain “head-to-head” prenyl synthases, although their biological functions remain to be elucidated.
Keywords: isoprenoid, enzyme, terpene, meningitis, squalene
Graphical abstract

Bacteria that cause meningitis, gonorrhea and some cases of endocarditis and septicemia all express terpene synthase-like proteins. We show that they make presqualene diphosphate or dehydrosqualene and report one X-ray structure with a bound inhibitor.
Introduction
Terpenes or isoprenoids are small molecules that contain one or more C5 (isoprene) building blocks[1]. There are ∼70,000 such species known[2], produced by a wide range of terpene synthases. Isoprenoids are of general interest since they are involved in the formation of important drugs [3]; bacterial cell wall biosynthesis[4]; fragrances[5]; quinones and hemes for electron transport[6] as well as the post-translational modification of proteins that are important in cellular signaling [7].
Although the number of terpenes is large, there are a much smaller number of protein classes involved in their biosynthesis[1]. Our interest here is in the so-called “head-to-head”[8] class of terpene synthases which are found in prokaryotes and eukaryotes, including humans. These enzymes condense two isoprenoid diphosphates, such as farnesyl diphosphate (FPP, 1, Scheme 1), at C1 to form e.g. presqualene diphosphate (PSPP, 2), hence the “head-to-head” designation, with PSPP then being converted to either dehydrosqualene (DHS, 3), hydroxysqualene (4) or squalene (SQ, 5). In contrast, “head-to-tail” condensations are involved in isoprenoid chain elongation with e.g. 1 reacting with isopentenyl diphosphate (IPP, 6) to form the C20 product geranylgeranyl diphosphate (GGPP, 7), which can then be converted by phytoene synthases to pre-phytoene diphosphate and phytoene, the building blocks of plant carotenoid biosynthesis. The structures of the head-to-head synthases, how they function, and how they might be inhibited are all important questions because several of these enzymes are drug targets[9] as well as, potentially, herbicide targets. Perhaps one of the simplest reactions (i.e. one step; no reductant) carried out by a head-to-head synthase is carried out by the enzyme HpnD (Hpn refers to genes involved in hopanoid biosynthesis) and involves the condensation of two FPP molecules to form PSPP (2), as in e.g. Zymomonas mobilis[10], Figure 1A. PSPP is then converted by HpnC to 12-hydroxysqualene (4) which, in turn, is converted by HpnE to squalene, used in hopanoid biosynthesis. In S. aureus, the enzyme CrtM converts two FPP molecules to PSPP and then to dehydrosqualene, the first committed step in biosynthesis of the carotenoid virulence factor, staphyloxanthin[11], Figure 1B. In eukaryotes such as fungi and protozoa, the enzyme squalene synthase (SQS) again first converts FPP to PSPP, but this is then converted to squalene in a second step, one that requires NADH/NADPH, Figure 1C. In the green alga, Botryococcus braunii Race B[12], a squalene synthase-like protein (SSL-1) also converts FPP to PSPP (Figure 1D), which can then be converted to squalene or to botryococcene (by SSL-3), making these prenyltransferases a potential source of biofuel.
Scheme 1.
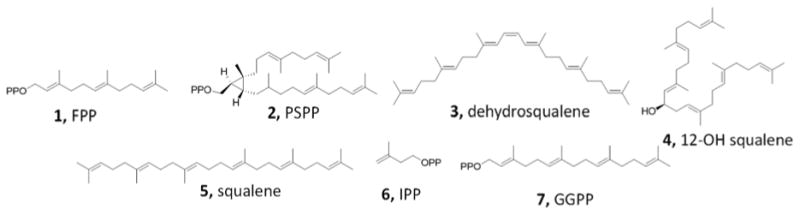
Structures of compounds discussed in the text.
Figure 1.
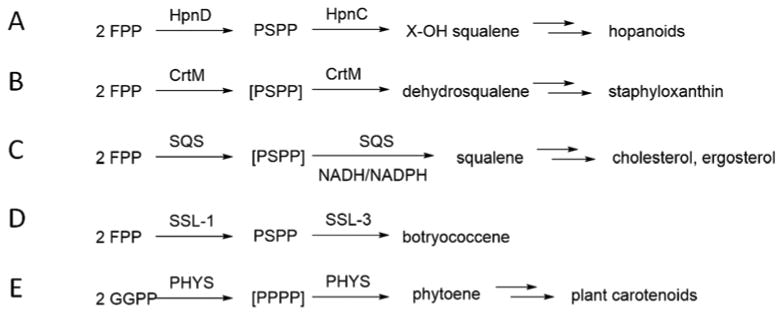
Some reactions catalyzed by “head-to-head” prenyl transferases. A, PSPP formation from FPP by HpnD in hopanoid biosynthesis. B, dehydrosqualene formation from FPP by S. aureus CrtM in staphyloxanthin biosynthesis. C, squalene formation from FPP and NADH/NADPH by SQS in sterol (e.g. cholesterol, ergosterol, sitosterol) biosynthesis. D, PSPP formation from FPP by Botryococcus braunii Race B SSL-1 in botryococcene biosynthesis. E, phytoene formation from GGPP by PHYS in plant carotenoid biosynthesis; PPPP = prephytoene diphosphate.
Finally, in plants, GGPP (7) is converted by phytoene synthase (PHY) to phytoene (the C40 analog of dehydrosqualene), Figure 1E, the precursor of carotenoid pigments such as β-carotene.
In this work, we identified homologs of the “head-to-head” terpene synthases in human pathogenic bacteria to investigate their structure and function. Earlier work[13] showed that S. aureus CrtM (SaCrtM; dehydrosqualene synthase) had a structure that was remarkably similar to that of human squalene synthase (HsSQS) and could be inhibited by known SQS inhibitors. This led to a novel approach to targeting S. aureus virulence in vivo[13] and so, we reasoned, learning more about the occurrence, structure, and function of related head-to-head terpene synthases in other pathogens might—in the future—result in novel therapeutic leads.
Results and Discussion
Bioinformatics analysis
We first carried out a BLAST (Basic Local Alignment Search Tool[14]) search of all bacterial genomes using SaCrtM as a query. There were several hits including genes from Neisseria meningitidis (the causative agent of meningitis), Neisseria gonorrhoeae (the causative agent of gonorrhea), and Enterococcus hirae (a causative agent of endocarditis and septicemia in humans). In an attempt to link primary sequence to predicted function, we then utilized the Enzyme Function Initiative (EFI)'s Sequence Similarity Network (SSN) tool[15] to map similarities and recognize clustering (Figure 2).
Figure 2. Network analysis of CrtM-like proteins.
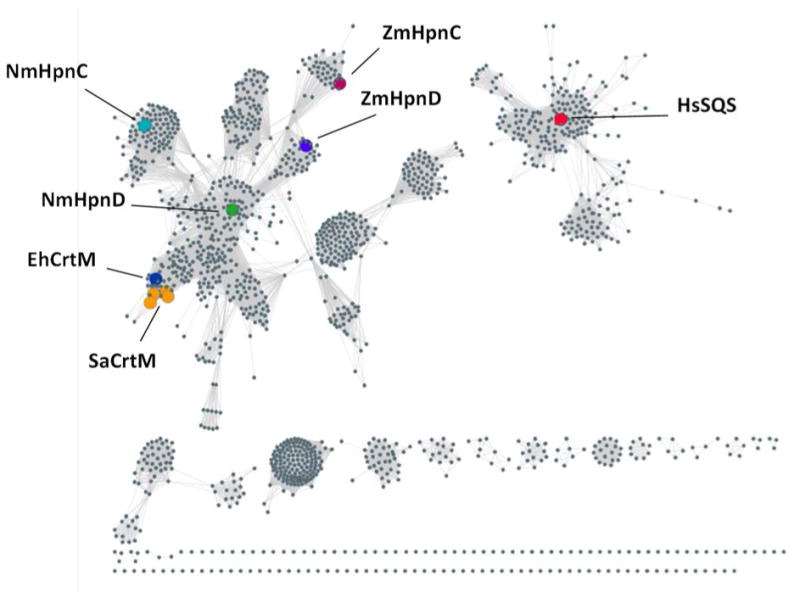
The Enzyme Function Initiative Enzyme Similarity Tool was used to generate a sequence similarity network for InterPro family IPR002060 (squalene/phytoene synthases) using an e-value cutoff of e-50 and an 85% amino acid sequence identity. SaCrtM = Staphylococcus aureus CrtM; EhCrtM = Enterococcus hirae CrtM; NmHpnD = Neisseria meningitidis HpnD; NmHpnC = Neisseria gonorrhoeae HpnC; ZmHpnD = Zymomonas mobilis HpnD; ZmHpnC = Zymomonas mobilis HpnC; HsSQS = Homo sapiens SQS.
From the SSN, which clusters proteins of related sequences, the S. aureus CrtM proteins (Figure 2, mid left; orange) form a tight cluster with a predicted E. hirae CrtM (EhCrtM; mid-left, blue) close-by, suggesting that the E. hirae protein might also produce dehydrosqualene. The adjacent cluster contains the Neisseria proteins (from N. meningitidis, N. gonorhoeae and N. sicca; green), annotated as HpnD due to proximity to the cluster containing Z. mobilis (top center cluster; blue). Previously characterized HpnD enzymes utilize a partner terpene synthase, HpnC, which converts the PSPP product of HpnD to 12-OH squalene and such HpnD/HpnC systems are also found in Neisseria spp. (but not in E. hirae), and are present in an operon together with HpnE, the flavoprotein reductase that in other organisms is known to convert HSQ to SQ. HpnCs and SQS are more distant from CrtM/HpnD, Figure 2.
We next sought to determine the structures and products of EhCrtM and Neisseria spp. HpnD and HpnC-like proteins which—based on the bioinformatics analysis—were expected to produce dehydrosqualene, PSPP or 12-OH squalene.
Protein expression and activity
We first cloned, expressed, purified and crystallized EhCrtM and solved its structure crystallographically. Full details are given in the Experimental Section and data acquisition and refinement details are given in Table 1. The protein produced dehydrosqualene from FPP, as anticipated based on the EFI analysis, and the two substrate analog S-thiolo-FPPs (FSPP) were found to bind to the active site, consistent with previous work on SaCrtM[13]. A view of the complete EhCrtM structure with two FSPPs bound is shown in Figure 3A, and a superposition of the EhCrtM (yellow) and SaCrtM (cyan) structures (with FSPP ligands) is shown in Figure 3B in which there is a 1.3 Å Cα rmsd over 266 residues. The SaCrtM and EhCrtM structures are therefore very similar, as are the FSPP (substrate analog) binding modes. We next cloned, expressed and purified the putative HpnDs from N. menigitidis, N. gonorrhoeae and N. sicca (Nm, Ng and Ns, respectively). All three proteins expressed well and were >95% pure as judged by SDS-PAGE electrophoresis (Figure 4A). However, given the sequence differences to SaCrtM, EhCrtM and ZmHpnD seen in the BLAST alignment and discussed more below, the actual substrates and products of the putative Neisseria spp. HpnDs were uncertain, so a range of potential isoprenoid diphosphate substrates were tested. There was no reaction with IPP, dimethylallyl diphosphate (DMAPP), geranyl diphosphate (GPP) or GGPP, but FPP was a good substrate (Figure 4B). There was no increase in activity with FPP together with either IPP, DMAPP, GPP or GGPP. The product of the putative HpnD with FPP as substrate was then found by TLC autoradiography to be PSPP, based on Rf values with authentic PSPP, as well as by its conversion to squalene with SQS/NADH addition (after complete conversion to PSPP), as shown in Figure 4C, with no longer-chain products observed. Figure 4D shows the LC-MS chromatograms of NmHpnD+FPP and HsSQS+FPP (no NADPH) reaction products, and the major products of both reactions are consistent with PSPP formation, based on their m/e values. To evaluate competency, the rates of the reactions of the NmHpnD, NgHpnD and NsHpnD were evaluated via a kinetic phosphate release assay, which produced similar results for the three Neisseria HpnDs, Figures 4E (for NmCrtM) and 4F (Km, Vmax and kcat/Km for all three proteins).
Table 1. Data collection and refinement statistics for EhCrtM in complex with FSPP.
| EhCrtM-FSPP | |
|---|---|
|
| |
| PDB code | 5IYS |
| Data collection | |
| space group | P21 |
| unit-cell | |
| a [Å] | 37.2 |
| b [Å] | 41.1 |
| c [Å] | 93.5 |
| α /β /γ (°) | 90.00/96.35/90.00 |
| resolution[Å] | 25-2.68 (2.78-2.68) |
| unique reflections | 21560 (2126) |
| redundancy | 5.2 (5.0) |
| completeness [%] | 99.7 (99.8) |
| average l/σ(l) | 24.43 (5.98) |
| R mergea [%] | 6.3 (26.9) |
| Refinement | |
| no. of reflections | 20009 (1465) |
| Rworka (95% of data) | 0.166 (0.198) |
| Rfree. a (5 % of data) | 0.219 (0.254) |
| r.m.s.d. bonds [Å] | 0.010 |
| r.m.s.d. angles [°] | 1.445 |
| dihedral angles | |
| most favored [%] | 98.9 |
| allowed [%] | 1.1 |
| disallowed [%] | 0.0 |
| no. of non-H atoms / average B | |
| [Å2] | |
| Protein | 2325/28.1 |
| Water | 203/40.1 |
| Ion | 13/58.2 |
| Ligand | 48/34.6 |
Values in parentheses are for the highest resolution shell.
Rmerge = ΣhklΣi|Ii(hkl)-<I(hkl)>| / ΣhklΣiIi(hkl).
Figure 3. Enterococcus hirae CrtM.

A, structure of EhCrtM (PDB entry 5IYS showing two FSPP ligands bound. B, Superposition of EhCrtM with S. aureus CrtM (PDB:3W7F, ref 13) showing a 1.3 Å Cα rmsd/266 residues. Yellow protein is EhCrtM; magenta FSPP is from EhCrtM; green Mg ions are from EhCrtM; cyan protein is SaCrtM; blue FSPP is from SaCrtM; grey Mg ions are from SaCrtM.
Figure 4. Neisseria HpnD protein expression and activity.
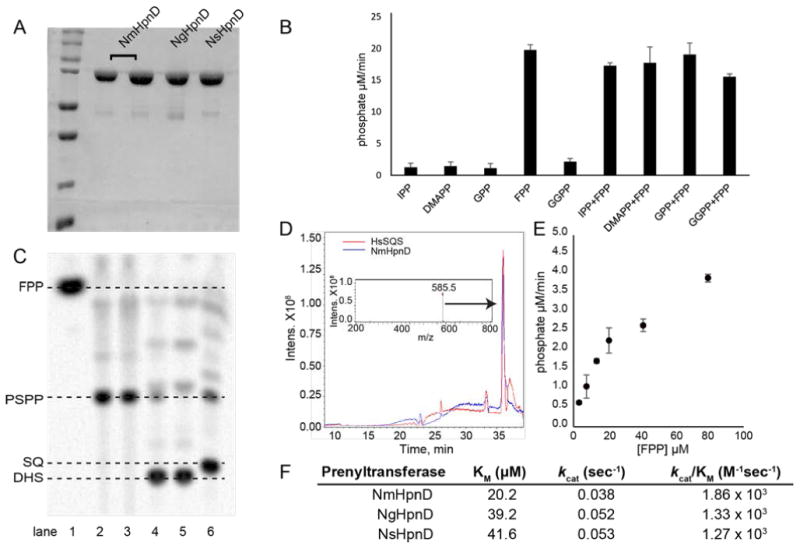
A, SDS-PAGE analysis of NmHpnD (two replicates), NgHpnD and NsHpnD. B, Activity of NmHpnD with various prenyl diphosphates as determined in an MESG/PNP/PPase assay for phosphate. C, TLC autoradiography of NmHpnD products with [u-14C-FPP]. Lane 1, FPP control; lane 2, MBP-NmHpnD plus FPP (PSPP product); lane 3, same as lane 2 but TEV cleaved protein to remove the MBP tag; lane 4, as lane 2 but SQS/NADPH added after PSPP reaction completion, which converts PSPP to SQ ; lane 5, SQS+NADPH (SQ product); lane 6, SaCrtM+FPP, DHS product. D. LC-MS traces showing reaction products of NmHpnD+FPP (in blue) and HsSQS+FPP (in red). Inset shows the mass spectrum of PSPP ([M-1]-=585.5 Da) from the NmHpnD reaction. E. Michaelis-Menten plot for MBP-NmHpnD with different FPP concentrations. F. Kinetic parameters for Neisseria spp.
We attempted to crystallize NmHpnD, but we were not successful. Furthermore, we were unable to obtain catalytically active HpnCs, as judged by the lack of new products in reaction mixtures containing HpnD plus HpnC.
We thus used the structure prediction program Phyre2[16] to predict possible HpnD structures. HpnD structures were predicted with “100% confidence” over 94-98% of their sequences when utilizing either an SaCrtM template, an Alicyclobacillus acidocaldarius squalene synthase HpnC (Protein Data Bank, PDB entry 4HD1), or Bacillus subtilis YisP (PDB: 3WE9[17]). While structure predictions are less reliable than are actual crystal structures, the similarities in the folds of SaCrtM, EhCrtM, AaHpnC and BsYisP all support the use of computational modeling to suggest which residues are likely to be in the NmHpnD active site region. And, when combined with the results of amino acid sequence alignment comparisons, both sets of results could suggest which residues might be involved in determining why NmHpnD produces only PSPP, and does not carry out the second half reaction found in CrtM, formation of dehydrosqualene.
We show in Figure 5A an alignment of the SaCrtM structure (cyan) containing PSPP (purple sticks) (PDB:3NPR[18]) with the NmHpnD model (yellow), and in Figure 5B the corresponding sequence alignment. As can be seen in Figure 5B, the only obvious difference in the active site region (residues within 4Å of PSPP, highlighted in yellow), shown in the red box, is that S19 in SaCrtM is R15 in NmHpnD model (Figure 5B). The presence of this charged residue could contribute to stabilizing PSPP (in HpnD), or, it might prevent the second-half reaction, conversion of PSPP to dehydrosqualene, for example by preventing formation of a [Mg2+]3 complex used to facilitate ionization/diphosphate release.
Figure 5. Role of NmHpnD R15/SaCrtM S19 in product formation.
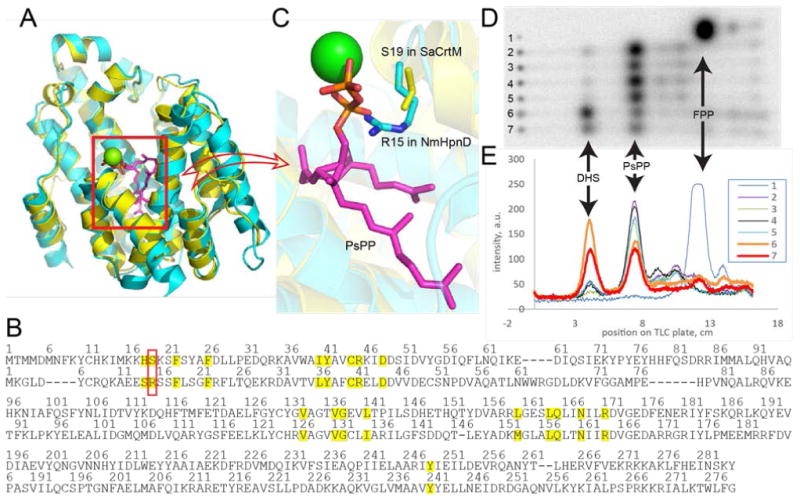
A, alignment of the SaCrtM structure containing PSPP (PDB:3NPR) on a Phyre2 model of NmHpnD. B. Structure-based sequence alignment of SaCrtM and NmHpnD based on A. The aligned active site residues (with 4 Å of PsPP) from both proteins are highlighted in yellow. Residue pair with different residues of interest (R15 in NmHpnD and S19 in SaCrtM) is boxed in red. C. A zoomed-in view of A showing the close proximity of the R15/S19 residues. D and E, Effects of mutations on product formation by HpnD and CrtM. Lane 1, FPP control; lane 2, MBP-NsHpnD, PSPP product; lane 3, MBP-NgHpnD, PSPP product; lane 4, MBP-NmHpnD, PSPP product; lane 5, MBP-NmHpnD R15S mutant, PSPP product; lane 6, SaCrtM, dehydosqualene product (gold); lane 7, SaCrtM S19R mutant showing large decrease in DHS formation (red) versus wt (lane 6).
To investigate the possible role of R15 in NmHpnD we replaced it with serine, R15S, in addition to substituting S19 in SaCrtM with arginine, S19R. The possibilities are that since the HpnD reaction is a “single-step ” reaction, 2FPP → PSPP, the R15S mutation in HpnD might have little effect on the reaction rate since it appears that R15 may coordinate to the PSPP product, Figure 5C. However, in CrtM, there are two reactions: 2FPP → PSPP, and PSPP → dehydrosqualene. If PSPP is stabilized by an S19R variant in CrtM with R19 binding to the PSPP diphosphate, as well as potentially having a repulsive Coulombic interaction with the [Mg2+]3, there could be a decrease in dehydrosqualene production. As can be seen in Figures 5D and 5E, in each of the wild type Neisseria spp., lanes 2-4, the major product is PSPP and the same is found with the R15S NmHpnD variant (Figures 5D and 5E, lane 5). However, there is significantly less dehydrosqualene formed using SaCrtM S19R (Figures 5D and 5E lane 7 in red), compared to the wild type protein, lane 6 in gold, supporting the idea that the presence of R19 favors PSPP product formation.
Conclusions
Using bioinformatics, we uncovered multiple “head-to-head” prenyl transferases in the bacterial pathogens that cause meningitis, gonorrhea, and some cases of endocarditis and septicemia. The products of these enzymes are presqualene diphosphate (from HpnD, in the Neisseria spp.) and dehydrosqualene (in Enterococcus hirae). We solved the structure of the E. hirae CrtM finding it to be very similar to the S. aureus enzyme. Computational modeling (and sequence similarity) suggested a role for R15 in stabilizing PSPP formation in HpnD, and in a CrtM S19R variant, an S→R substitution decreased formation of dehydrosqualene. Further work will be required in order to deduce the biological role of the “Hpn” cluster enzymes (HpnC, HpnD and HpnE) in Neisseria because neither squalene nor hopanoids have been reported in these species with virulence, adhesion/entry as well as biofilm formation (as with YisP) being intriguing possibilities. In addition, unlike S. aureus, E. hirae is not pigmented, so the role of its CrtM likewise remains to be determined. What is clear, however, is that HpnD is the first example of a presqualene diphosphate synthase in both pathogenic Neisseria species, warranting further investigation into their biological function.
Experimental Section
SDS-PAGE analysis of recombinant proteins
Proteins were expressed as N-terminal MBP fusions in BL21(DE3-RIPL) Escherichia coli. Protein purity was assessed by SDS-PAGE and Coomassie staining using 12% gels. Briefly, proteins were overexpressed by inoculating 1 L of LB with 50 μg/mL kanamycin and 34 μg/mL chloramphenicol and monitoring growth until OD600=0.6, then placing cultures on ice for 15 minutes. Cultures were then induced with 0.6 mM IPTG for 8 hours at 30 °C. Typical yields were 28-32 mg/L as determined by A280 and Bradford assay. Comparison of kinetic competence showed that with MBP tags, the proteins were stable at -80 °C for ∼1 year.
Time-course cleavage of MBP with TEV (tobacco etch virus) protease
A solution of MBP-HpnD was treated with 1/100 (w/w) of TEV protease at 23 °C and 37 °C and samples were checked for proteolytic progress at 1, 2, and 3 h by SDS-PAGE. Complete removal of the MBP fusion partner was seen at the last time point. Experiments using TEV-cleaved MBP-fusion proteins were treated in the same way, to affect full cleavage.
Substrate selectivity of Neisseria HpnD
Substrate selectivity was determined by using a continuous colorimetric inorganic phosphatase/purine nucleoside phosphorylase (PNP) coupled assay for diphosphate release. In reaction mixtures containing either 50 μM isopentenyl diphosphate (IPP, C5), dimethylallyl diphosphate (DMAPP, C5), geranyl diphosphate (GPP, C10), farnesyl diphosphate (FPP, C15) or geranylgeranyl diphosphate (GGPP, C20), 200 μM of methylthioguanosine (MESG), 0.5 U of purine nucleoside phosphorylase and 0.5 U of inorganic diphosphatase were added. Reactions were initiated with the addition of 2 μM MBP-NmHpnD. Reaction buffers contained 50 mM Tris (7.4), 125 mM NaCl and 20 mM MgCl2 and reactions were allowed to proceed at room temperature for up to 5 minutes, monitoring the absorbance at 360 nm. Initial velocities were calculated using an extinction coefficient ε = 11000 M-1 cm-1. In a second set of experiments, FPP was co-incubated with either IPP, DMAPP, GPP or GGPP, all at 50 μM concentrations, and PPi release determined as described above.
Autoradiography of products
NmHpnD reactions were monitored by autoradiography/thin-layer chromatography using 14C-labeled substrates. Reactions were terminated by the addition of potato acid phosphatase (PAP), diluted with 1 volume of satd. NaCl, then extracted with 2 volumes of chloroform. Extracts were dried, then resuspended in the minimal volume of hexanes (∼50 μL), spotted on reverse-phase C18 thin-layer chromatography plates and developed with a mobile phase of 8:2 acetone/methanol, dried and exposed overnight on a storage phosphor screen. A BioRad Quantity One instrument was then used to image and analyze the exposed screens.
Kinetic analysis of NmHpnD
To reaction mixtures containing either 2.5, 5, 10, 20, 40 or 80 μM FPP, 0.5 U of purine nucleoside phosphorylase, 0.5 U of inorganic diphosphatase and 200 μM of MESG, 2 μM of MBP-NmHpnD was added and absorbance at 360 nm monitored for up to 5 minutes. Initial velocities were calculated as a function of substrate concentration and Km and kcat calculated using Origin software (OriginLab Corporation, MA).
Sequence similarity analysis
The Enzyme Function Initiative Enzyme Similarity Tool was used to generate a sequence similarity network for InterPro family IPR002060 squalene/phytoene synthase using an e-value cutoff of e-50 and 85% sequence identity convergence to a node.
EhCrtM purification, crystallization and structure determination
The gene encoding EhCrtM (dehydrosqualene synthase from Enterococcus hirae ATCC 9790, GenBank accession number: AFM70029.1) was chemically synthesized by Beijing Genomics Institute (Beijing, China) and ligated into a pET28a vector. Plasmids were transformed into E. coli BL21(DE3) and cultivated at the 5 L scale. Protein was induced with 0.2 mM isopropyl- β-D-thiogalactopyranoside (IPTG) in LB medium at 16 °C for 24 hours. Purification was carried out at 4 °C, as follows. Cells were harvested by centrifugation at 5,000 × g for 10 minutes, re-suspended in lysis buffer containing 25 mM Tris-Cl, pH 8.0, 500 mM NaCl and 20 mM imidazole, then homogenized using a French Press. Cell debris was removed by centrifugation at 17,000 × g for 1 hour. Supernatants were applied to an FPLC system (GE Healthcare) containing a Ni-NTA column and the target protein was eluted at ∼150 mM imidazole when using a 20-500 mM imidazole gradient. The protein was dialyzed against buffer containing 25 mM Tris, 500 mM NaCl, pH 8.0, and subjected to TEV protease digestion overnight to remove the 6× His tag. The mixture was then passed through another Ni-NTA column and the untagged protein eluted with 25 mM Tris, pH 8.0 and 500 mM NaCl. The protein was further purified by using a Superdex-200 gel-filtration column with a buffer containing 20 mM Tris, pH 8.0 and 500 mM NaCl. The purified protein was finally concentrated to 10 mg/mL in buffer containing 25 mM Tris, pH 8.5, 500 mM NaCl, 5 mM MgCl2 and 10 mM DTT for crystallization trials.
Using the sitting-drop vapor diffusion crystallization method, 1 μL 8 mg/mL EhCrtM was mixed with 1 μL of reservoir solution in 24-well Cryschem Plates and equilibrated against 500 μL of the reservoir solution at 25 °C. Crystals were first seen in the No. 75 condition (0.2 M lithium sulfate, 0.1 M bis-Tris pH 6.5, 25 % w/v polyethylene glycol 3350) of the Crystal Screen Index kit (Hampton Research). The crystals reached dimensions suitable for X-ray diffraction within 2-3 days in an optimized reservoir solution containing 0.1 M bis-Tris pH 6.5, 24%-28% PEG 3350, and 0.1 M Li2SO4. EhCrtM-S-thiolo-diphosphate (FSPP) crystals were obtained by soaking EhCrtM crystals in the mother liquor containing 20 mM FSPP for 2 hours prior to data collection. Crystals were mounted in a cryo-loop and flash-cooled by liquid nitrogen for data collection. Data sets were collected at beam line BL13C1 of the National Synchrotron Radiation Research Center (NSRRC, Hsinchu, Taiwan) and processed by using the HKL2000 program[19]. Structures were solved by molecular replacement using the Phaser program in the of CCP4 suite of programs[20] using the structure of SaCrtM (PDB: 4E9U) as the search template. Structure refinements were carried out using the programs Refmac5[21] and Coot[22]. All Figures were prepared by using the PyMOL program (http://pymol.sourceforge.net/).
Acknowledgments
This work was supported in part by a NIH Director's New Innovator Award (DP2 OD008463 to D.A.M.), the David and Lucile Packard Fellowship for Science and Engineering (to D.A.M.); the National Natural Science Foundation of China (grants 31200053, 31300615, 31400678 and 31470240); the Chinese Academy of Sciences (grant KSZD-EW-Z-015-2); the United States Public Health Service (NIH grants CA158191 and GM065307 to E.O.); a Harriet A. Harlin Professorship, and the University of Illinois Foundation/Oldfield Research Fund. C.J.S. is a member of the NIH Chemistry-Biology Interface Training Program (Grant NRSA 1-T32-GM070421). We thank the National Synchrotron Radiation Research Center of Taiwan for beam-time allocation and data-collection assistance.
References
- 1.Oldfield E, Lin FY. Angew Chem Int Ed. 2012;51:1124–1137. doi: 10.1002/anie.201103110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buckingham J. Dictionary of Natural Products on DVD. CRC Press; 2007. [Google Scholar]
- 3.Tu Y. Nat Med. 2011;17:1217–1220. doi: 10.1038/nm.2471. [DOI] [PubMed] [Google Scholar]
- 4.Matsumoto Y, Yasukawa J, Ishii M, Hayashi Y, Miyazaki S, Sekimizu K. Sci Rep. 2016;6:22894. doi: 10.1038/srep22894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu M, Chen CC, Chen L, Xiao X, Zheng Y, Huang JW, Liu W, Ko TP, Cheng YS, Feng X, Oldfield E, Guo RT, Ma Y. Angew Chem Int Ed. 2016;55:4721–4724. doi: 10.1002/anie.201600656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Debnath J, Siricilla S, Wan B, Crick DC, Lenaerts AJ, Franzblau SG, Kurosu M. J Med Chem. 2012;55:3739–3755. doi: 10.1021/jm201608g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia Y, Liu YL, Xie Y, Zhu W, Guerra F, Shen S, Yeddula N, Fischer W, Low W, Zhou X, Zhang Y, Oldfield E, Verma IM. Sci Transl Med. 2014;6:263ra161. doi: 10.1126/scitranslmed.3010382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poulter CD, Muscio OJ, Goodfellow RJ. Biochemistry. 1974;13 doi: 10.1021/bi00704a032. [DOI] [PubMed] [Google Scholar]
- 9.Shang N, Li Q, Ko TP, Chan HC, Li J, Zheng Y, Huang CH, Ren F, Chen CC, Zhu Z, Galizzi M, Li ZH, Rodrigues-Poveda CA, Gonzalez-Pacanowska D, Veiga-Santos P, de Carvalho TM, de Souza W, Urbina JA, Wang AH, Docampo R, Li K, Liu YL, Oldfield E, Guo RT. PLoS Pathog. 2014;10:e1004114. doi: 10.1371/journal.ppat.1004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.a) Pan JJ, Ramamoorthy G, Poulter CD. Org Lett. 2016;18:512–515. doi: 10.1021/acs.orglett.5b03546. [DOI] [PubMed] [Google Scholar]; b) Pan JJ, Solbiati JO, Ramamoorthy G, Hillerich BS, Seidel RD, Cronan JE, Almo SC, Poulter CD. ACS Cent Sci. 2015;1:77–82. doi: 10.1021/acscentsci.5b00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu GY, Essex A, Buchanan JT, Datta V, Hoffman HM, Bastian JF, Fierer J, Nizet V. J Exp Med. 2005;202:209–215. doi: 10.1084/jem.20050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell SA, Niehaus TD, Nybo SE, Chappell J. Biochemistry. 2014;53:7570–7581. doi: 10.1021/bi501264s. [DOI] [PubMed] [Google Scholar]
- 13.Liu CI, Luiu GY, Song Y, Yin F, Hensler ME, Jeng WY, Nizet V, Wang AH, Oldfield E. Science. 2008;319:1391–1394. doi: 10.1126/science.1153018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 15.Gerlt JA, Bouvier JT, Davidson DB, Imker HJ, Sadkhin B, Slater DR, Whalen KL. Biochim Biophys Acta. 2015;1854:1019–1037. doi: 10.1016/j.bbapap.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelley LA, Sternberg MJ. Nat Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- 17.Feng X, Hu Y, Zheng Y, Zhu W, Li K, Huang CH, Ko TP, Ren F, Chan HC, Nega M, Bogue S, López D, Kolter R, Götz F, Guo RT, Oldfield E. Chem & Biol. 2014;21:1557–1563. doi: 10.1016/j.chembiol.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin FY, Liu CI, Liu YL, Zhang Y, Wang K, Jeng WY, Ko TP, Cao R, Wang AHJ, Oldfield E. Proc Natl Acad Sci USA. 2010;107:21337–21342. doi: 10.1073/pnas.1010907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otwinowski Z, Minor W. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 20.a) Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AG, McCoy A, McNicholas SJ, Murshudov GN, Pannu NS, Potterton EA, Powell HR, Read RJ, Vagin A, Wilson KS. Acta Crystallog Sect D. 2011;67:235–242. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. J Appl Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murshudov GN, Vagin AA, Dodson EJ. Acta Crystallog Sect D. 1997;53(Pt3):240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 22.Emsley P, Lohkamp B, Scott WG, Cowtan K. Acta Crystallog Sect D. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]


