1. Introduction
The saliva circulating in the mouth comprises a mixture of secretions from the major and minor salivary glands and traces of gingival crevicular fluid. Saliva contains proteins, glycoproteins, electrolytes, small organic molecules (1), and constituents of non-salivary origin including blood cells (2–4) and desquamated epithelial cells. Saliva also contains serum-derived components resulting from passive diffusion via gingival crevices (5). Therefore, saliva may be a good surrogate for serum/plasma samples in biochemical and immunological analysis (6).
There has been increasing interest in the use of saliva and other oral samples for the diagnosis of oral and systemic diseases. Saliva is low-cost, noninvasive, and easier to collect by individuals with minimal training (7–10). It is a highly accessible bodily fluid for biomarker detection in clinical applications. Little attention has been given to nitrate and nitrite in human saliva until recently (11). Saliva nitrate concentrations are about ten-fold higher than serum. It is now known that approximately 25% of the circulating nitrate in blood is actively taken up, concentrated, and secreted into saliva by the salivary glands (12,13). Salivary nitrate is reduced to nitrite by nitrate-reductase expressing bacteria residing primarily on the tongue. Nitrite is then ingested where it may be further reduced to nitric oxide (NO) to support or maintain NO-signaling especially in acidic and ischemic tissues (14). The functionality of this entero-salivary nitrate-reduction to nitrite system has been demonstrated in humans and experimental systems and is considered to operate in conjunction with nitric oxide synthase dependent NO-formation to modulate NO-bioavailability and affect cardiovascular function, cellular energetics, immune function, neurotransmission, and more (15,16). Elucidation of this pathway has opened new therapeutic possibilities for the prevention, diagnosis and treatment of numerous cardiovascular disorders (17). NO production in the oral cavity may also affect oral health and periodontal diseases (18).
Understanding that dietary nitrate may improve NO-bioavailability and exercise performance for example has led to use of nitrite-detecting strips to evaluate nitrate rich dietary products and supplements, compliance to diet, and for improving exercise performance by athletes (19). Commercially available strips (Neogenesis or Berkeley) for estimation of nitrite (a potential marker of NO-bioavailability) in saliva are sold over the counter. These simple to use and inexpensive POC tests for measuring nitrite are attractive as a cheap and easy way to estimate NO-bioavailability and may also have potential use in research as a low burden low cost test but it is important to validate these POC tests against standard lab-based methods. To our knowledge, saliva strips have been validated in only one study (20) where plasma from healthy donors was used as the standard. Compared to plasma, serum is considered the gold standard for many clinical assays (21,22), and demonstrates a higher sensitivity in biomarker detection (23). Moreover, reliability of metabolites is slightly higher in serum compared to plasma (24). No study to date has validated the strips against lab measures using both saliva and serum, nor in patients with cardiometabolic disease. This study validates two commonly used commercial strips: Nitric Oxide Test Strips (Berkeley Test) and Nitric Oxide Indicator Strips (Neogenesis; Austin, TX) compared to standard lab measures for saliva and serum nitrite/nitrate.
2. Materials and methods
2.1 Study population
The present study utilizes baseline data and samples from the San Juan Overweight Adults Longitudinal Study (SOALS). The study was approved by the University of Puerto Rico Institutional Review Board. Detailed description of the study have been published elsewhere (25). Participants who were free of previously diagnosed diabetes, between 40 and 65 years, and overweight or obese (BMI ≥ 25.0 kg/m2) were recruited primarily from the San Juan municipality area. Participants with previous diagnosis of diabetes as well as those that had braces or less than four teeth (since one of the primary goals of SOALS was relating periodontitis and glucose abnormalities), pregnant, some systemic conditions (such as physician-diagnosed hypoglycemia, congenital heart murmurs, heart valve disease, congenital heart disease, endocarditis, rheumatic fever, hemophilia or bleeding disorders), or inability to complete study procedures were excluded from the study. Most exclusion criteria were related to health conditions that could potentially increase the risk of acute systemic complications from a periodontal examination. Participants were also excluded if they met any of the American Diabetes Association criteria for diabetes: fasting plasma glucose (FPG) ≥126 mg/dL, two-hour oral glucose tolerance test (OGTT) ≥ 200 mg/dL, or HbA1c ≥ 6.5% at the baseline exam (26). A total of 1,206 eligible participants completed the SOALS baseline exam. For this validation study, we excluded current or past smokers, and the sampling was restricted to never smokers, since smoking is highly associated with altered NO metabolism (27–31). From the never smokers, a subgroup of twenty participants with complete baseline data and saliva samples were selected randomly after stratifying on the basis of two factors related to nitric oxide metabolism, namely mouthwash use (never vs. twice-a-day or more) and metabolic syndrome defined using ATP III with 5 participants selected from within each of the four strata. Our objective was to achieve a good range of nitric oxide metabolites (nitrate/nitrite) in serum and in saliva and have appropriate representation and ability to control for these two major factors, that may impact nitric oxide metabolism (32).
2.2 Saliva Collection
Unstimulated saliva samples were collected using an established protocol (33). Participants were asked to refrain from eating, drinking, smoking and from oral hygiene practices (using mouthwash/brushing) for at least an hour prior to the visit. The participants were asked to rinse their mouth with drinking water 5 minutes prior to sample collection. After that, they were asked to drool/spit into a 50 cc container placed on a table, until approximately 6cc of saliva had been collected. Samples were later taken to the lab to be centrifuged at 2600 × g for 15 minutes at 4 °C. Saliva supernatant was divided into 500 ul vials and preserved with Protease inhibitor (made from a combination of Aprotinin, Na3OV4 and PMSF) before freezing in −80. (34).
2.3 Biomarker assessment using strips
Baseline saliva samples from the selected 20 participants were sent on dry ice to University of Alabama at Birmingham (UAB) for analysis. 10μl of each sample was thawed in the dark on ice and then placed onto the absorption pad at end of each strip. The test strip changed color within 45 seconds, and was compared with the Nitric Oxide test strip color chart provided by the strip manufacturers.
2.4 Nitrite and Nitrate lab measurements
After thawing, saliva was centrifuged (5000 × g, 10min) and supernatant nitrite and nitrate measured by HPLC-coupled to the Griess reaction (Eicom). Nitrite levels were also measured by triodide reduction to NO and measurement by reaction with ozone using the Sievers NO-analyzer (35). Serum nitrite and nitrate concentrations were measured after methanol extraction by HPLC-coupled Griess reaction.
2.5 Covariates
We used standard cut-offs from the literature as described below for classifying metabolic syndrome and its components (32). Participants were classified as having elevated blood pressure if they had systolic blood pressure ≥ 130 mm Hg or diastolic blood pressure ≥ 85 mm Hg or reported antihypertensive medication use. Elevated triglycerides were defined as levels ≥ 150 mg/dL or a history of medication use for elevated triglycerides. Low HDL-C was defined as levels < 40 mg/dL in men and levels < 50 mg/dL in women or history of drug treatment for reduced HDL-C. Elevated fasting glucose was defined as levels ≥ 100 mg/dL or a history of medication use for elevated glucose.
Glucose and insulin levels were evaluated at fasting and after administration of a 75-g glucose load at 30, 60, and 120 minutes. Glucose was measured using an enzymatic colorimetric assay. Plasma insulin concentrations were analyzed using an immunochemiluminometric assay. Insulin resistance was estimated using HOMA-IR [Fasting glucose × Fasting insulin/405]. Glycosylated hemoglobin (HbA1C) was measured with an assay based on a latex immunoagglutination inhibition method (DCA 2000+ Analyzer, Siemens Healthcare Diagnostics, NY, US). Pre-diabetes was defined using standard cutoffs as fasting plasma glucose between 100–125 mg/dL, 2-hr OGTT 140–199 mg/dL, or HbA1C of 5.7–6.4% (36).
2.6 Statistical analysis
Lab measures are continuous variables with skewed distributions, and the strips provide ordinal data. Hence, Spearman’s rank correlation and Spearman’s rank partial correlation coefficients were calculated, to evaluate the NO results from the strips (categorical, ordinal variable) with serum and saliva nitrate and nitrite values (continuous variable). Analyses were conducted using SPSS version 21.
3. Results
Table 1 describes the study population. Seventy-five percent of the participants were women, 55% married, 60% were obese, and the mean age was 50.4 years (SD=7.0). Sixty percent of the population was not physically active, and more than half of them (57.9%) were not drinking alcohol.
Table 1.
Descriptive characteristics of study sample
| Characteristic | N (%) or Mean ± SD |
|---|---|
| Age | 50.40 ± 7.01 |
| Gender | |
| Female | 15 (75%) |
| Male | 5 (25%) |
| Education | |
| More than high school | 2 (10%) |
| High school | 6 (30%) |
| Less than high school | 12 (60%) |
| Annual income | |
| ≤ $20,000 | |
| ≥ $20,000 | 5 (25%) |
| 15 (75%) | |
| Marital Status | |
| Married | 11 (55%) |
| Unmarried | 9 (45%) |
| Smoking status | |
| Never | 20(100%) |
| Alcohol intake | |
| Abstainer | 9 (47.4%) |
| Former | 2 (10.5%) |
| Current | 8 (42.1%) |
| Physical activity | |
| Yes | 8 (40%) |
| No | 12 (60%) |
| BMI (mean) | 33.95 ± 6.81 |
| Overweight (25.0 – 29.9) | 8 (40%) |
| Obese (≥30.0) | 12 (60%) |
Nitrate concentrations in the saliva ranged between 6.7 and 525.2 uM (mean 135.6±132.0 uM) and in serum between 7.3 to 37.7 uM (mean 14.6±7.0 uM) (Figures 1A and 1B). Nitrite concentrations in saliva ranged between 13.1 to 180.1 uM (mean 62.1±46.3 uM) and in the serum between 0.4 to 7.5 uM (mean 1.7±1.7 uM). The concentration of both nitrite and nitrate was significantly higher in saliva compared to serum (Wilcoxon Signed Ranks Test p < 0. 01). Saliva nitrite levels had a significant positive correlation with saliva nitrate levels (r=0.49, Figure 2A) and with serum nitrate levels (r=0.54, Figure 2B). After controlling for metabolic syndrome and mouthwash use, the correlation of saliva nitrite with saliva nitrate increased (r=0.55) while the correlation with serum nitrate decreased slightly (r=0.52). Saliva nitrate had a significant positive correlation with serum nitrite levels (r=0.46, Figure 2C) and remained similar even after controlling for mouthwash use and metabolic syndrome. No significant correlation was observed between saliva nitrite and serum nitrite levels (Figure 2D; r=0.17 or r=0.22 after controlling for mouthwash and metabolic syndrome).
Figure 1.
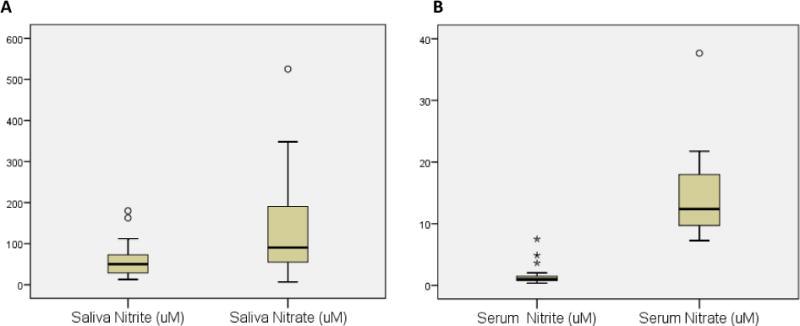
Distribution of nitrite and nitrate in saliva (A) and in serum (B)
Figure 2.
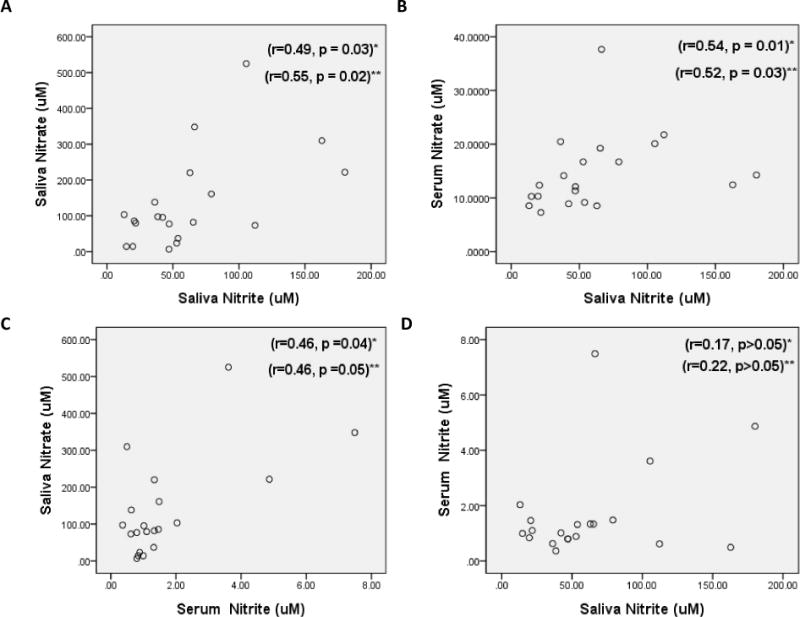
Scatter Plot relating Nitrate and Nitrite concentrations in Saliva and Serum
* Spearman’s rank correlation, ** Spearman’s rank partial correlation
The distributions of the Neogenesis and Berkeley strip scores are cross-tabulated in Table 2. Spearman correlations revealed moderate to high correlation between the two strips (r=0.72, p<0.001) and it increased (r=0.75, p<0.001) with the Spearman partial correlations. Both strips showed a significant correlation with the salivary nitrite measurements (Table 3), with Berkeley strips showing a higher correlation (r=0.76, p<0.001) than the Neogenesis strips (r=0.59, p<0.01). Neither of the strips showed any significant correlation with salivary nitrate levels, or with the nitrite or nitrate levels in serum (p>0.01).
Table 2.
NO Score distributions for Neogenesis Saliva Strips vs. Berkeley Saliva Strips
| Berkeley Saliva Strip | |||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Depleted | Low | Threshold | Target | High | Total | ||
|
|
|||||||
| Neogenesis | Depleted | 2 | 2 | 0 | 0 | 0 | 4 |
| Saliva | Low | 0 | 3 | 2 | 3 | 1 | 9 |
| Strip | Normal | 0 | 0 | 2 | 1 | 4 | 7 |
| Total | 2 | 5 | 4 | 4 | 5 | 20 | |
Table 3.
Spearman’s rank correlation & Spearman’s rank partial correlation coefficients relating NO strip results and laboratory nitrite and nitrate measurements in saliva and in serum.
| Saliva Nitrite |
Saliva Nitrate |
Serum Nitrite |
Serum Nitrate |
|
|---|---|---|---|---|
| Saliva Strips | Correlation Coefficient | |||
|
Neogenesis (Spearman
correlation) (Spearman partial correlation) |
0.53 0.59* |
0.30 0.29 |
0.33 0.34 |
0.24 0.25 |
|
Berkeley (Spearman
correlation) (Spearman partial correlation) |
0.71** 0.76** |
0.35 0.36 |
0.17 0.17 |
0.35 0.37 |
p-value ≤ 0.01
p-value ≤ 0.001
Remaining correlations are not significant (p-value > 0.05)
4. Discussion
Nitric oxide (NO) is an endogenous signaling molecule with multiple biological roles in the human body, including blood pressure regulation (37), improved memory function (38–40) and ability to fight infections (41–43). Decreased NO bioavailability is also associated with insulin resistance and diabetes (44). NO can be synthesized endogenously from arginine by NO synthase enzymes in endothelial, neural and inflammatory cells, and also exogenously from the metabolism of nitrate by oral bacteria. Consequently, there has been increased interest in the importance of consuming adequate amounts of nitrate-rich foods, such as green leafy vegetables and beet-root juice in improving tolerance to physical exercise (45). However, few studies exist in the literature validating and comparing these products as indicators of NO bioavailability in the body.
Our study found that Berkeley strip and Neogenesis strip results are strongly correlated with each other and with the laboratory measures of nitrite in saliva, even after controlling the metabolic syndrome and mouthwash. The Berkeley strips had a stronger correlation with salivary nitrite compared to the Neogenesis strips, but since the data output from the strips do not provide absolute concentrations, an accurate quantitative assessment of the relationships between the strips and the lab measurements is precluded. Neither of the strips had a significant correlation with the levels of serum nitrite, which is considered a marker of endogenous endothelial nitric oxide synthase activity and a substrate for NO production. Therefore, the saliva NO test strips are unlikely to be of use for assessment of circulating NO-bioavailability. This is consistent with a recent report showing no correlation between saliva and plasma nitrite (20) and likely reflects the fact that circulating nitrite levels can be modulated by multiple reactions and processes including endogenous formation via NO-oxidation, swallowing of salivary nitrite and stomach metabolism and transport into the circulation, versus nitrite oxidation or reduction (by metalloproteins) and nitrite excretion in sweat and urine (46).
The range of concentrations of nitrate and nitrite in our study were similar to those reported in a previous study (20); however, the range of serum nitrite levels in our study was higher compared to the previous report (0.37 to 7.49 uM compared to 0.06 to 0.184 uM). This could be due to the fact that our population consisted of overweight to obese people. Among the obese and overweight individuals, the overall production and levels of NO are increased along with high nitrite excretion (47). Consistent with previous studies (20), there were significant correlations between the levels of nitrate and nitrite in the saliva, and between salivary nitrite and serum nitrate levels. In contrast to the previous report there was no significant correlation between the salivary and serum concentrations of nitrate, but we did observe a significant correlation between salivary nitrate and serum nitrite levels. A possible explanation for the difference with the previous study (20) is that we used serum instead of plasma samples. Serum is considered the gold standard for many clinical assays (21,22), and demonstrates a higher sensitivity and reliability in biomarker detection compared to plasma (24).
The population of this study was stratified on metabolic syndrome and mouthwash use, hence we controlled for metabolic syndrome and mouthwash use. After adjusting for these factors, the correlation between the strips and lab results increased. The sample is restricted to overweight and obese non-smokers, which may limit generalizability. The limitations of the study also include a small sample size. The reasonable validity of the Berkeley and Neogenesis strips, enables individuals to easily assess their salivary nitrite levels on their own and see the impact of dietary and lifestyle changes they implement. There is some limited potential to incorporate saliva nitrite measures in research studies using the strips as a semi-quantitative measure and surrogate for the lab measures of salivary nitrite, where these are secondary measures or covariates in the study. The ability to assess and monitor nitrite levels in patients may have a profound implication for cardiometabolic health and well-being (48). The findings of this study indicate that saliva strips are strongly correlated with lab measures of saliva nitrite but are weakly related with serum nitrite and serum nitrate.
5. Conclusions
Easy to use Point-of-care (POC) tests commercially available Berkeley and Neogenesis strips for measuring NO-metabolites in saliva, show good validity for salivary nitrite, compared to standard lab measures, but not for serum measures.
Research Highlights.
Berkeley and Neogenesis NO strip measurements correlate with each other.
Lab measures of Saliva and Serum nitrate do not correlate significantly.
NO strips measurements provide a reasonable surrogate for salivary, but not systemic nitrite levels
Acknowledgments
We would like to acknowledge the SOALS team (Ms. Elaine Rodríguez, Ms. Rosalyn Roman, Ms. Yadiris Santaella, Ms. Grace Velez) for their help with the study. We would also like to acknowledge the PRCTRC laboratory personnel (Mrs. Nilda González and Mrs. Aracelis Arroyo) for their help during the blood and saliva processing Dr. David Wong. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Research reported in this publication was supported by the National Institute of Dental and Craniofacial Research Grant R01DE020111, the National Institute on Minority Health and Health Disparities Grant U54MD007587 and the P30 DK079626/DK/NIDDK NIH, UAB Diabetes Research Center Bio-Analytical Redox Biology (BARB) Core, NIH grants UH2 TR000923, U01DE17593, R01 CA139596, R01 DE17170, R21 CA0126733, PRSTT 2013-000016, the Barnes Fund for Head & Neck Cancer Research and the O’Keefe Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Saliva: its role in health and disease. Working Group 10 of the Commission on Oral Health, Research and Epidemiology (CORE) Int Dent J ENGLAND. 1992 Aug;42(4 Suppl 2):287–304. [PubMed] [Google Scholar]
- 2.Rajendran A, Sivapathasundharam B. Shafer’s Textbook of Oral Pathology [Internet] Elsevier; Health Sciences APAC: 2014. Available from: https://books.google.com.pr/books?id=WnhtAwAAQBAJ. [Google Scholar]
- 3.Lavelle CLB. Applied Oral Physiology. Elsevier; 1988. Applied Oral Physiology [Internet] pp. 128–141. [cited 2016 Jun 6] Available from: http://www.sciencedirect.com/science/article/pii/B9780723608189500194. [Google Scholar]
- 4.Pandey P, Reddy NV, Rao VAP, Saxena A, Chaudhary CP. Estimation of salivary flow rate, pH, buffer capacity, calcium, total protein content and total antioxidant capacity in relation to dental caries severity, age and gender. 6(Suppl 1) doi: 10.4103/0976-237X.152943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffiths GS. Periodontol 2000. Vol. 31. Denmark: 2003. Formation, collection and significance of gingival crevice fluid; pp. 32–42. [DOI] [PubMed] [Google Scholar]
- 6.Bodis S, Haregewoin A. Evidence for the release and possible neural regulation of nitric oxide in human saliva. Biochem Biophys Res Commun [Internet] 1993 Jul 15;194(1):347–50. doi: 10.1006/bbrc.1993.1826. [cited 2016 Jun 6] Available from: http://www.sciencedirect.com/science/article/pii/S0006291X83718267. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson DB. Current diagnostic uses of saliva. J Dent Res UNITED STATES. 1987 Feb;66(2):420–4. doi: 10.1177/00220345870660020601. [DOI] [PubMed] [Google Scholar]
- 8.Malamud D. Saliva as a diagnostic fluid. Dent Clin North Am United States. 2011 Jan;55(1):159–78. doi: 10.1016/j.cden.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufman E, Lamster IB. The diagnostic applications of saliva–a review. Crit Rev Oral Biol Med United States. 2002;13(2):197–212. doi: 10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- 10.Mukhopadhyay R. Devices to drool for. Anal Chem United States. 2006 Jul;78(13):4255–9. doi: 10.1021/ac069420i. [DOI] [PubMed] [Google Scholar]
- 11.Friis ML, Johnsen T, Larsen NE, Hvidberg EF, Pakkenberg H. Eur J Clin Pharmacol. 2. Vol. 18. Springer-Verlag; 1980. Bromocriptine concentration in saliva and plasma after long-term treatment of patients with Parkinson’s disease; pp. 171–4. [DOI] [PubMed] [Google Scholar]
- 12.Jin L, Qin L, Xia D, Liu X, Fan Z, Zhang C, et al. Active secretion and protective effect of salivary nitrate against stress in human volunteers and rats. Free Radic Biol Med United States. 2013 Apr;57:61–7. doi: 10.1016/j.freeradbiomed.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manuscript A. No Title. 2012:61–7. [Google Scholar]
- 14.Moncada S, Rees DD, Schulz R, Palmer RM. Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc Natl Acad Sci U S A [Internet] 1991;88(6):2166–70. doi: 10.1073/pnas.88.6.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: a historical overview. J Physiol Pharmacol Poland. 2002 Dec;53(4 Pt 1):503–14. [PubMed] [Google Scholar]
- 16.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev UNITED STATES. 1991 Jun;43(2):109–42. [PubMed] [Google Scholar]
- 17.Oxide N, Unique ASA, Molecule S, The IN. :503–14. Review article. [Google Scholar]
- 18.Batista AC, Silva TA, Chun JH, Lara VS. Nitric oxide synthesis and severity of human periodontal disease. Oral Dis Denmark. 2002 Sep;8(5):254–60. doi: 10.1034/j.1601-0825.2002.02852.x. [DOI] [PubMed] [Google Scholar]
- 19.Berkeley Test, LLC. Med Devices Surg Technol Week [Internet] Vol. 26. Atlanta: NewsRx; 2016. Jun, p. 1047. (Patent Issued for Compositions, Apparatus and Methods for Monitoring Biomarkers (USPTO 9360490)). Available from: http://search.proquest.com/docview/1797382347?accountid=44825. [Google Scholar]
- 20.Clodfelter WH, Basu S, Bolden C, Dos PC, King SB, Kim-shapiro DB. Nitric Oxide The relationship between plasma and salivary NO x. Nitric Oxide [Internet] Vol. 47. Elsevier Inc; 2015. pp. 85–90. Available from: http://dx.doi.org/10.1016/j.niox.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serum or Plasma? [Internet] [cited 2016 Jun 6]. Available from: http://www.austincc.edu/mlt/phb/Pulse_serum_or_plasma-2.pdf.
- 22.Organization WH. WORLD HEALTH ORGANIZATION USE OF ANTICOAGULANTS IN DIAGNOSTIC.
- 23.Prehn C, He Y, Belcredi P, Mo G, Yu Z, Kastenmu G. Differences between Human Plasma and Serum Metabolite Profiles. 2011 Jul;:1–6. doi: 10.1371/journal.pone.0021230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breier M, Wahl S, Prehn C, Fugmann M, Ferrari U, Weise M, et al. Targeted Metabolomics Identifies Reliable and Stable Metabolites in Human Serum and Plasma Samples. 2014 Feb;:1–11. doi: 10.1371/journal.pone.0089728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andriankaja OM, Jimenez JJ, Munoz-Torres FJ, Perez CM, Vergara JL, Joshipura K. Lipid lowering agents (LLA) use and systemic and oral inflammation in overweight or obese adult Puerto Ricans: the SOALS Study. J Clin Periodontol. 2015 Sep; doi: 10.1111/jcpe.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diabetes DOF. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32(SUPPL. 1) doi: 10.2337/dc09-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heiss C, Kleinbongard P, Dejam A, Perr?? S, Schroeter H, Sies H, et al. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J Am Coll Cardiol. 2005;46(7):1276–83. doi: 10.1016/j.jacc.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 28.Node K, Kitakaze M, Yoshikawa H, Kosaka H, Hori M. Reduced plasma concentrations of nitrogen oxide in individuals with essential hypertension. Hypertension [Internet] 1997;30(3 Pt 1):405–8. doi: 10.1161/01.hyp.30.3.405. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9314424. [DOI] [PubMed] [Google Scholar]
- 29.Motoyama T, Kawano H, Kugiyama K, Hirashima O, Ohgushi M, Yoshimura M, et al. Endothelium-dependent vasodilation in the brachial artery is impaired in smokers: effect of vitamin C. Am J Physiol [Internet] 1997;273(4 Pt 2):H1644–50. doi: 10.1152/ajpheart.1997.273.4.H1644. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9362226. [DOI] [PubMed] [Google Scholar]
- 30.Barua RS, Ambrose JA, Eales-Reynolds L-J, DeVoe MC, Zervas JG, Saha DC. Dysfunctional Endothelial Nitric Oxide Biosynthesis in Healthy Smokers With Impaired Endothelium-Dependent Vasodilatation. Circulation [Internet] 2001;104(16):1905–10. doi: 10.1161/hc4101.097525. Available from: http://circ.ahajournals.org/content/104/16/1905\nhttp://circ.ahajournals.org/content/104/16/1905.full.pdf\nhttp://circ.ahajournals.org/content/104/16/1905.long\nhttp://www.ncbi.nlm.nih.gov/pubmed/11602492. [DOI] [PubMed] [Google Scholar]
- 31.Tsuchiya M, Asada A, Kasahara E, Sato EF, Shindo M, Inoue M. Smoking a single cigarette rapidly reduces combined concentrations of nitrate and nitrite and concentrations of antioxidants in plasma. Circulation. 2002;105(10):1155–7. doi: 10.1161/hc1002.105935. [DOI] [PubMed] [Google Scholar]
- 32.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 33.Henson BS, Wong DaT. Collection, Storage, and Processing of Saliva Samples for Downstream Molecular Applications. In: Seymour GJ, Cullinan MP, Heng NCK, editors. Oral Biology;Molecular Techniques and Applications. Humana Press; 2010. pp. 21–30. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Farrell JJ, Zhou H, Elashoff D, Akin D, Park NH, et al. Salivary Transcriptomic Biomarkers for Detection of Resectable Pancreatic Cancer. Gastroenterology. 2010;138(3) doi: 10.1053/j.gastro.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lang JD, Teng X, Chumley P, Crawford JH, Isbell TS, Chacko BK, et al. Inhaled NO accelerates restoration of liver function in adults following orthotopic liver transplantation. J Clin Invest [Internet] 2007;117(9):2583–91. doi: 10.1172/JCI31892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diabetes VII, In C. Standards of medical care in diabetes-2011. Diabetes Care. 2011;34(SUPPL 1) doi: 10.2337/dc11-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hermann M, Flammer A, Luscher TF. J Clin Hypertens (Greenwich) 12 Suppl 4. Vol. 8. United States: 2006. Dec, Nitric oxide in hypertension; pp. 17–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choopani S, Moosavi M, Naghdi N. Involvement of nitric oxide in insulin induced memory improvement. Peptides [Internet] 2008 Jun;29(6):898–903. doi: 10.1016/j.peptides.2008.01.005. Available from: http://www.sciencedirect.com/science/article/pii/S0196978108000120. [DOI] [PubMed] [Google Scholar]
- 39.Kennedy DO. Plants and the Human Brain [Internet] OUP; USA: 2014. Available from: https://books.google.com.pr/books?id=YUNDAgAAQBAJ. [Google Scholar]
- 40.Paul V, Ekambaram P. Involvement of nitric oxide in learning & memory processes. 2011 May;:471–8. [PMC free article] [PubMed] [Google Scholar]
- 41.Olekhnovitch R, Ryffel B, Müller AJ, Bousso P. Collective nitric oxide production provides tissue-wide immunity during Leishmania infection. J Clin Invest. 2014;124(4):1711–22. doi: 10.1172/JCI72058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Who T. Nitric oxide Bhramari Pranayam and deafness. 2016:20–3. [Google Scholar]
- 43.Curtis V, Armenakis H. M. Report Information from ProQuest. Art Persuas. 2015 Jan [Google Scholar]
- 44.Vanizor B, Orem A, Karahan SC, Kiran E, Erem C, Aliyazicioglu R, et al. Decreased nitric oxide end-products and its relationship with high density lipoprotein and oxidative stress in people with type 2 diabetes without complications. Diabetes Res Clin Pract [Internet] 2001 Oct;54(1):33–9. doi: 10.1016/s0168-8227(01)00281-9. [cited 2016 Aug 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/11532328. [DOI] [PubMed] [Google Scholar]
- 45.Bescos R, Sureda A, Tur JA, Pons A. Sports Med. 2. Vol. 42. New Zealand: 2012. Feb, The effect of nitric-oxide-related supplements on human performance; pp. 99–117. [DOI] [PubMed] [Google Scholar]
- 46.No W. Nitric Oxide Increased NO scavenging. 2015;45:4–6. [Google Scholar]
- 47.Alemany M. Metabolic Syndrome: A Multifaceted Disease of Affluence. J Endocrinol Metab [Internet] 2012;2(4–5):155–65. [cited 2016 Aug 28] Available from: http://www.jofem.org/index.php/jofem/article/view/116. [Google Scholar]
- 48.Bryan NS. The potential use of salivary nitrite as a marker of NO status in humans. Nitric Oxide - Biol Chem. 2015;45:4–6. doi: 10.1016/j.niox.2014.12.011. [DOI] [PubMed] [Google Scholar]


