Abstract
Decades of public health research have documented that smoking in pregnancy poses significant health risks to both mother and child. More recent studies have shown that even passive maternal exposure to secondhand smoke associates with negative birth outcomes. However, the mechanisms linking exposure to outcomes have remained obscure. As a first step toward defining the metabolic consequence of low-level nicotine exposure on fetal development, we conducted an untargeted metabolomic analysis of 81 paired samples of maternal serum and amniotic fluid collected from karyotypically normal pregnancies in the second trimester. By comparing the m/z and retention times of our mass spectral features with confirmed standards, we identified cotinine, a nicotine derivative, and used the calculated cotinine concentrations to classify our maternal serum samples into exposure groups using previously defined cut-offs. We found that cotinine levels consistent with low-level maternal exposure to nicotine associated with distinct metabolic perturbations, particularly in amniotic fluid. In fact, the metabolic effects in amniotic fluid of ostensibly low-level exposed mothers showed greater overlap with perturbations previously observed in the sera of adult smokers than did the perturbations observed in the corresponding maternal sera. Dysregulated fetal pathways included aspartate and asparagine metabolism, pyrimidine metabolism, and metabolism of other amino acids. We also observed a strong negative association between level of maternal serum cotinine and acetylated polyamines in the amniotic fluid. Combined, these results confirm that low-level maternal nicotine exposure, indicated by a maternal serum cotinine level of 2–10 ng/mL, is associated with striking metabolic consequences in the fetal compartment, and that the affected pathways overlap those perturbed in the sera of adult smokers.
Keywords: nicotine, cotinine, tobacco smoke, pregnancy, prenatal exposure, metabolomics, amniotic fluid
1 Introduction
The link between maternal smoking and negative birth outcomes, including small-for-gestational age birth, preterm delivery, stillbirth, and sudden infant death syndrome (SIDS) has been well documented over past decades by careful epidemiological studies (Cnattingius, 2004; Rogers, 2009). Increased public awareness of the dangers of prenatal smoke exposure has led to a marked drop in the rate of maternal smoking in the United States (US) (Curtin & Matthews, 2016). However, the percentage of pregnant women who are passively exposed to secondhand smoke, also known as environmental tobacco smoke, remains high. In one large study of exposure patterns in the US, 30% of participating women indicated that they were exposed to secondhand smoke just before or during pregnancy (Anderka et al., 2010).
The full impact of low-level maternal smoke exposure on birth outcomes remains poorly understood, but a growing literature documents that the consequences of passive smoke exposure overlap those seen with active maternal smoking. Specifically, studies have associated maternal secondhand smoke exposure with low infant birth weight and congenital anomalies (Salmasi, Grady, Jones, McDonald, & Knowledge Synthesis, 2010), increased risk of stillbirth, small infant head circumference (Crane, Keough, Murphy, Burrage, & Hutchens, 2011), and SIDS (U.S. Department of Health and Human Services, 2006).
Prenatal exposure to tobacco smoke can be assessed in different ways. Historically, researchers simply asked mothers about exposure status; unfortunately, this approach was fraught with complications, especially when exposures were low or indirect (Polanska, Hanke, Laudanski, & Kalinka, 2007). A more objective approach now available involves quantifying biomarkers of tobacco exposure directly in biological samples. One such marker is nicotine, the primary addictive component in tobacco smoke. Once absorbed into the blood stream, nicotine is quickly metabolized into cotinine, which has a half-life in blood of close to 16 hours. Because of its longer half-life, cotinine is often preferred over nicotine as a biomarker for tobacco exposure (Benowitz, Hukkanen, & Jacob, 2009). Cotinine has been reliably detected at high levels (>10 ng/mL) in serum samples from smokers, at low levels (2–10 ng/mL) in serum samples from “light” smokers or those passively exposed to secondhand smoke, and at baseline levels (<2 ng/mL) in samples from individuals with no known smoke exposure (Hanke, Sobala, & Kalinka, 2004; Florescu et al., 2009).
Prior studies of both actively smoking (Luck, Nau, Hansen, & Steldinger, 1985) and passively exposed (Jauniaux, Gulbis, Acharya, Thiry, & Rodeck, 1999) pregnant women document that nicotine and/or cotinine not only pass into the fetal compartment (Jordanov, 1990) but concentrate there. Whether this pattern reflects active concentration of nicotine metabolites across the placenta, or a lower turnover rate in the fetal compartment, remains unknown. Either way, this observation raises the alarming possibility that for any given level of maternal nicotine exposure, the fetal exposure, and therefore the fetal metabolic consequences, may be compounded.
To test this hypothesis, we conducted high-resolution untargeted metabolomic analysis of 81 pairs of maternal serum and amniotic fluid samples collected from women in the second trimester of pregnancy. During most of the second trimester, the fetal skin remains un-keratinized, allowing for rapid and bi-directional diffusion across the fetal skin and surfaces of the umbilical cord, placenta, and amnion. The composition of amniotic fluid is therefore similar to that of fetal plasma during this time (Underwood, Gilbert, & Sherman, 2005), and metabolites found in amniotic fluid collected during the second trimester can serve as a good indicator of metabolic status of the fetal compartment.
We compared the pathway profiles of samples characterized by maternal serum cotinine level and found clear perturbations that associated with maternal cotinine in the range of 2–10 ng/mL. Our data therefore confirmed that even low-level nicotine exposure associates with significant changes in fetal metabolism related to aspartate and asparagine metabolism, pyrimidine metabolism, and amino group metabolism, and these perturbations show striking overlap with the dysregulated pathways previously observed in the sera of actively smoking adults (serum cotinine >10 ng/mL). Although this is an implied causality framework in which the associations do not establish a cause-effect relationship, the results presented here are both compelling and disturbing, and extend from previous studies to provide a first glimpse into potential mechanisms of fetal consequence following even low-level maternal exposure to nicotine.
2 Materials and methods
2.1 Paired maternal serum and amniotic fluid samples from women with karyotypically normal pregnancies
We conducted this study using 81 pairs of second-trimester amniotic fluid and matched maternal serum samples collected in the US between 2004–2014; these samples derived from pregnant women who underwent amniocentesis and prenatal testing and whose results confirmed normal fetal karyotype (46,XX or 46,XY). Specifically, samples were obtained as de-identified banked clinical laboratory discards from the Greenwood Genetic Center (GGC, Greenwood, SC, USA), which served as a referral lab for the samples. All women whose samples were used in this study had previously consented to have their de-identified clinical sample leftovers made available for research.
Of the 81 women whose samples we studied here, 62 had been referred for amniocentesis and testing due to an increased risk of Down syndrome (often because of a positive screening result), and 19 were referred due to advanced maternal age. For all paired samples, we were provided the following information: maternal age at serum collection, gestational age at the time of both serum and amniotic fluid collection, serum and amniotic fluid collection year, maternal race/ethnicity, reason for referral, maternal self-reported smoking status, fetal gender, and fetal karyotype. Of note, the 81 pairs of samples used in this study were selected from a larger set to serve as matched controls for a separate study of chromosomally abnormal pregnancies.
Individual samples were collected at the location of the woman’s referring physician or laboratory and transported the same day, or overnight, at ambient temperature to the prenatal testing laboratory at GGC. Maternal serum samples were collected most commonly in Becton-Dickinson (BD) red top, red/black top (serum separator, SST), or gold top (SST) vacutainer tubes. Amniotic fluid samples were collected in standard BD plastic syringes and transported in clear or amber polystyrene tubes. Upon arrival in the laboratory at GGC all samples were inspected for correct identification, sample type, and sample condition, and each received a unique sample identification number in compliance with accession protocols.
Serum samples were received at GGC either as isolated serum, which had been previously removed from the red blood cell clot by centrifugation, or as a clotted whole blood sample. Clotted whole blood samples were centrifuged at 2200 rpm for 10 minutes and the serum transferred to polypropylene vials for storage. Serum samples were stored at 2–8°C for up to 48 hours before clinical testing and then frozen at −20°C (± 10°C) for long-term storage. Amniotic fluid samples received in the cytogenetic laboratory were centrifuged at 1000 rpm for 10 minutes. The supernatant was removed and maintained at −20°C (± 10°C) for long-term storage.
The maternal serum and amniotic fluid samples selected for this study were thawed, aliquotted to fresh vials, and shipped on dry ice to Emory University by overnight courier. At Emory, the samples were stored at −80°C and then thawed and subjected to liquid chromatography-mass spectrometry (LC-MS), as described below.
2.2 High-resolution liquid chromatography-mass spectrometry
Sample analysis was performed as previously described (Jones et al., 2016; Soltow et al., 2013). Serum samples were analyzed with three technical replicates on a Thermo Scientific LTQ Velos Orbitrap mass spectrometer, coupled with dual liquid chromatography, alternating data collection between HILIC and C18 columns. Analyses were performed with positive electrospray ionization mode, an injection volume of 10 μL, mass-to-charge ratio (m/z) scan range of 85 to 2,000, and resolution of 60,000 (FWHM). Serum samples were randomized and run in batches of 20, with pooled reference plasma (Q-Standard) samples analyzed prior to and following each batch to enable quality control and metabolite quantification, as described previously (Go, Walker, et al., 2015). Data extraction was performed using apLCMS (Yu, Park, Johnson, & Jones, 2009) and xMSanalyzer (Uppal et al., 2013). Amniotic fluid samples were processed and analyzed separately from serum samples but under parallel protocols. We performed principal component analysis (PCA) to evaluate potential batch effects (Yang et al., 2008) and corrected for these effects, where necessary, using ComBat (Johnson, Li, & Rabinovic, 2007) and xMSanalyzer.
The resulting data matrices contained individual features defined by accurate mass m/z, retention time (RT), and ion intensities. We averaged the non-zero intensities of technical replicates and performed log2 transformation. Confirmed metabolite identities were based on accurate mass m/z, coelution with authentic standards, and MS/MS criteria (Go, Liang, et al., 2015; Go, Walker, et al., 2015; Jones et al., 2016). To compare the global metabolic profiles of serum and amniotic fluid, we generated additional tentative matches for all m/z features to metabolites in the Kyoto Encyclopedia for Genes and Genomes (KEGG) database (Kanehisa & Goto, 2000) using xMSannotator (Uppal, Walker, & Jones, 2016), restricting matches to adducts M+H and M+Na with mass error within ±10 ppm. We identified overlapping metabolites across data sets from the same column using m/z (±10 ppm) and retention time (±30 seconds) using xMSanalyzer.
2.3 Quantification of maternal serum cotinine levels and sample pair categorization
The LC-MS peak of cotinine (m/z = 177.1014, RT = 174 seconds) was confirmed by comparing m/z and retention time against the cotinine chemical standard run on the C18 column with MS/MS confirmation (Jones et al., 2016). The corresponding peak from the HILIC column was identified by matched m/z and correlation of intensities with C18 analyses. The raw intensities of cotinine separated by the C18 and HILIC columns were highly correlated within individual samples (Pearson’s r = 0.86, maternal serum; Pearson’s r = 0.90, amniotic fluid). The absolute concentration of cotinine in each sample was calculated by reference standardization (Go, Walker, et al., 2015) using the concentration (0.0197 μM) in the Q-Standard pooled reference plasma, which was run together with each batch of samples in this study, as previously described (Go, Walker, et al., 2015). A two-sided t-test showed that the calculated levels of cotinine in the maternal serum samples did not differ significantly between the C18 and HILIC-derived data sets (p = 0.19).
Subjects were classified according to serum cotinine thresholds of 2 ng/mL, indicative of low-level exposure from light smoking or secondhand smoke, and 10 ng/mL, indicative of smoking (Hanke, Sobala, & Kalinka, 2004; Jones et al., 2016; Misra & Nguyen, 1999). Fifteen samples with cotinine levels close to the defined cutoffs that fell into different exposure groups between chromatography columns were excluded from the primary analysis. These samples were, however, included in an additional sensitivity analysis where serum cotinine levels of all 81 samples derived from the HILIC column were not dichotomized and instead were treated as a continuous variable.
2.4 Statistical analyses
We summarized the clinical and demographic characteristics of our study volunteers using descriptive statistics and compared these between cotinine exposure groups (classified according to measured maternal serum cotinine as explained above) using the non-parametric two-sided Fisher’s exact test for categorical variables, including maternal race/ethnicity, fetal gender, and collection year (2004–2008–2009–2014). For continuous variables, including maternal age and gestational age, we used the two-sided Mann-Whitney U-test.
We filtered LC-MS features from both the C18 and HILIC columns to retain only those with at least 80% non-zero values across samples of one or more cotinine groups. We obtained covariate-adjusted intensities for each of our four data sets (serum [C18 and HILIC columns], amniotic fluid [C18 and HILIC columns]) using residuals from linear regression against relevant potential confounders identified by the two-sided tests described above.
We performed initial PCA on the preprocessed, covariate-adjusted feature intensities to visualize the variation in feature intensity profiles across all study samples (Jolliffe, 2002). Next, we conducted feature selection using partial least squares discriminant analysis (PLSDA), a supervised, multivariate statistical technique that seeks to maximize the covariance between exposure groups and the intensity profiles of the samples (Wold, Sjöström, & Eriksson, 2001). The top features from the PLSDA model were selected by the variable importance in projection (VIP) scores (VIP ≥ 2) and further evaluated by Support Vector Machines using 10-fold cross-validation. We performed hierarchical cluster analysis (HCA) using the R computing environment (R Core Team, 2015).
2.5 Pathway analysis and annotation of significant features
Features identified from the PLSDA model, described above, as discriminating between cotinine exposure groups by a VIP score of ≥2 were selected for use in pathway enrichment analysis using Mummichog (Li et al., 2013). Pathways were considered to differ between groups for p ≤ 0.05. We further augmented Mummichog output by annotating features selected by PLSDA using xMSannotator (Uppal et al., 2016), first obtaining putative matches to known metabolites in the Human Metabolome Database (HMDB) (Wishart et al., 2007) on the basis of accurate mass m/z, with a mass error threshold of ±10 ppm (Level 5 identification using criteria of (Schymanski et al., 2014)). When searching HMDB, we considered multiple adducts (e.g., M+Na, M+H, M+H-H2O, M+ACN+H, M+2Na-H, 2M+H, M+2H, 2M+Na, M+NH4, 2M+ACN+H, M+H-2H2O, M+ACN+Na). We then used xMSannotator’s multilevel scoring algorithm to assign confidence levels to all annotations and accepted levels 2 (medium confidence) and 3 (high confidence).
3 Results
3.1 Categorization of study samples by maternal nicotine exposure level
We detected non-zero cotinine levels in all maternal serum samples (n = 81) analyzed by both C18 and HILIC chromatography and classified sample pairs according to previously defined cutoffs: high-level nicotine exposure (>10 ng/mL), low-level exposure (2–10 ng/mL), and minimal to no exposure (<2 ng/mL). For 66 samples (81.5% of our total), exposure classification was unambiguous; of these, 47 (71.2%) were categorized as minimally or not exposed (median cotinine = 0.351 ± 0.361 ng/mL [HILIC]) and 18 (27.3%) were categorized as low-level exposed (median cotinine = 4.971 ± 3.979 ng/mL). Please see Supplemental Figure 1 for a comparison of cotinine-derived exposure classifications and self-reported maternal smoking habits. Of the 47 women categorized by serum cotinine level as minimally or not exposed, we had self-reported smoking status information for 46, and all self-reported as non-smokers. Of the 18 women categorized by serum cotinine as low-level exposed, 15 self-reported as light smokers and three self-reported as non-smokers. Only one subject had a serum cotinine level consistent with smoking and was excluded from further study. Of note, this woman had self-reported as a non-smoker. The 15 samples with cotinine levels that fell at the border between groups, making classification ambiguous, were excluded from categorical analysis but used in a follow-up sensitivity analysis in which maternal serum cotinine was used as a continuous variable, as described below.
Characteristics of the remaining study population (n = 65) are summarized in Table 1 and the corresponding LC-MS peak intensity matrices are provided in Supplemental Table 1A (amniotic fluid peaks from the C18 column), Supplemental Table 1B (amniotic fluid peaks from the HILIC column), Supplemental Table 1C (maternal serum peaks from the C18 column), and Supplemental Table 1D (maternal serum peaks from the HILIC column). Of these samples, 63 were collected in the second trimester, and two were collected early in the third trimester (29.5 and 30 weeks gestation); for simplicity, conclusions are therefore expressed in the context of second trimester collection. The median length of time between serum and amniotic fluid collection for these 65 women was 16 days; however, amniocentesis preceded maternal serum collection by one week for two subjects.
Table 1.
Characteristics of study subjects categorized by maternal exposure to nicotine.
| All, n = 65 | Maternal serum cotinine levela
|
|||
|---|---|---|---|---|
| <2 ng/mL, n = 47 | 2–10 ng/mL, n = 18 | p-valueb | ||
| Maternal race/ethnicity [n (%)] | <0.001 | |||
| African American | 18 (27.7) | 17 (36.2) | 1 (5.6) | |
| Caucasian | 38 (58.5) | 21 (44.7) | 17 (94.4) | |
| Other | 9 (13.8) | 9 (19.2) | 0 (0.0) | |
| Fetal gender [n (%)] | >0.9 | |||
| Female | 41 (63.1) | 30 (63.8) | 11 (61.1) | |
| Male | 24 (36.9) | 17 (36.2) | 7 (38.9) | |
| Collection year, MS [n (%)] | >0.9 | |||
| 2004–2008 | 35 (53.8) | 25 (53.2) | 10 (55.6) | |
| 2009–2014 | 30 (46.2) | 22 (46.8) | 8 (44.4) | |
| Collection year, AF [n (%)] | >0.9 | |||
| 2004–2008 | 34 (52.3) | 25 (53.2) | 9 (50.0) | |
| 2009–2014 | 31 (47.7) | 22 (46.8) | 9 (50.0) | |
| Maternal age [years, (median ± IQR)] | 34.0 ± 12.0 | 33.0 ± 13.0 | 34.0 ± 11.3 | 0.5 |
| Gestational age, MS [weeks, (median ± IQR)] | 17.0 ± 1.9 | 16.7 ± 1.9 | 17.6 ± 2.0 | 0.3 |
| Gestational age, AF [weeks, (median ± IQR)] | 20.0 ± 3.7 | 19.3 ± 3.4 | 21.4 ± 3.7 | 0.005 |
One sample pair with measured maternal serum cotinine >10 ng/mL, indicative of high-level nicotine exposure, was excluded as an isolated case.
Two-sided Fisher’s exact test and Mann–Whitney U-test were performed for nominal and continuous variables, respectively, between cotinine exposure groups.
Abbreviations: MS, maternal serum; AF, amniotic fluid; IQR, interquartile range.
Most available subject characteristics, including fetal gender, collection year, maternal age, and gestational age at serum collection, were similar across the two exposure groups. However, maternal race/ethnicity and gestational age at amniocentesis differed significantly (p < 0.05). Specifically, all but one of the low-level exposed mothers were Caucasian, compared with less than half (44.7%) of the minimally or not exposed mothers. Further, amniotic fluid samples were collected about 2 weeks later in gestation from low-level exposed women (median = 21.4 ± 3.7 weeks) than from minimally or not exposed women (19.3 ± 3.4 weeks).
3.2 Mapping maternal serum and amniotic fluid features to KEGG metabolic pathways
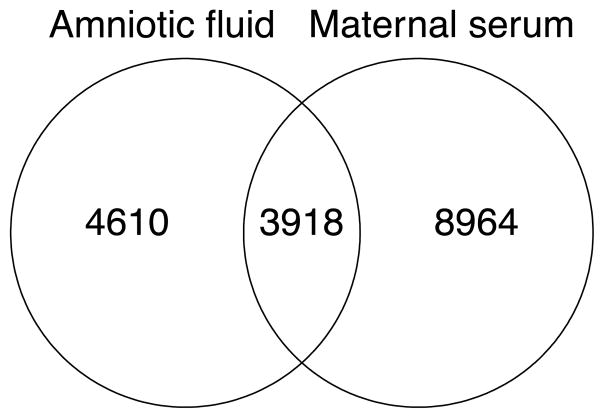
Feature matching between HILIC maternal serum and amniotic fluid showed 3,918 features common to both matrices (Fig. 1). To explore the global metabolic differences between maternal serum and amniotic fluid samples, we first mapped the 12,884 metabolite features in maternal serum and the 8,541 metabolite features in amniotic fluid detected by HILIC chromatography to known compounds and pathways in the KEGG database. In serum, we found tentative matches for 6,366 unique KEGG compounds and of these 634 compounds mapped to 188 human pathways. In amniotic fluid, we found tentative matches for 5,988 unique KEGG compounds, and of these 648 compounds mapped to 200 distinct KEGG human pathways. Among metabolic pathways, we saw greater baseline representation of matches to amino acid and carbohydrate metabolism in the amniotic fluids compared to maternal sera, while serum matches showed greater global representation of lipid metabolism (Supplemental Figure 2).
Fig. 1.
Overlap of features detected by HILIC chromatography in maternal serum and amniotic fluid samples. Matching was performed using a mass error threshold of 10 ppm and a retention time threshold of 30 seconds.
3.3 Metabolic association with low-level maternal nicotine exposure reveals dysregulated amino acid pathways in amniotic fluid
Of the features detected in amniotic fluid by C18 and HILIC chromatography, 3,097 and 6,369, respectively, were present in ≥80% of all samples from either exposure group. To identify those features contributing to differences between the minimally or not exposed (maternal serum cotinine <2 ng/mL) and low-level exposed (maternal serum cotinine 2–10 ng/mL) groups, we applied PLSDA after adjusting feature intensities for maternal race/ethnicity and gestational age. We selected the 145 C18 features and 201 HILIC features with VIP ≥ 2 and assessed 10-fold CV accuracy of these discriminatory features using balanced classification rate (BCR) due to the unbalanced group sizes. This analysis confirmed that while both sets of data effectively discriminated the classes, the PLSDA-selected HILIC features performed better (mean balanced classification rate was 88 for HILIC and 70 for C18).
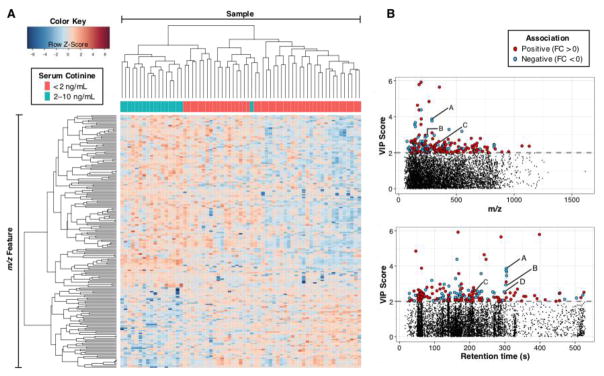
Approximately two-thirds of the 201 discriminatory HILIC features were positively associated with low-level maternal nicotine exposure (Fig. 2A), and the expression patterns of 94% of the exposed samples clustered separately from the minimally or not exposed samples. Manhattan plots (Fig. 2B) illustrated that most of the top features had m/z <500 and retention time <350 seconds. Group-wise average intensities, fold change, and VIP score of selected features are provided in Supplemental Table 2.
Fig. 2.
(A) A heat map showing two-way hierarchical cluster analysis of metabolites detected in amniotic fluid of minimally or not exposed versus low-level nicotine exposed mothers; adjusted intensities of 201 features selected by PLSDA from amniotic fluid (HILIC column) are presented. Clustering of features is shown on the left axis and clustering of samples is shown on the top axis. (B) Manhattan plot of corresponding VIP scores as a function of m/z (upper panel) and retention time (lower panel). Dashed line in each panel indicates selection cutoff (VIP ≥ 2). Features positively associated with low-level maternal exposure are shown in red; features negatively associated are shown in blue. Select metabolites involved in aspartate and asparagine metabolism are annotated as follows: A, N1,N12-diacetylspermine; B, N1-acetylspermine; C, adenosine monophosphate; D, acetylspermidine. Abbreviations: FC, fold change.
We used Mummichog (Li et al., 2013) to map the discriminatory features in amniotic fluid to metabolic pathways. The resulting set of dysregulated pathways (Table 2) showed considerable overlap with results from a prior study that applied metabolomic analysis to adult serum samples from members of the US armed services obtained from the Department of Defense Serum Repository (DoDSR) (Jones et al., 2016). Of note, five of the top nine pathways altered in sera from military personnel categorized as smokers (serum cotinine ≥ 10 ng/mL) versus light or non-smokers (serum cotinine <10 ng/mL) were also disrupted in the amniotic fluid samples from women showing low-level exposure to nicotine (serum cotinine 2–10 ng/mL). These included aspartate and asparagine metabolism, pyrimidine metabolism, urea/amino group metabolism, arginine and proline metabolism, and xenobiotic metabolism. Interestingly, most pathways overlapping with the DoDSR study seen in our amniotic fluid samples were not also seen in our maternal serum samples (Table 2), confirming that the metabolomic consequences of low-level nicotine exposure were compounded in the fetal compartment.
Table 2.
Metabolic pathways identified as perturbed in amniotic fluid and maternal serum from women categorized as low-level exposed to nicotine versus minimally or not exposed. Pathways listed here all had Mummichog p-value < 0.05 and at least four overlap metabolites. Pathway size indicates the number of metabolites detected for each pathway. Overlap size indicates the number of metabolites that were found to differ significantly between low-level exposed and minimally or not exposed subjects.
| Amniotic fluid | ||||
|---|---|---|---|---|
| Pathway | Overlap size | Pathway size | p-value | Column |
|
| ||||
| Aspartate and asparagine metabolisma | 14 | 65 | 0.001 | HILIC |
| 5 | 46 | 0.009 | C18 | |
| Pyrimidine metabolisma | 8 | 41 | 0.001 | HILIC |
| Butanoate metabolism | 4 | 24 | 0.002 | HILIC |
| Lysine metabolism | 4 | 27 | 0.003 | HILIC |
| TCA cycle | 4 | 11 | 0.003 | C18 |
| Vitamin A (retinol) metabolism | 4 | 29 | 0.003 | HILIC |
| Urea cycle/amino group metabolisma | 5 | 48 | 0.005 | HILIC |
| 4 | 32 | 0.010 | C18 | |
| Arginine and proline metabolisma | 4 | 26 | 0.006 | C18 |
| Methionine and cysteine metabolism | 4 | 28 | 0.007 | C18 |
| Xenobiotics metabolisma | 4 | 40 | 0.020 | C18 |
| Tyrosine metabolism | 5 | 73 | 0.038 | HILIC |
|
| ||||
| Maternal serum | ||||
|
| ||||
| Pathway | Overlap size | Pathway size | p-value | Column |
|
| ||||
| Leukotriene metabolism | 16 | 48 | 0.001 | HILIC |
| Eicosapentaenoic acid derived metabolites | 8 | 21 | 0.002 | HILIC |
| Linoleate metabolism | 6 | 19 | 0.006 | HILIC |
| Porphyrin metabolism | 6 | 23 | 0.017 | HILIC |
| Vitamin A (retinol) metabolism | 8 | 35 | 0.027 | HILIC |
| Vitamin E metabolism | 5 | 32 | 0.027 | C18 |
Pathways were significantly enriched in Jones et al. (2016) analysis comparing serum metabolomes of actively smoking and light- or non-smoking military personnel.
As a follow-up sensitivity analysis, we performed PLS regression using the original set of all 81 amniotic fluid samples with maternal serum cotinine concentration included as a continuous variable, as described above. Metabolic pathways identified as significantly perturbed in the amniotic fluid in association with higher maternal serum cotinine levels from this analysis (Supplemental Table 3) were consistent with those presented in Table 2.
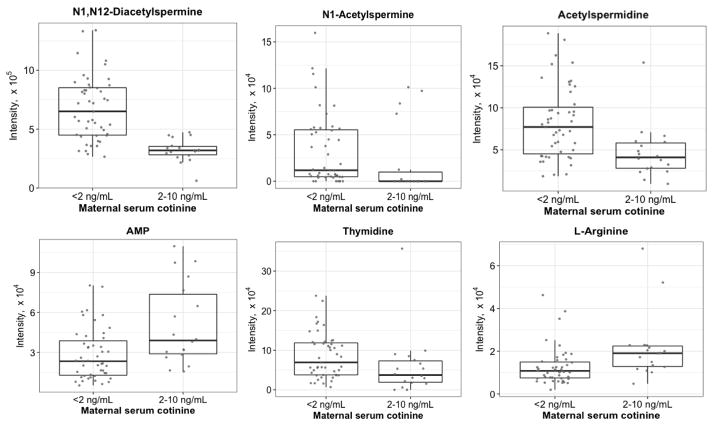
Within the pathway identified as most significantly perturbed in amniotic fluid in association with low-level maternal nicotine exposure -- aspartate and asparagine metabolism -- we observed decreased N1,N12-diacetylspermine, acetylspermidine, and N1-acetylspermine, while other metabolites such as adenosine monophosphate (AMP) were increased. Other representatives of nucleic acid metabolism (matches to thymidine and cytosine) were decreased in amniotic fluid in association with low-level maternal nicotine exposure, while a feature matching cytidine triphosphate was increased. Among differentially expressed confirmed amino acids, we observed decreased proline and increased arginine in association with low-level nicotine exposure, and all m/z matches to pathways of xenobiotic metabolism showed increased intensity with exposure. Box plots of representative metabolites from dysregulated pathways are shown in Fig. 3.
Fig. 3.
Box plots of representative amniotic fluid metabolites with differential abundance between maternal cotinine exposure groups. All metabolites were identified either by confirmed m/z and retention time or by xMSannotator confidence ≥2. Abbreviations: AMP, adenosine monophosphate.
3.4 Low-level nicotine exposure associates with inflammatory pathways in maternal serum
To assess the impact of low-level nicotine exposure on the maternal serum metabolome, we analyzed the 6,010 features from the C18 column and 9,596 features from the HILIC column detected with 80% minimum presence in maternal serum samples of either exposure group. We performed PLSDA after first adjusting feature intensities for maternal race/ethnicity, resulting in 227 features from C18 and 406 features from HILIC with VIP ≥ 2 (Supplemental Table 2). Expression patterns of these features within both exposure groups were variable, and in sharp contrast to the amniotic fluid samples, hierarchical cluster analysis did not achieve clear separation between the two exposure groups (Supplemental Figure 3 and Supplemental Figure 4).
We again used Mummichog (Li et al., 2013) to investigate which, if any, metabolic pathways were perturbed in our cohort of low-level nicotine exposed mothers relative to non- or minimally exposed mothers. This analysis demonstrated that leukotriene metabolism is the most affected pathway, with 16 of the 48 mapped features having VIP ≥ 2. All top pathways in maternal serum (bottom panel of Table 2) including leukotrienes, linoleates and eicosapentaenoic acid derived metabolites, are involved in inflammation. Recent literature also increasingly suggests the involvement of porphyrin/heme metabolism, retinol and vitamin E metabolism in the immune system (Erkelens & Mebius, 2017; Soares & Hamza, 2016). These data confirm that the mothers exposed to low-level nicotine showed increased inflammatory activities.
4 Discussion
The growth of exposomics in recent years has generated tremendous momentum in the identification and analysis of both rare and common environmental exposures. In this study, we applied the power of high-resolution metabolomics to explore the metabolic impact of one of the best-studied environmental exposures, nicotine, on one of the most vulnerable populations, the developing human fetus (Barr, Bishop, & Needham, 2007). We found that maternal serum cotinine levels consistent with low-level nicotine exposure associate with significant metabolic perturbations in amniotic fluid. Elucidating the metabolomic consequence of low-level maternal nicotine exposure on amniotic fluid marks an important step toward uncovering the mechanism of impact of light maternal smoking or secondhand smoke exposure on fetal development and birth outcomes.
Our pathway analysis of amniotic fluid samples showed dysregulated metabolism of both amino and nucleic acids in association with low-level maternal nicotine exposure, with the most affected pathways involving aspartate, asparagine, and pyrimidines. These pathways were also identified as significant when we conducted a separate PLS analysis of our full set of 81 amniotic fluid samples using maternal serum cotinine level as a continuous response variable (Supplemental Table 3) rather than as a categorical exposure variable.
These findings are markedly similar to results from a recent study that explored the impact of smoking, defined by serum cotinine concentrations of >10 ng/mL, on metabolomic profiles in de-identified serum samples from the DoDSR (Jones et al., 2016). Specifically, five of nine metabolic pathways found to be dysregulated in the sera of smokers from the DoDSR (Jones et al., 2016) were also perturbed in amniotic fluid samples from pregnant women showing only low-level nicotine exposure. Given that these women all had <10 ng/mL cotinine in their serum it is not surprising that we did not see comparable overlap of dysregulated pathways in the maternal serum samples. Gender composition of the sample cohorts might also have played a role here; all of the serum samples in our study were collected from women, but 81% of the DoDSR samples were from men.
Our findings described here extend from prior reports that concentrations of nicotine metabolites in amniotic fluid exceed those found in the corresponding maternal serum (Ruhle, Graf von Ballestrem, Pult, & Gnirs, 1995). Specifically, we demonstrate that the metabolic consequences of exposure are also compounded in the fetal compartment. This is a compelling result, but also complex. For example, of the overlapping pathways perturbed in our exposed amniotic fluid samples and also in the sera of DoDSR smokers (Jones et al., 2016), the individual metabolites implicating these pathways were sometimes distinct between the sample types. Further, for some metabolites such as arginine that were clearly altered in both sample types, the direction of perturbation sometimes differed. This may reflect the distinct composition of adult serum versus second trimester amniotic fluid and potentially also the distinct kinetics of some metabolic reactions in these sample types. Given that the serum and amniotic fluid samples were run in separate batches over both the C18 and HILIC columns, it is also possible that technical factors beyond our control differed between the runs. Finally, considering that the predominant pathways we found altered between exposure groups in maternal serum samples related to inflammation, that these pathways did not also show up as significantly altered in amniotic fluid may reflect the immunological privilege of the fetal compartment (Weetman, 1999). Further research will be needed to more thoroughly explore all of these points.
Although research over the past fifty years has built an undeniable case documenting that both maternal smoking and secondhand smoke exposure associate with negative birth outcomes, the biological mechanisms underlying these associations remain largely unknown. By applying the power of untargeted metabolomics we have begun to identify candidate explanations. For example, of the dysregulated metabolic pathways in our amniotic fluid samples of low-level nicotine exposed mothers, we note that at least three -- aspartate and asparagine metabolism, arginine and proline metabolism, and methionine metabolism -- impact pathways that include polyamines, a class of compounds recognized as critical in fetal development (Lefevre, Palin, & Murphy, 2011). One m/z feature in our amniotic fluid data set that matched with high confidence to N1,N12-diacetylspermine (DiAcSpm) showed strong association with low-level maternal nicotine exposure. DiAcSpm and its metabolic precursor, N1-acetylspermine, are catabolized from spermine by the regulatory enzyme spermidine/spermine N1-acetyltransferase (SSAT1) (Park & Igarashi, 2013). These acetylated spermines, along with a feature matching acetylspermidine, were significantly decreased in the amniotic fluids from women in our study cohort exposed to low-level nicotine. Altered enzymatic activity in de novo polyamine synthesis offers one potential explanation for these observations. Further, previous studies in rat have documented that inhibiting the activity of ornithine decarboxylase (ODC), which synthesizes the precursor of spermidine, results in fetal intrauterine growth restriction and decreased placental weight (Ishida, Hiramatsu, Masuyama, Mizutani, & Kudo, 2002), two phenotypes clearly reminiscent of the negative birth outcomes associated with maternal exposure to direct or secondhand smoke in humans.
While powerful, our study had a number of important limitations. These included the limited number of usable sample pairs (65) in our categorical analyses and the limited information we had available on each. Our study cohort also demonstrated differences in racial distribution between the exposure groups, such that African American women constituted 36% of the minimally or not exposed group, but only 6% of the exposed group. Supplemental Figure 5 shows the distribution of cotinine intensities across both sample types and chromatography columns by maternal race. Given that African Americans tend to metabolize and clear nicotine more slowly than Caucasians (Perez-Stable, Herrera, Jacob, & Benowitz, 1998), this is unlikely to explain the racial disparity in our cohort. To explore the possible impact of maternal race on our results, we conducted a sensitivity analysis by which feature selection and pathway analysis were repeated for amniotic fluid samples from Caucasian mothers only (n = 38); all fetal pathways listed as significantly perturbed in association with higher cotinine levels in Table 2 again achieved p<0.05 in this analysis (Supplemental Table 4), confirming the effect was not determined by race.
Another limitation of our study stems from the reality that while we could accurately quantify the level of cotinine in a woman’s serum, we could only guess at the true source of her nicotine exposure. According to prior research (Hanke, Sobala, & Kalinka, 2004; Jones et al., 2016; Misra & Nguyen, 1999), serum cotinine levels between 2–10ng/mL may be indicative of maternal exposure to secondhand smoke. In our cohort, we found that of the 18 women whose serum cotinine fell within this range, 15 self-reported as smokers and three self-reported as non-smokers (Supplemental Figure 1). These three self-reported non-smokers might have been exposed to secondhand smoke or may have encountered nicotine through an alternate route, such as a nicotine patch or nicotine chewing gum (Shipton et al., 2009). Not knowing the true source of the exposure, and having data from only a single time-point for each woman, limits our interpretation of results.
Finally, as an observational rather than interventional study, our results tested association between maternal serum cotinine level and metabolomic patterns in amniotic fluid, but we did not have the power to test causation. It is therefore possible that some exposure or factor other than nicotine, but associated with nicotine in our study cohort, caused the metabolic differences we observed. Future studies will be required to test this possibility.
Conclusions
Our results presented here reveal a complex and extensive set of compounds and metabolic pathways perturbed in second-trimester amniotic fluid in association with low-level maternal exposure to nicotine. We further note a strong overlap between the dysregulated pathways in amniotic fluid reported here, including aspartate and asparagine metabolism, pyrimidine metabolism, and urea/amino group metabolism, with pathways previously shown to be perturbed in the sera of adult smokers. The directionality of these changes suggested a pronounced decrease in specific polyamines known to play a crucial role in fetal development. Our results highlight the importance of accounting for even low maternal nicotine exposure in studies of the prenatal exposome. Our results further lay a foundation for targeted metabolomic studies of relevant pathways aimed at defining the extent to which the metabolic consequence of low-level maternal nicotine exposure in the fetal compartment mirrors that of smoking in adults.
Supplementary Material
Highlights.
low-level maternal exposure to nicotine leads to significant metabolic disturbance in amniotic fluid
amniotic fluid perturbations of low-level nicotine exposed mothers mirror changes seen in serum of actively smoking adults
perturbed pathways include asp/asn, pyrimidine, and amino group metabolism
Acknowledgments
We gratefully acknowledge the many colleagues at Emory University who provided guidance and support at different stages of this project, especially Doug Walker, Tianwei Yu, and Karan Uppal. This work was supported by a Pilot Award granted by the HERCULES Program with funding from the National Institute of Environmental Health Sciences of the National Institutes of Health (award number P30ES019776 to Gary Miller), a shared instrumentation grant NIH S10 OD18006 (to DP Jones), and the Rollins Earn and Learn (REAL) Program, Rollins School of Public Health, Emory University, Atlanta, GA.
Abbreviations
- SIDS
Sudden Infant Death Syndrome
- LC-MS
liquid chromatography-mass spectrometry
- m/z
mass/charge
- PCA
principal component analysis
- RT
retention time
- KEGG
Kyoto Encyclopedia for Genes and Genomes
- MS/MS
tandem mass spectrometry
- PLSDA
partial least squares discriminant analysis
- VIP
variable importance in projection
- HCA
hierarchical cluster analysis
- MS
maternal serum
- AF
amniotic fluid
- IQR
interquartile range
- DoDSR
Department of Defense Serum Repository
- AMP
adenosine monophosphate
- DiAcSpm
N1,N12-diacetylspermine
Footnotes
Conflicts of interest
Stephanie Sherman: none
Judith L. Fridovich-Keil: none
Taylor Fischer: none
Loukia N. Lili: none
Shuzhao Li: none
ViLinh Tran: none
Kim Stewart: none
Charles E. Schwartz: none
Dean P. Jones: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderka M, Romitti PA, Sun L, Druschel C, Carmichael S, Shaw G National Birth Defects Prevention S. Patterns of tobacco exposure before and during pregnancy. Acta Obstet Gynecol Scand. 2010;89(4):505–514. doi: 10.3109/00016341003692261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Bishop A, Needham LL. Concentrations of xenobiotic chemicals in the maternal-fetal unit. Reprod Toxicol. 2007;23(3):260–266. doi: 10.1016/j.reprotox.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Hukkanen J, Jacob P., 3rd Nicotine chemistry, metabolism, kinetics and biomarkers. Handb Exp Pharmacol. 2009;(192):29–60. doi: 10.1007/978-3-540-69248-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, St Helen G, Dempsey DA, Jacob P, 3rd, Tyndale RF. Disposition kinetics and metabolism of nicotine and cotinine in African American smokers: impact of CYP2A6 genetic variation and enzymatic activity. Pharmacogenet Genomics. 2016;26(7):340–350. doi: 10.1097/FPC.0000000000000222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6(Suppl 2):S125–140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- Crane JM, Keough M, Murphy P, Burrage L, Hutchens D. Effects of environmental tobacco smoke on perinatal outcomes: a retrospective cohort study. BJOG. 2011;118(7):865–871. doi: 10.1111/j.1471-0528.2011.02941.x. [DOI] [PubMed] [Google Scholar]
- Curtin SC, Matthews TJ. Smoking Prevalence and Cessation Before and During Pregnancy: Data From the Birth Certificate, 2014. Natl Vital Stat Rep. 2016;65(1):1–14. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26905977. [PubMed] [Google Scholar]
- Erkelens MN, Mebius RE. Retinoic Acid and Immune Homeostasis: A Balancing Act. Trends Immunol. 2017;38(3):168–180. doi: 10.1016/j.it.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Florescu A, Ferrence R, Einarson T, Selby P, Soldin O, Koren G. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: focus on developmental toxicology. Ther Drug Monit. 2009;31(1):14–30. doi: 10.1097/FTD.0b013e3181957a3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Liang Y, Uppal K, Soltow QA, Promislow DE, Wachtman LM, Jones DP. Metabolic Characterization of the Common Marmoset (Callithrix jacchus) PLoS One. 2015;10(11):e0142916. doi: 10.1371/journal.pone.0142916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go YM, Walker DI, Liang Y, Uppal K, Soltow QA, Tran V, … Jones DP. Reference Standardization for Mass Spectrometry and High-resolution Metabolomics Applications to Exposome Research. Toxicol Sci. 2015;148(2):531–543. doi: 10.1093/toxsci/kfv198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke W, Sobala W, Kalinka J. Environmental tobacco smoke exposure among pregnant women: impact on fetal biometry at 20–24 weeks of gestation and newborn child’s birth weight. Int Arch Occup Environ Health. 2004;77(1):47–52. doi: 10.1007/s00420-003-0475-0. [DOI] [PubMed] [Google Scholar]
- Ishida M, Hiramatsu Y, Masuyama H, Mizutani Y, Kudo T. Inhibition of placental ornithine decarboxylase by DL-alpha-difluoro-methyl ornithine causes fetal growth restriction in rat. Life Sci. 2002;70(12):1395–1405. doi: 10.1016/s0024-3205(01)01510-7. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11883715. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Gulbis B, Acharya G, Thiry P, Rodeck C. Maternal tobacco exposure and cotinine levels in fetal fluids in the first half of pregnancy. Obstet Gynecol. 1999;93(1):25–29. doi: 10.1016/s0029-7844(98)00318-4. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9916950. [DOI] [PubMed] [Google Scholar]
- Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8(1):118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- Jolliffe IT. Principal Component Analysis. 2. New York, NY: Springer New York; 2002. [Google Scholar]
- Jones DP, Walker DI, Uppal K, Rohrbeck P, Mallon CT, Go YM. Metabolic Pathways and Networks Associated With Tobacco Use in Military Personnel. J Occup Environ Med. 2016;58(8 Suppl 1):S111–116. doi: 10.1097/JOM.0000000000000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordanov JS. Cotinine concentrations in amniotic fluid and urine of smoking, passive smoking and non-smoking pregnant women at term and in the urine of their neonates on 1st day of life. Eur J Pediatr. 1990;149(10):734–737. doi: 10.1007/BF01959534. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2209668. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28(1):27–30. doi: 10.1093/nar/28.1.27. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10592173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre PL, Palin MF, Murphy BD. Polyamines on the reproductive landscape. Endocr Rev. 2011;32(5):694–712. doi: 10.1210/er.2011-0012. [DOI] [PubMed] [Google Scholar]
- Li S, Park Y, Duraisingham S, Strobel FH, Khan N, Soltow QA, … Pulendran B. Predicting network activity from high throughput metabolomics. PLoS Comput Biol. 2013;9(7):e1003123. doi: 10.1371/journal.pcbi.1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck W, Nau H, Hansen R, Steldinger R. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev Pharmacol Ther. 1985;8(6):384–395. doi: 10.1159/000457063. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/4075937. [DOI] [PubMed] [Google Scholar]
- Misra DP, Nguyen RH. Environmental tobacco smoke and low birth weight: a hazard in the workplace? Environ Health Perspect. 1999;107(Suppl 6):897–904. doi: 10.1289/ehp.99107s6897. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10592147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, Igarashi K. Polyamines and their metabolites as diagnostic markers of human diseases. Biomol Ther (Seoul) 2013;21(1):1–9. doi: 10.4062/biomolther.2012.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Stable EJ, Herrera B, Jacob P, 3rd, Benowitz NL. Nicotine metabolism and intake in black and white smokers. JAMA. 1998;280(2):152–156. doi: 10.1001/jama.280.2.152. [DOI] [PubMed] [Google Scholar]
- Polanska K, Hanke W, Laudanski T, Kalinka J. Serum cotinine level as a biomarker of tobacco smoke exposure during pregnancy. Ginekol Pol. 2007;78(10):796–801. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18200972. [PubMed] [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. Retrieved from https://www.R-project.org/ [Google Scholar]
- Rogers JM. Tobacco and pregnancy. Reprod Toxicol. 2009;28(2):152–160. doi: 10.1016/j.reprotox.2009.03.012. [DOI] [PubMed] [Google Scholar]
- Ruhle W, Graf von Ballestrem CL, Pult HM, Gnirs J. Correlation of cotinine level in amniotic fluid, umbilical artery blood and maternal blood. Geburtshilfe Frauenheilkd. 1995;55(3):156–159. doi: 10.1055/s-2007-1022795. [DOI] [PubMed] [Google Scholar]
- Salmasi G, Grady R, Jones J, McDonald SD Knowledge Synthesis G. Environmental tobacco smoke exposure and perinatal outcomes: a systematic review and meta-analyses. Acta Obstet Gynecol Scand. 2010;89(4):423–441. doi: 10.3109/00016340903505748. [DOI] [PubMed] [Google Scholar]
- Schymanski EL, Jeon J, Gulde R, Fenner K, Ruff M, Singer HP, Hollender J. Identifying small molecules via high resolution mass spectrometry: communicating confidence. Environ Sci Technol. 2014;48(4):2097–2098. doi: 10.1021/es5002105. [DOI] [PubMed] [Google Scholar]
- Shipton D, Tappin DM, Vadiveloo T, Crossley JA, Aitken DA, Chalmers J. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. BMJ. 2009;339:b4347. doi: 10.1136/bmj.b4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares MP, Hamza I. Macrophages and Iron Metabolism. Immunity. 2016;44(3):492–504. doi: 10.1016/j.immuni.2016.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltow QA, Strobel FH, Mansfield KG, Wachtman L, Park Y, Jones DP. High-performance metabolic profiling with dual chromatography-Fourier-transform mass spectrometry (DC-FTMS) for study of the exposome. Metabolomics. 2013;9(1 Suppl):S132–S143. doi: 10.1007/s11306-011-0332-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, GA: 2006. https://www.ncbi.nlm.nih.gov/pubmed/20669524. [Google Scholar]
- Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: not just fetal urine anymore. J Perinatol. 2005;25(5):341–348. doi: 10.1038/sj.jp.7211290. [DOI] [PubMed] [Google Scholar]
- Uppal K, Soltow QA, Strobel FH, Pittard WS, Gernert KM, Yu T, Jones DP. xMSanalyzer: automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data. BMC Bioinformatics. 2013;14:15. doi: 10.1186/1471-2105-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal K, Walker DI, Jones DP. xMSannotator: an R package for network-based annotation of high-resolution metabolomics data. Anal Chem. 2016 doi: 10.1021/acs.analchem.6b01214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weetman AP. The immunology of pregnancy. Thyroid. 1999;9(7):643–646. doi: 10.1089/thy.1999.9.643. [DOI] [PubMed] [Google Scholar]
- Wishart DS, Tzur D, Knox C, Eisner R, Guo AC, Young N, … Querengesser L. HMDB: the Human Metabolome Database. Nucleic Acids Res. 2007;35(Database issue):D521–526. doi: 10.1093/nar/gkl923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wold S, Sjöström M, Eriksson L. PLS-regression: a basic tool of chemometrics. Chemometrics and Intelligent Laboratory Systems. 2001;58(2):109–130. doi: http://dx.doi.org/10.1016/S0169-7439(01)00155-1. [Google Scholar]
- Yang H, Harrington CA, Vartanian K, Coldren CD, Hall R, Churchill GA. Randomization in laboratory procedure is key to obtaining reproducible microarray results. PLoS One. 2008;3(11):e3724. doi: 10.1371/journal.pone.0003724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Park Y, Johnson JM, Jones DP. apLCMS--adaptive processing of high-resolution LC/MS data. Bioinformatics. 2009;25(15):1930–1936. doi: 10.1093/bioinformatics/btp291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.