Abstract
Resistance to thyrotropin (RTSH) is broadly defined as reduced sensitivity of thyroid follicle cells to stimulation by biologically active TSH due to genetic defects. Affected individuals have elevated serum TSH in the absence of goiter, with the severity ranging from nongoitrous isolated hyperthyrotropinemia to severe congenital hypothyroidism with thyroid hypoplasia. Conceptually, defects leading to RTSH impair both aspects of TSH-mediated action, namely thyroid hormone synthesis and gland growth. These include inactivating mutations in the genes encoding the TSH receptor and the PAX8 transcription factor. A common third cause has been genetically mapped to a locus on chromosome 15, but the underlying pathophysiology has not yet been elucidated. This review provides a succinct overview of currently defined causes of nonsyndromic RTSH, their differential diagnoses (autoimmune; partial iodine organification defects; syndromic forms of RTSH) and implications for the clinical approach to patients with RTSH.
Keywords: Thyrotropin receptor, TSHR, paired domain, PAX8, mutations, congenital hypothyroidism, subclinical hypothyroidism, hormone resistance
1. Introduction
Thyroid-stimulating hormone (thyrotropin; TSH) is secreted by the specialized cells (thyrotrophs) residing in the anterior pituitary and acts on follicular thyroid cells via binding to its cognate receptor (TSHR) to stimulate hormone production and secretion as well as differentiation and growth of the thyroid gland. It is thereby integral part of the pituitary-thyroid feedback control of thyroid function. Resistance to TSH (RTSH) is broadly defined as reduced sensitivity of thyroid follicle cells to stimulation by biologically active TSH due to genetic defects. This definition would exclude autoimmunity with TSHR-blocking antibodies mimicking the RTSH phenotype. Affected individuals have elevated serum TSH levels with normal or low levels of thyroid hormones (triiodothyronine, T3 and thyroxine, T4) in the presence of a eutopic, hypoplastic or normal-sized thyroid glands. They are frequently identified at birth through TSH-based neonatal screening for congenital hypothyroidism (CH).
Conceptually, defects leading to RTSH impair both aspects of TSH-mediated action: thyroid hormone synthesis and thyroid gland growth, and can be envisioned to be caused by either 1) inactivating mutations in the TSHR gene, 2) reduced quantity of TSHR secondary to defects in factors controlling TSHR expression, 3) postreceptor defects in signal transduction, e.g. defect in G proteins, and 4) defects in transcriptional master regulators required for both normal differentiated function and growth of thyroid cells.
2. RTSH due to loss-of-function mutations in TSHR
2.1. TSHR physiology
The TSHR is a G-protein coupled receptor expressed at the basolateral surface of thyroid follicle cells. It consists of a classical seven transmembrane domain (TMD) connected via a linker region (hinge region) to a large extracellular domain (ECD) principally composed of a sequence of several leucine-rich repeat regions (LRR) (Fig. 1). The latter assemble into a horseshow-like structure with the beta-strands of the LRRs forming a concave surface for ligand binding. The TMD consists of alpha helical transmembrane spanning segments connected by extracellular loops in contact with the liganded ECD, and intracellular loops involved in G-protein coupling.
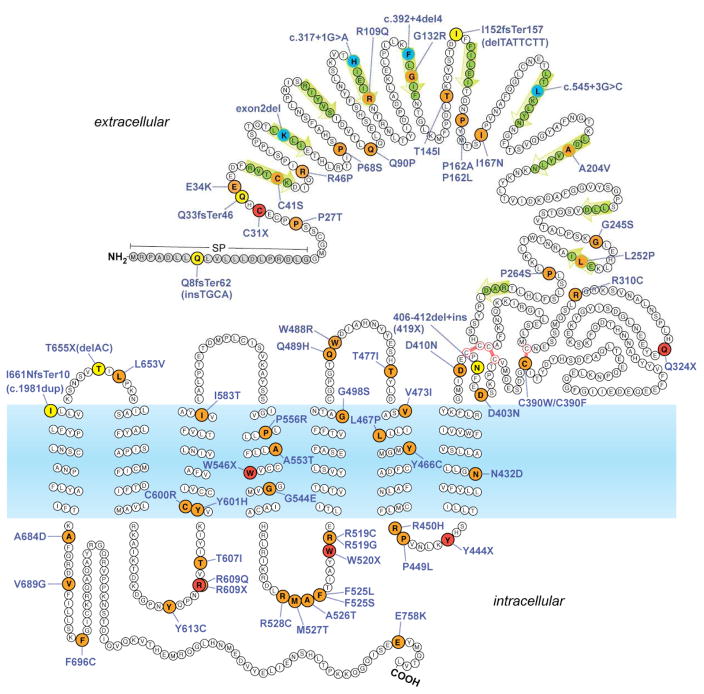
Fig. 1.
Topology model of TSHR with location of confirmed or putative inactivating mutations identified in subjects with RTSH. Missense mutations are indicated in orange, nonsense mutations in red, insertions/deletions in the coding sequence in yellow, and intronic mutations in blue. Residues forming the beta strands of the leucine-rich repeats within the extracellular ligand-binding domain are marked by green arrows. SP, signal peptide sequence (removed in mature protein).
Activation by TSH binding generates a complex structural rearrangement transmitted to the intracellular G-protein binding surface formed by TMD and intracellular loops (reviewed in [1]). TSHR can signal through both Gs and Gq G-proteins. Thus, in terms of second messenger, binding of TSH activates both the cAMP pathway (via Gsα) as wells as the phosphoinositol/calcium (IP/Ca2+; via Gq) signaling cascades. While the former is linked to iodide uptake, thyroid hormone secretion, and gland growth and differentiation, the IP/Ca2+ pathway is rate-limiting for hormone synthesis by stimulating iodide organification.
Another feature relevant for TSHR physiology and the manifestation of TSHR defects is the propensity of TSHR to form dimers and/or oligomers at the surface of thyroid cells. This phenomenon provides an explanation for the interference observed with some mutant receptors when coexpressed with the wild type and should be relevant for the observed dominant transmission of some heterozygous TSHR defects [2].
2.2. Inactivating mutations of TSHR
First described in 1995 [3], at least 68 distinct TSHR loss-of-function (LOF) mutations have now been reported in patients with RTSH phenotype (Fig. 1). Except for rare deletions [4–6], the described mutations have been either point or small indel mutations in the coding sequence causing amino acid replacement (missense) or truncation (nonsense or frameshift) of the predicted protein [7–54]. TSHR LOF mutations are found throughout the receptor structure, in contrast to the gain-of-function mutations causing hyperthyroidism, which are located primarily in the TMD of the receptor. Decreased action of TSH results in reduced T4 and T3 synthesis and secretion, with compensatory increase in TSH secretion. The absence of goiter despite high serum level of biologically active TSH is compatible with the dominant role of TSHR-induced cAMP signaling on the growth of the thyroid gland. Although the majority of TSHR LOF mutations impair overall receptor expression level and/or ligand binding, some mutations have differential effects on the coupling of either Gs or Gq proteins. In a small number of patients, Gq-dominant mutations have been linked to an RTSH phenotype with paradoxically increased thyroidal iodine uptake, a feature associated with impaired iodine organification (“nonclasscial RTSH”) [16, 55]. Since TSHR LOF mutations have to date been rarely evaluated for both Gs and Gq coupling, it remains an open question whether there are clear clinical correlates to mutations with differential effects on dual G protein coupling.
The magnitude of functional impairment of TSHR correlates to some degree with the severity of the RTSH phenotype: complete loss of TSHR function due to biallelic complete LOF mutations produces severe CH [7]. In these cases, severe hypoplasia with absent radiotracer uptake can be mistaken for athyreosis, but serum thyroglobulin (TG) is always detectable (“apparent athyreosis”). Biallelic defects (compound heterozygous or homozygous) with residual receptor function allow for either partial compensation (mild hypothyroidism) or full compensation (isolated hyperthyrotropinemia, approximately one third of cases) by high serum TSH. The inheritance of RTSH due to TSHR defects is typically considered recessive, since monoallelic TSHR defects are not regularly detected in neonatal screening using TSH cut-off value >20 uU/ml [10, 52]. Heterozygous TSHR mutations do, however, play a more prominent role in the pathogenesis of isolated non-autoimmune hyperthyrotropinemia (NAHT) diagnosed after the neonatal period. For instance, in the largest cohort of pediatric NAHT patients studied so far [10], about 12% of the patients carried potentially pathogenic heterozygous mutations (compared to a estimated frequency of <1% of heterozygous mutation carriers in the general population) [33, 52].
The mutational spectrum of TSHR mutations differs among different populations, in part due to the frequency of population-specific founder mutations. It is thus not surprising that the reported overall prevalence of TSHR mutations in patients with non-autoimmune hyperthyrotropinema varies widely between studies of different populations. In various East-Asian cohorts with nonsyndromic congenital hyperthyrotropinemia (hypothyroidism), between 4.2% and 9.4% harbored mono- or biallelic TSHR mutations. About 75% of which were of the R450H variant that is found at a prevalence of about 0.5% in the corresponding general populations [33, 34, 46, 49]. TSHR LOF mutations are the most common cause of non-goitrous CH in consanguineous families [28] and specific founder mutations have been found in over half of patients with subclinical hypothyroidism in a consanguineous Arab-Muslim population [31]. Genetic analysis of the TSHR gene should therefore especially be considered if there is parental consanguinity or a family history suggestive of autosomal recessive inheritance of the RTSH phenotype.
3. RTSH due to loss-of-function mutations in PAX8
3.1. PAX physiology
PAX8 is a member of the paired box domain containing transcription factors that plays an essential role in the morphogenesis of the thyroid gland, the maintenance of a thyroid-differentiated phenotype [56], and the survival of differentiated thyroid follicle cells [57, 58]. PAX8, together with the homeobox protein NKX2-1, is the earliest marker of thyroid cell specification in the median thyroid anlage of both human and mice. The essential role of PAX8 for thyroid development was first shown in Pax8 knockout mice, in which the thyroid is hypoplastic with residual tissue only containing C cells derived from the lateral thyroid anlage [59]. In synergy with NKX2-1, PAX8 expression promotes the differentiation of functional thyroid tissue from embryonic stem cells and with the aid of TSH regulates expression of terminal differentiation markers, including thyroglobulin (TG), thyroid peroxidase (TPO), and the sodium-iodide symporter (SLC5A5; NIS) producing a fully functional thyroid gland synthesizing T4 [60].
3.2. PAX8 mutations in RTSH
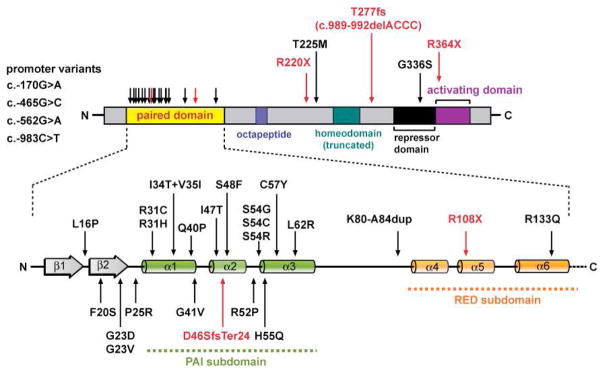
Although initially associated with thyroid dysgenesis [61], PAX8 mutations are not a relevant cause of sporadic thyroid ectopy or genuine agenesis [62–64] but found in a minority of cases (e.g. 1/28 German, 1/16 Chinese) within the normotopic hypoplasia subgroup [65–68]. More generally, heterozygous PAX8 LOF mutations have to be considered as another cause of RTSH that is clinically and by thyroid function tests indistinguishable from that caused by TSHR mutations. The clinical severity can thus range from subclinical hypothyroidism with normal-sized gland to overt hypothyroidism with severe thyroid gland hypoplasia. The most common mechanism involves mutations in the paired box domain disrupting binding to target sites, thereby leading to reduced expression of target genes (Fig. 2). The presence of RTSH-associated PAX8 promoter variants [69–71], the observation of a frameshift mutation with demonstrated protein instability [72], and the autoregulation of PAX8 by binding to its own promoter [73] are also consistent with a haploinsufficiency mechanism. A noteworthy mutational hotspot is the CpG dinucleotide at codon 31, for which frequent mutational events (R31H and R31C) have been reported [61, 65, 67, 74–77]. For some of the reported mutations, the primary defect is the impaired synergism with other thyroid transcription factors (NKX2-1) or insufficient recruitment of coactivators (p300) without altering DNA binding [78–80].
Fig. 2.
PAX8 gene mutations identified in patients with RTSH phenotype. The PAX8 structure comprises an N-terminal paired box (prd) DNA-binding domain and C-terminal region crucial for transactivation activity. Colored boxes indicate the relative positions of prd domain, conserved octapeptide sequence, (partial) homeodomain-homolog region, and of regions containing repressor or activator activity [108]. The expanded view of the prd domain reveals two subdomains (PAI and RED), each defined by trihelical helix-turn-helix motifs with independent DNA-binding activities. Missense mutations within the PAI subdomain interfering with the DNA-binding induced-fit of the helix-turn-helix motif are the most common mutational events in PAX8-associated RTSH.
Inheritance of PAX8 linked RTSH follows an autosomal dominant segregation pattern [81], but often shows highly variable expressivity within affected members of the same family [82]. Thus, there is no clear correlation between the activity of mutant PAX8 proteins in vitro and the severity of RTSH in patients. In addition, incomplete penetrance [83], parental mosaicism [84], and late-onset of RTSH phenotype due to insufficient postnatal thyroid growth [64, 77, 85, 86] have been shown to potentially mask the inherited nature of the condition.
PAX8 is also expressed during mammalian kidney development and, at least in mice, plays a redundant role with PAX2 in formation of the initial pronephros [87]. Thus, kidney organogenesis in Pax8 mutant mice is generally normal [59]. Yet, several human carriers of PAX8 gene mutation were reported to have associated kidney and urogenital abnormalities [28, 77, 85, 88]. It is tempting to speculate that the PAX8 mutations may have contributed to these non-thyroidal developmental defects.
4. RTSH linked to a defect on the long arm of chromosome 15
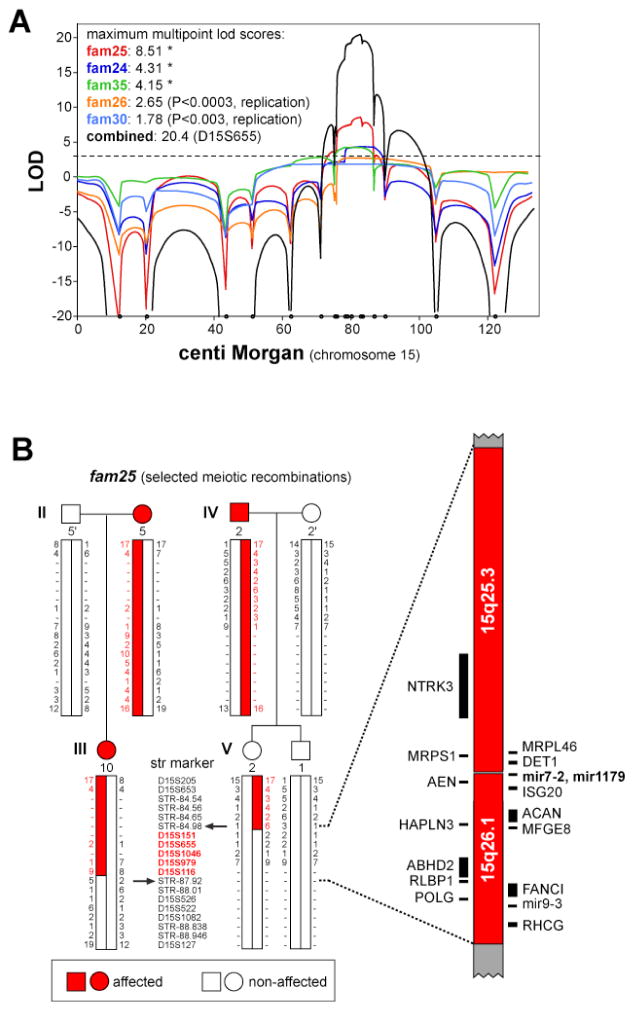
Mutations in TSHR or PAX8 have only been found in a relatively small proportion of screened patients with RTSH phenotype suggesting that additional etiologies remain to be discovered. With expected locus heterogeneity in RTSH, one approach is to focus on large RTSH kindreds with sufficient statistical power for genome-wide linkage scans. Among six multigenerational families, in which non-syndromic RTSH segregated in autosomal dominant fashion with high penetrance, yet variable expressivity, only one harbored a mutation in the PAX8 candidate gene [78, 89]. In the remaining five families, the defect was mapped to a single, 2.9 Megabase interval on chromosome 15q25.3–26.1 (combined LOD score of 14.6) [90] (Fig. 3). Since there were no genealogical links or evidence for shared ancestral haplotypes, genetic defects in this locus are expected to be a rather prevalent event in RTSH. While none of the protein-coding genes in this interval appeared to be a plausible candidate gene, recent genome-wide association studies have found a significant association between common single nucleotide polymorphisms in the center of the linked region containing a micro-RNA cluster and TSH serum level in the general population [91, 92]. Thus, elucidating the precise genetic cause for this form of RTSH may shed light on a novel thyroid-specific expressed modulator of TSH-responsiveness.
Fig. 3.
Autosomal-dominant RTSH linked to a locus on the long arm of chromosome 15. A) Multipoint genetic linkage analysis of chromosome 15 in five extended families with dominant inherited RTSH. The analysis shown includes data from additional family members not available in the original publication [90]. B) Example of fine mapping of the critical recombinants in family 25 using short tandem repeat markers. The RTSH-associated haplotype can be narrowed to a 2.9 megabase interval containing sixteen positional candidate genes. Note that common single nucleotide polymorphisms within the central micro RNA cluster (mir7-2, mir1179) have recently been found to be significantly associated with serum TSH level in the general population [91, 92]. Adapted from ref. [90] with permission.
5. RTSH as part of complex syndromes
Abnormal thyroid function consistent with RTSH is also found as a feature of complex syndromes that obligatorily involve other organs. In these patients, the non-thyroidal abnormalities dominate the clinical presentation and the underlying genetic defects should not be considered candidate genes for patients with isolated RTSH phenotype.
5.1. RTSH caused by mutations in GNAS1 (Albright hereditary osteodystrophy)
Heterozygous germline mutations in the gene encoding the alpha subunit of G stimulatory protein (Gsα, GNAS1) cause hypocalcemia and hyperphosphatemia due to impaired signaling transduction from the parathormone receptor (pseudohypoparathyroidism, PHP Ia) [93]. Haploinsufficiency for GNAS1 also explains the resistance to other hormones, specifically gonadotropins and TSH. Clinically this syndrome is referred to as Albright hereditary osteodystrophy characterized by typical physical features (short stature, short neck, round face, obesity, brachymetacarpy, subcutaneous ossification) and mental retardation.
5.2. RTSH caused by mutations in NKX2-1
NKX2-1 (also known as thyroid transcription factor 1, TITF1) is a homeobox transcription factor critical for the development of thyroid gland, basal ganglia and lung parenchyma. It is involved in maintaining the expression of thyroid-specific genes (TPO, TG, TSHR) in apparent synergism with PAX8. Haploinsufficiency for NKX2-1, due to either chromosomal deletions encompassing the gene locus [94] or deleterious gene mutations ([95], [96], and recently reviewed in ref. [97]), produces a “brain-thyroid-lung” syndrome. The severity of the individual components of the syndrome is very variable, and includes: 1) RTSH (70% of patients), 2) “benign hereditary chorea” (90% of patients) manifesting as neonatal hypotonia preceding the development of juvenile choreoathetosis and ataxia, 3) respiratory distress (55% of patients) due to lung hypoplasia causing significantly increased mortality. Inheritance of the defect is autosomal dominant with variable penetrance, however, most of the reported mutations have apparently arisen de novo. The RTSH phenotype, if present, is in the majority of cases compensated (i.e., isolated hyperthyrotropinema) [98].
6. Organification defects presenting with hallmarks of RTSH
Defects in thyroid hormonogenesis due to impairment of the enzymatic machinery in iodine organification are classically associated with thyroid gland enlargement. However, in partial defects of iodine organification, goiter is frequently absent despite elevated serum TSH [99–102]. These patients thus present with the hallmarks of mild RTSH (elevated TSH, low or normal T4, normal-sized gland). The common genetic defects in these patients are in DUOX2 and DUOXA2, which encode the heterodimeric dual oxidase enzyme complex that is rate limiting in the iodine organification [103]. For instance, only one out of twelve Korean CH patients with DUOX2 or DUOXA2 mutations was noted to have thyroid gland enlargement [101]. In contrast to genuine RTSH, whose postnatal course is either stable (TSHR, Chr15-associated) or tends to be progressive (PAX8) due to insufficient thyroid growth, partial defects in the DUOX2 system are often self-limiting only manifesting during the newborn period (transient CH) [100].
In this context the recent report on SLC26A4 (Pendrin) mutations in two patients with apparent RTSH and thyroid gland hypoplasia is noteworthy [104]. Biallelic SLC26A4 mutations are a cause of sensorineural hearing loss with bilateral enlargement of the vestibular aqueduct in combination with goiter and/or CH (Pendred syndrome). In the follicular thyroid cells, SLC26A4 is localized to the apical membrane and mediates the iodine efflux into the follicular lumen where organification takes place. It is believed that reduced thyroid gland size in these patients is a consequence of severe iodide deficiency within the follicular lumen concomitant with upregulation of the H2O2-generating enzymes leading to oxidative stress and secondary epithelial atrophy [105].
7. Recommendations for treatment and genetic screening
Individuals with uncompensated RTSH should be treated with levothyroxine (L-T4), like any other patient with primary hypothyroidism. Since these subjects have normal responsiveness to thyroid hormone, the goal is to normalize their serum TSH concentration. Immediate initiation of replacement therapy with L-T4 is crucial in all infants diagnosed with CH by neonatal screening, if the elevated blood TSH is confirmed on a serum sample on day 3 or 6 of life and is accompanied by low T4.
In individuals with compensated RTSH (euthyroid hyperthyrotropinema), longitudinal studies of individuals with TSHR LOF mutation or with RTSH linked to Chr15 indicate that the elevated TSH concentrations stimulate an adequate production of thyroid hormones and L-T4 therapy should thus be dispensable [10, 42, 89, 106, 107]. In fact, compared to a patient cohort receiving L-T4 supplementation, untreated patients with compensated RTSH had no obvious signs of growth or neurological abnormalities [10]. There was also no evidence for tissue hypothyroidism or the development of pituitary hyperplasia as a consequence of chronic thyrotroph hyperstimulation [10].
In patients with TSHR LOF mutations and those in whom the defect has been linked to the chromosome 15q locus, the RTSH phenotype appears to be stable over time. In contrast, mutations in PAX8 have been repeatedly reported to manifest RTSH that progresses during postnatal growth of the thyroid gland indicating that the defect in growth and/or survival of follicular thyroid cells cannot be permanently compensated [64, 77, 85, 86]. Genetic analysis may therefore provide a diagnostic tool to guide therapy and follow-up of RTSH patients.
PRACTICE POINTS.
RTSH should be considered in the differential diagnosis of all patients with non-autoimmune, nongoitrous hyperthyrotropinemia with or without low serum iodothyronines or clinical stigmata of hypothyroidism
Genetic analysis has the potential to provide a definitive diagnosis with relevance for prognosis, follow-up and genetic counseling.
Standard L-T4 replacement aiming to normalize serum TSH level is required in all hypothyroid patients, but the need of therapy is questionable in individuals with fully compensated RTSH and isolated hyperthyrotropinemia.
RESEARCH AGENDA.
Elucidating the precise genetic cause for RTSH linked to the Chr15q locus may shed light on a novel modulator of TSH-responsiveness in health and disease.
The role of Gq signaling in TSHR LOF mutations and its potential relevance for distinct clinical RTSH subtypes requires more systematic investigation.
Acknowledgments
This work was supported in part by Grants R37DK15070 from the National Institutes of Health USA and the Seymour J. Abrams fund for thyroid research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes And Digestive And Kidney Diseases or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- *1.Kleinau G, Neumann S, Gruters A, Krude H, Biebermann H. Novel insights on thyroid-stimulating hormone receptor signal transduction. Endocr Rev. 2013;34:691–724. doi: 10.1210/er.2012-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *2.Persani L, Calebiro D, Bonomi M. Technology insight: Modern methods to monitor protein-protein interactions reveal functional tsh receptor oligomerization. Nat Clin Pract Endocrinol Metab. 2007;3:180–90. doi: 10.1038/ncpendmet0401. [DOI] [PubMed] [Google Scholar]
- 3.Sunthornthepvarakul T, Gottschalk ME, Hayashi Y, Refetoff S. Brief report - resistance to thyrotropin caused by mutations in the thyrotropin-receptor gene. New Engl J Med. 1995;332:155–60. doi: 10.1056/NEJM199501193320305. [DOI] [PubMed] [Google Scholar]
- 4.Cangul H, Morgan NV, Forman JR, Saglam H, Aycan Z, Yakut T, et al. Novel tshr mutations in consanguineous families with congenital nongoitrous hypothyroidism. Clin Endocrinol. 2010;73:671–7. doi: 10.1111/j.1365-2265.2010.03849.x. [DOI] [PubMed] [Google Scholar]
- 5.Cangul H, Schoenmakers NA, Saglam H, Doganlar D, Saglam Y, Eren E, et al. A deletion including exon 2 of the tshr gene is associated with thyroid dysgenesis and severe congenital hypothyroidism. J Pediatr Endocr Met. 2014;27:731–5. doi: 10.1515/jpem-2014-0011. [DOI] [PubMed] [Google Scholar]
- 6.Kumorowicz-Czoch M, Madetko-Talowska A, Tylek-Lemanska D, Pietrzyk JJ, Starzyk J. Identification of deletions in children with congenital hypothyroidism and thyroid dysgenesis with the use of multiplex ligation-dependent probe amplification. J Pediatr Endocr Met. 2015;28:171–6. doi: 10.1515/jpem-2014-0040. [DOI] [PubMed] [Google Scholar]
- 7.Abramowicz MJ, Duprez L, Parma J, Vassart G, Heinrichs C. Familial congenital hypothyroidism due to inactivating mutation of the thyrotropin receptor causing profound hypoplasia of the thyroid gland. J Clin Invest. 1997;99:3018–24. doi: 10.1172/JCI119497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biebermann H, Schoneberg T, Krude H, Schultz G, Gudermann T, Gruters A. Mutations of the human thyrotropin receptor gene causing thyroid hypoplasia and persistent congenital hypothyroidism. J Clin Endocr Metab. 1997;82:3471–80. doi: 10.1210/jcem.82.10.4286. [DOI] [PubMed] [Google Scholar]
- *9.Tonacchera M, Di Cosmo C, De Marco G, Agretti P, Banco M, Perri A, et al. Identification of tsh receptor mutations in three families with resistance to tsh. Clin Endocrinol. 2007;67:712–8. doi: 10.1111/j.1365-2265.2007.02950.x. [DOI] [PubMed] [Google Scholar]
- 10.Calebiro D, Gelmini G, Cordella D, Bonomi M, Winkler F, Biebermann H, et al. Frequent tsh receptor genetic alterations with variable signaling impairment in a large series of children with nonautoimmune isolated hyperthyrotropinemia. J Clin Endocr Metab. 2012;97:E156–E60. doi: 10.1210/jc.2011-1938. [DOI] [PubMed] [Google Scholar]
- 11.Tonacchera M, Perri A, De Marco G, Agretti P, Banco ME, Di Cosmo C, et al. Low prevalence of thyrotropin receptor mutations in a large series of subjects with sporadic and familial nonautoimmune subclinical hypothyroidism. J Clin Endocr Metab. 2004;89:5787–93. doi: 10.1210/jc.2004-1243. [DOI] [PubMed] [Google Scholar]
- 12.Tonacchera M, Agretti P, De Marco G, Perri A, Pinchera A, Vitti P, et al. Thyroid resistance to tsh complicated by autoimmune thyroiditis. J Clin Endocr Metab. 2001;86:4543–6. doi: 10.1210/jcem.86.9.7791. [DOI] [PubMed] [Google Scholar]
- 13.Camilot M, Teofoli F, Gandini A, Franceschi R, Rapa A, Corrias A, et al. Thyrotropin receptor gene mutations and tsh resistance: Variable expressivity in the heterozygotes. Clin Endocrinol. 2005;63:146–51. doi: 10.1111/j.1365-2265.2005.02314.x. [DOI] [PubMed] [Google Scholar]
- 14.Rapa A, Monzani A, Moia S, Vivenza D, Bellone S, Petri A, et al. Subclinical hypothyroidism in children and adolescents: A wide range of clinical, biochemical, and genetic factors involved. J Clin Endocr Metab. 2009;94:2414–20. doi: 10.1210/jc.2009-0375. [DOI] [PubMed] [Google Scholar]
- 15.Nicoletti A, Bal M, De Marco G, Baldazzi L, Agretti P, Menabo S, et al. Thyrotropin-stimulating hormone receptor gene analysis in pediatric patients with non-autoimmune subclinical hypothyroidism. J Clin Endocr Metab. 2009;94:4187–94. doi: 10.1210/jc.2009-0618. [DOI] [PubMed] [Google Scholar]
- 16.Narumi S, Nagasaki K, Ishii T, Muroya K, Asakura Y, Adachi M, et al. Nonclassic tsh resistance: Tshr mutation carriers with discrepantly high thyroidal iodine uptake. J Clin Endocr Metab. 2011;96:E1340–E5. doi: 10.1210/jc.2011-0070. [DOI] [PubMed] [Google Scholar]
- 17.Gagne N, Parma J, Deal C, Vassart G, Van Vliet G. Apparent congenital athyreosis contrasting with normal plasma thyroglobulin levels and associated with inactivating mutations in the thyrotropin receptor gene: Are athyreosis and ectopic thyroid distinct entities? J Clin Endocr Metab. 1998;83:1771–5. doi: 10.1210/jcem.83.5.4771. [DOI] [PubMed] [Google Scholar]
- 18.Alberti L, Proverbio MC, Costagliola S, Romoli R, Boldrighini B, Vigone MC, et al. Germline mutations of tsh receptor gene as cause of nonautoimmune subclinical hypothyroidism. J Clin Endocr Metab. 2002;87:2549–55. doi: 10.1210/jcem.87.6.8536. [DOI] [PubMed] [Google Scholar]
- 19.deRoux N, Misrahi M, Brauner R, Houang M, Carel JC, Granier M, et al. Four families with loss of function mutations of the thyrotropin receptor. J Clin Endocr Metab. 1996;81:4229–35. doi: 10.1210/jcem.81.12.8954020. [DOI] [PubMed] [Google Scholar]
- 20.Jeziorowska A, Pniewska-Siark B, Brzezianska E, Pastuszak-Lewandoska D, Lewinski A. A novel mutation in the thyrotropin (thyroid-stimulating hormone) receptor gene in a case of congenital hypothyroidism. Thyroid. 2006;16:1303–9. doi: 10.1089/thy.2006.16.1303. [DOI] [PubMed] [Google Scholar]
- 21.Yuan ZF, Mao HQ, Luo YF, Wu YD, Shen Z, Zhao ZY. Thyrotropin receptor and thyroid transcription factor-1 genes variant in chinese children with congenital hypothyroidism. Endocr J. 2008;55:415–23. doi: 10.1507/endocrj.k07e-064. [DOI] [PubMed] [Google Scholar]
- 22.CliftonBligh RJ, Gregory JW, Ludgate M, John R, Persani L, Asteria C, et al. Two novel mutations in the thyrotropin (tsh) receptor gene in a child with resistance to tsh. J Clin Endocr Metab. 1997;82:1094–100. doi: 10.1210/jcem.82.4.3863. [DOI] [PubMed] [Google Scholar]
- 23.Jordan N, Williams N, Gregory JW, Evans C, Owen M, Ludgate M. The w546x mutation of the thyrotropin receptor gene: Potential major contributor to thyroid dysfunction in a caucasian population. J Clin Endocr Metab. 2003;88:1002–5. doi: 10.1210/jc.2002-021301. [DOI] [PubMed] [Google Scholar]
- 24.Park SM, Clifton-Bligh RJ, Betts P, Chatterjee VKK. Congenital hypothyroidism and apparent athyreosis with compound heterozygosity or compensated hypothyroidism with probable hemizygosity for inactivating mutations of the tsh receptor. Clin Endocrinol. 2004;60:220–7. doi: 10.1111/j.1365-2265.2004.01967.x. [DOI] [PubMed] [Google Scholar]
- 25.Tiosano D, Pannain S, Vassart G, Parma J, Gershoni-Baruch R, Mandel H, et al. The hypothyroidism in an inbred kindred with congenital thyroid hormone and glucocorticoid deficiency is due to a mutation producing a truncated thyrotropin receptor. Thyroid. 1999;9:887–94. doi: 10.1089/thy.1999.9.887. [DOI] [PubMed] [Google Scholar]
- 26.Richter-Unruh A, Hauffa BP, Pfarr N, Pohlenz J. Congenital primary hypothyroidism in a turkish family caused by a homozygous nonsense mutation (r609x) in the thyrotropin receptor gene. Thyroid. 2004;14:971–4. doi: 10.1089/thy.2004.14.971. [DOI] [PubMed] [Google Scholar]
- 27.Shibayama K, Ohyama Y, Hishinuma A, Yokota Y, Kazahari K, Kazahari M, et al. Subclinical hypothyroidism caused by a mutation of the thyrotropin receptor gene. Pediatr Int. 2005;47:105–8. doi: 10.1111/j.1442-200x.2005.02020.x. [DOI] [PubMed] [Google Scholar]
- 28.Cangul H, Aycan Z, Saglam H, Forman JR, Cetinkaya S, Tarim O, et al. Tshr is the main causative locus in autosomal recessively inherited thyroid dysgenesis. J Pediatr Endocr Met. 2012;25:419–26. doi: 10.1515/jpem-2012-0053. [DOI] [PubMed] [Google Scholar]
- 29.Bretones P, Duprez L, Parma J, David M, Vassart G, Rodien P. A familial case of congenital hypothyroidism caused by a homozygous mutation of the thyrotropin receptor gene. Thyroid. 2001;11:977–80. doi: 10.1089/105072501753211064. [DOI] [PubMed] [Google Scholar]
- 30.Lado-Abeal J, Castro-Piedras I, Palos-Paz F, Labarta-Aizpun JI, Albero-Gamboa R. A family with congenital hypothyroidism caused by a combination of loss-of-function mutations in the thyrotropin receptor and adenylate cyclase-stimulating g alpha-protein subunit genes. Thyroid. 2011;21:103–9. doi: 10.1089/thy.2010.0187. [DOI] [PubMed] [Google Scholar]
- 31.Tenenbaum-Rakover Y, Grasberger H, Mamanasiri S, Ringkananont U, Montanelli L, Barkoff MS, et al. Loss-of-function mutations in the thyrotropin receptor gene as a major determinant of hyperthyrotropinemia in a consanguineous community. J Clin Endocr Metab. 2009;94:1706–12. doi: 10.1210/jc.2008-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sriphrapradang C, Tenenbaum-Rakover Y, Weiss M, Barkoff MS, Admoni O, Kawthar D, et al. The coexistence of a novel inactivating mutant thyrotropin receptor allele with two thyroid peroxidase mutations: A genotype-phenotype correlation. J Clin Endocr Metab. 2011;96:E1001–E6. doi: 10.1210/jc.2011-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narumi S, Muroya K, Abe Y, Yasui M, Asakura Y, Adachi M, et al. Tshr mutations as a cause of congenital hypothyroidism in japan: A population-based genetic epidemiology study. J Clin Endocr Metab. 2009;94:1317–23. doi: 10.1210/jc.2008-1767. [DOI] [PubMed] [Google Scholar]
- 34.Lee ST, Lee DH, Kim JY, Kwon MJ, Kim JW, Hong YH, et al. Molecular screening of the tsh receptor (tshr) and thyroid peroxidase (tpo) genes in korean patients with nonsyndromic congenital hypothyroidism. Clin Endocrinol. 2011;75:715–21. doi: 10.1111/j.1365-2265.2011.04156.x. [DOI] [PubMed] [Google Scholar]
- 35.Russo D, Betterle C, Arturi F, Chiefari E, Girelli ME, Filetti S. A novel mutation in the thyrotropin (tsh) receptor gene causing loss of tsh binding but constitutive receptor activation in a family with resistance to tsh. J Clin Endocr Metab. 2000;85:4238–42. doi: 10.1210/jcem.85.11.6985. [DOI] [PubMed] [Google Scholar]
- 36.Nagashima T, Murakami M, Onigata K, Morimura T, Nagashima K, Mori M, et al. Novel inactivating missense mutations in the thyrotropin receptor gene in japanese children with resistance to thyrotropin. Thyroid. 2001;11:551–9. doi: 10.1089/105072501750302859. [DOI] [PubMed] [Google Scholar]
- 37.Kanda K, Mizuno H, Sugiyama Y, Imamine H, Togari H, Onigata K. Clinical significance of heterozygous carriers associated with compensated hypothyroidism in r450h, a common inactivating mutation of the thyrotropin receptor gene in japanese. Endocrine. 2006;30:383–8. doi: 10.1007/s12020-006-0018-z. [DOI] [PubMed] [Google Scholar]
- 38.Tsunekawa K, Onigata K, Morimura T, Kasahara T, Nishiyama S, Kamoda T, et al. Identification and functional analysis of novel inactivating thyrotropin receptor mutations in patients with thyrotropin resistance. Thyroid. 2006;16:471–9. doi: 10.1089/thy.2006.16.471. [DOI] [PubMed] [Google Scholar]
- 39.Tonacchera M, Agretti P, Pinchera A, Rosellini V, Perri A, Collecchi P, et al. Congenital hypothyroidism with impaired thyroid response to thyrotropin (tsh) and absent circulating thyroglobulin: Evidence for a new inactivating mutation of the tsh receptor gene. J Clin Endocr Metab. 2000;85:1001–8. doi: 10.1210/jcem.85.3.6460. [DOI] [PubMed] [Google Scholar]
- 40.Sura-Trueba S, Aumas C, Carre A, Durif S, Leger J, Polak M, et al. An inactivating mutation within the first extracellular loop of the thyrotropin receptor impedes normal posttranslational maturation of the extracellular domain. Endocrinology. 2009;150:1043–50. doi: 10.1210/en.2008-1145. [DOI] [PubMed] [Google Scholar]
- 41.Fricke-Otto S, Pfarr N, Muhlenberg R, Pohlenz J. Mild congenital primary hypothyroidism in a turkish family caused by a homozygous missense thyrotropin receptor (tshr) gene mutation (a593 v) Exp Clin Endocr Diab. 2005;113:582–5. doi: 10.1055/s-2005-865914. [DOI] [PubMed] [Google Scholar]
- 42.Lucas-Herald A, Bradley T, Hermanns P, Jones J, Attaie M, Thompson E, et al. Novel heterozygous thyrotropin receptor mutation presenting with neonatal hyperthyrotropinaemia, mild thyroid hypoplasia and absent uptake on radioisotope scan. J Pediatr Endocrinol Metab. 2013;26:583–6. doi: 10.1515/jpem-2012-0308. [DOI] [PubMed] [Google Scholar]
- 43.Moia S, Godi M, Walker GE, Roccio M, Agretti P, Tonacchera M, et al. The w520x mutation in the tshr gene brings on subclinical hypothyroidism through an haploinsufficiency mechanism. J Endocrinol Invest. 2013;36:716–21. doi: 10.3275/8930. [DOI] [PubMed] [Google Scholar]
- 44.Cangul H, Saglam H, Saglam Y, Eren E, Dogan D, Kendall M, et al. An essential splice site mutation (c. 317+1g > a) in the tshr gene leads to severe thyroid dysgenesis. J Pediatr Endocr Met. 2014;27:1021–5. doi: 10.1515/jpem-2014-0048. [DOI] [PubMed] [Google Scholar]
- 45.Bas VN, Cangul H, Agladioglu SY, Kendall M, Cetinkaya S, Maher ER, et al. Mild and severe congenital primary hypothyroidism in two patients by thyrotropin receptor (tshr) gene mutation. J Pediatr Endocr Met. 2012;25:1153–6. doi: 10.1515/jpem-2012-0211. [DOI] [PubMed] [Google Scholar]
- 46.Chang WC, Liao CY, Chen WC, Fan YC, Chiu SJ, Kuo HC, et al. R450h tsh receptor mutation in congenital hypothyroidism in taiwanese children. Clinica Chimica Acta. 2012;413:1004–7. doi: 10.1016/j.cca.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 47.Cangul H, Bas VN, Saglam Y, Kendall M, Barrett TG, Maher ER, et al. A nonsense thyrotropin receptor gene mutation (r609x) is associated with congenital hypothyroidism and heart defects. J Pediatr Endocr Met. 2014;27:1101–5. doi: 10.1515/jpem-2014-0025. [DOI] [PubMed] [Google Scholar]
- 48.Jin HY, Heo SH, Kim YM, Kim GH, Choi JH, Lee BH, et al. High frequency of duox2 mutations in transient or permanent congenital hypothyroidism with eutopic thyroid glands. Horm Res Paediat. 2014;82:252–60. doi: 10.1159/000362235. [DOI] [PubMed] [Google Scholar]
- 49.Tsunekawa K, Yanagawa Y, Aoki T, Morimura T, Araki O, Kimura T, et al. Frequency and clinical implication of the r450h mutation in the thyrotropin receptor gene in the japanese population detected by smart amplification process 2. Biomed Res Int. 2014 doi: 10.1155/2014/964635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Satoh M, Aso K, Ogikubo S, Yoshizawa-Ogasawara A, Saji T. Hypothyroidism caused by the combination of two heterozygous mutations: One in the tsh receptor gene the other in the duox2 gene. J Pediatr Endocr Met. 2015;28:657–61. doi: 10.1515/jpem-2014-0078. [DOI] [PubMed] [Google Scholar]
- 51.Cerqueira TLO, Carre A, Chevrier L, Szinnai G, Tron E, Leger J, et al. Functional characterization of the novel sequence variant p.S304r in the hinge region of tshr in a congenital hypothyroidism patients and analogy with other formerly known mutations of this gene portion. J Pediatr Endocr Met. 2015;28:777–84. doi: 10.1515/jpem-2014-0194. [DOI] [PubMed] [Google Scholar]
- 52.Labadi A, Grassi ES, Gellen B, Kleinau G, Biebermann H, Ruzsa B, et al. Loss-of-function variants in a hungarian cohort reveal structural insights on tsh receptor maturation and signaling. J Clin Endocr Metab. 2015;100:E1039–E45. doi: 10.1210/jc.2014-4511. [DOI] [PubMed] [Google Scholar]
- 53.Fu C, Wang J, Luo S, Yang Q, Li Q, Zheng H, et al. Next-generation sequencing analysis of tshr in 384 chinese subclinical congenital hypothyroidism (ch) and ch patients. Clinica Chimica Acta. 2016;462:127–32. doi: 10.1016/j.cca.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 54.Qiu Y-L, Ma S-G, Liu H, Yue H-N. Two novel tshr gene mutations (p.R528c and c. 392+4del4) associated with congenital hypothyroidism. Endocrine Research. 2016;41:180–4. doi: 10.3109/07435800.2015.1124438. [DOI] [PubMed] [Google Scholar]
- 55.Grasberger H, Van Sande J, Mahameed AHD, Tenenbaum-Rakover Y, Refetoff S. A familial thyrotropin (tsh) receptor mutation provides in vivo evidence that the inositol phosphates/ca(2+) cascade mediates tsh action on thyroid hormone synthesis. J Clin Endocr Metab. 2007;92:2816–20. doi: 10.1210/jc.2007-0366. [DOI] [PubMed] [Google Scholar]
- 56.De Felice M, Di Lauro R. Minireview: Intrinsic and extrinsic factors in thyroid gland development: An update. Endocrinology. 2011;152:2948–56. doi: 10.1210/en.2011-0204. [DOI] [PubMed] [Google Scholar]
- 57.Marotta P, Amendola E, Scarfo M, De Luca P, Zoppoli P, Amoresano A, et al. The paired box transcription factor pax8 is essential for function and survival of adult thyroid cells. Mol Cell Endocrinol. 2014;396:26–36. doi: 10.1016/j.mce.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 58.Di Palma T, Filippone MG, Pierantoni GM, Fusco A, Soddu S, Zannini M. Pax8 has a critical role in epithelial cell survival and proliferation. Cell Death Dis. 2013;4:e729. doi: 10.1038/cddis.2013.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mansouri A, Chowdhury K, Gruss P. Follicular cells of the thyroid gland require pax8 gene function. Nat Genet. 1998;19:87–90. doi: 10.1038/ng0598-87. [DOI] [PubMed] [Google Scholar]
- *60.Antonica F, Kasprzyk DF, Opitz R, Iacovino M, Liao XH, Dumitrescu AM, et al. Generation of functional thyroid from embryonic stem cells. Nature. 2012;491:66–71. doi: 10.1038/nature11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Macchia PE, Lapi P, Krude H, Pirro MT, Missero C, Chiovato L, et al. Pax8 mutations associated with congenital hypothyroidism caused by thyroid dysgenesis. Nat Genet. 1998;19:83–6. doi: 10.1038/ng0598-83. [DOI] [PubMed] [Google Scholar]
- 62.Mahjoubi F, Mohammadi MM, Montazeri M, Aminii M, Hashemipour M. Mutations in the gene encoding paired box domain (pax8) are not a frequent cause of congenital hypothyroidism (ch) in iranian patients with thyroid dysgenesis. Arq Bras Endocrinol Metabol. 2010;54:555–9. doi: 10.1590/s0004-27302010000600008. [DOI] [PubMed] [Google Scholar]
- 63.Brust ES, Beltrao CB, Chammas MC, Watanabe T, Sapienza MT, Marui S. Absence of mutations in pax8, nkx2. 5, and tsh receptor genes in patients with thyroid dysgenesis. Arq Bras Endocrinol Metabol. 2012;56:173–7. doi: 10.1590/s0004-27302012000300004. [DOI] [PubMed] [Google Scholar]
- 64.Al Taji E, Biebermann H, Limanova Z, Hnikova O, Zikmund J, Dame C, et al. Screening for mutations in transcription factors in a czech cohort of 170 patients with congenital and early-onset hypothyroidism: Identification of a novel pax8 mutation in dominantly inherited early-onset non-autoimmune hypothyroidism. Eur J Endocrinol. 2007;156:521–9. doi: 10.1530/EJE-06-0709. [DOI] [PubMed] [Google Scholar]
- 65.Liu SG, Zhang SS, Zhang LQ, Li WJ, Zhang AQ, Lu KN, et al. Screening of pax8 mutations in chinese patients with congenital hypothyroidism. J Endocrinol Invest. 2012;35:889–92. doi: 10.3275/8239. [DOI] [PubMed] [Google Scholar]
- 66.Lanzerath K, Bettendorf M, Haag C, Kneppo C, Schulze E, Grulich-Henn J. Screening for pax8 mutations in patients with congenital hypothyroidism in south-west germany. Horm Res. 2006;66:96–100. doi: 10.1159/000093799. [DOI] [PubMed] [Google Scholar]
- 67.Ramos HE, Carre A, Chevrier L, Szinnai G, Tron E, Cerqueira TL, et al. Extreme phenotypic variability of thyroid dysgenesis in six new cases of congenital hypothyroidism due to pax8 gene loss-of-function mutations. Eur J Endocrinol. 2014;171:499–507. doi: 10.1530/EJE-13-1006. [DOI] [PubMed] [Google Scholar]
- 68.Kumorowicz-Czoch M, Madetko-Talowska A, Dudek A, Tylek-Lemanska D. Genetic analysis of the paired box transcription factor (pax8) gene in a cohort of polish patients with primary congenital hypothyroidism and dysgenetic thyroid glands. J Pediatr Endocrinol Metab. 2015;28:735–43. doi: 10.1515/jpem-2014-0310. [DOI] [PubMed] [Google Scholar]
- 69.Camilot M, Teofoli F, Vincenzi M, Federici F, Perlini S, Tato L. Implementation of a congenital hypothyroidism newborn screening procedure with mutation detection on genomic DNA extracted from blood spots: The experience of the italian northeastern reference center. Genet Test. 2007;11:387–90. doi: 10.1089/gte.2007.0033. [DOI] [PubMed] [Google Scholar]
- 70.Hermanns P, Grasberger H, Cohen R, Freiberg C, Dorr HG, Refetoff S, et al. Two cases of thyroid dysgenesis caused by different novel pax8 mutations in the DNA-binding region: In vitro studies reveal different pathogenic mechanisms. Thyroid : official journal of the American Thyroid Association. 2013;23:791–6. doi: 10.1089/thy.2012.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Perone D, Medeiros-Neto G, Nogueira CR, Chagas AJ, Alves Dias VM, Viana MF, et al. Analysis of the pax8 gene in 32 children with thyroid dysgenesis and functional characterization of a promoter variant. J Pediatr Endocrinol Metab. 2016;29:193–201. doi: 10.1515/jpem-2015-0199. [DOI] [PubMed] [Google Scholar]
- 72.Narumi S, Araki S, Hori N, Muroya K, Yamamoto Y, Asakura Y, et al. Functional characterization of four novel pax8 mutations causing congenital hypothyroidism: New evidence for haploinsufficiency as a disease mechanism. Eur J Endocrinol. 2012;167:625–32. doi: 10.1530/EJE-12-0410. [DOI] [PubMed] [Google Scholar]
- 73.di Gennaro A, Spadaro O, Baratta MG, De Felice M, Di Lauro R. Functional analysis of the murine pax8 promoter reveals autoregulation and the presence of a novel thyroid-specific DNA-binding activity. Thyroid : official journal of the American Thyroid Association. 2013;23:488–96. doi: 10.1089/thy.2012.0357. [DOI] [PubMed] [Google Scholar]
- 74.Komatsu M, Takahashi T, Takahashi I, Nakamura M, Takada G. Thyroid dysgenesis caused by pax8 mutation: The hypermutability with cpg dinucleotides at codon 31. J Pediatr. 2001;139:597–9. doi: 10.1067/mpd.2001.117071. [DOI] [PubMed] [Google Scholar]
- 75.Jo W, Ishizu K, Fujieda K, Tajima T. Congenital hypothyroidism caused by a pax8 gene mutation manifested as sodium/iodide symporter gene defect. J Thyroid Res. 2010;2010:619013. doi: 10.4061/2010/619013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fu C, Chen R, Zhang S, Luo S, Wang J, Chen Y, et al. Pax8 pathogenic variants in chinese patients with congenital hypothyroidism. Clinica chimica acta; international journal of clinical chemistry. 2015;450:322–6. doi: 10.1016/j.cca.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 77.Lof C, Patyra K, Kuulasmaa T, Vangipurapu J, Undeutsch H, Jaeschke H, et al. Detection of novel gene variants associated with congenital hypothyroidism in a finnish patient cohort. Thyroid : official journal of the American Thyroid Association. 2016;26:1215–24. doi: 10.1089/thy.2016.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grasberger H, Ringkananont U, Lefrancois P, Abramowicz M, Vassart G, Refetoff S. Thyroid transcription factor 1 rescues pax8/p300 synergism impaired by a natural pax8 paired domain mutation with dominant negative activity. Mol Endocrinol. 2005;19:1779–91. doi: 10.1210/me.2004-0426. [DOI] [PubMed] [Google Scholar]
- 79.Tonacchera M, Banco ME, Montanelli L, Di Cosmo C, Agretti P, De Marco G, et al. Genetic analysis of the pax8 gene in children with congenital hypothyroidism and dysgenetic or eutopic thyroid glands: Identification of a novel sequence variant. Clin Endocrinol. 2007;67:34–40. doi: 10.1111/j.1365-2265.2007.02831.x. [DOI] [PubMed] [Google Scholar]
- 80.Di Palma T, Zampella E, Filippone MG, Macchia PE, Ris-Stalpers C, de Vroede M, et al. Characterization of a novel loss-of-function mutation of pax8 associated with congenital hypothyroidism. Clin Endocrinol. 2010;73:808–14. doi: 10.1111/j.1365-2265.2010.03851.x. [DOI] [PubMed] [Google Scholar]
- 81.Vilain C, Rydlewski C, Duprez L, Heinrichs C, Abramowicz M, Malvaux P, et al. Autosomal dominant transmission of congenital thyroid hypoplasia due to loss-of-function mutation of pax8. J Clin Endocrinol Metab. 2001;86:234–8. doi: 10.1210/jcem.86.1.7140. [DOI] [PubMed] [Google Scholar]
- 82.Congdon T, Nguyen LQ, Nogueira CR, Habiby RL, Medeiros-Neto G, Kopp P. A novel mutation (q40p) in pax8 associated with congenital hypothyroidism and thyroid hypoplasia: Evidence for phenotypic variability in mother and child. J Clin Endocrinol Metab. 2001;86:3962–7. doi: 10.1210/jcem.86.8.7765. [DOI] [PubMed] [Google Scholar]
- *83.de Sanctis L, Corrias A, Romagnolo D, Di Palma T, Biava A, Borgarello G, et al. Familial pax8 small deletion (c. 989_992delaccc) associated with extreme phenotype variability. J Clin Endocrinol Metab. 2004;89:5669–74. doi: 10.1210/jc.2004-0398. [DOI] [PubMed] [Google Scholar]
- 84.Narumi S, Yoshida A, Muroya K, Asakura Y, Adachi M, Fukuzawa R, et al. Pax8 mutation disturbing thyroid follicular growth: A case report. J Clin Endocrinol Metab. 2011;96:E2039–44. doi: 10.1210/jc.2011-1114. [DOI] [PubMed] [Google Scholar]
- 85.Meeus L, Gilbert B, Rydlewski C, Parma J, Roussie AL, Abramowicz M, et al. Characterization of a novel loss of function mutation of pax8 in a familial case of congenital hypothyroidism with in-place, normal-sized thyroid. J Clin Endocrinol Metab. 2004;89:4285–91. doi: 10.1210/jc.2004-0166. [DOI] [PubMed] [Google Scholar]
- 86.Narumi S, Muroya K, Asakura Y, Adachi M, Hasegawa T. Transcription factor mutations and congenital hypothyroidism: Systematic genetic screening of a population-based cohort of japanese patients. J Clin Endocrinol Metab. 2010;95:1981–5. doi: 10.1210/jc.2009-2373. [DOI] [PubMed] [Google Scholar]
- 87.Bouchard M, Souabni A, Mandler M, Neubuser A, Busslinger M. Nephric lineage specification by pax2 and pax8. Genes Dev. 2002;16:2958–70. doi: 10.1101/gad.240102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *88.Carvalho A, Hermanns P, Rodrigues AL, Sousa I, Anselmo J, Bikker H, et al. A new pax8 mutation causing congenital hypothyroidism in three generations of a family is associated with abnormalities in the urogenital tract. Thyroid : official journal of the American Thyroid Association. 2013;23:1074–8. doi: 10.1089/thy.2012.0649. [DOI] [PubMed] [Google Scholar]
- *89.Grasberger H, Mimouni-Bloch A, Vantyghem MC, van Vliet G, Abramowicz M, Metzger DL, et al. Autosomal dominant resistance to thyrotropin as a distinct entity in five multigenerational kindreds: Clinical characterization and exclusion of candidate loci. J Clin Endocrinol Metab. 2005;90:4025–34. doi: 10.1210/jc.2005-0572. [DOI] [PubMed] [Google Scholar]
- 90.Grasberger H, Vaxillaire M, Pannain S, Beck JC, Mimouni-Bloch A, Vatin V, et al. Identification of a locus for nongoitrous congenital hypothyroidism on chromosome 15q25.3–26. 1. Hum Genet. 2005;118:348–55. doi: 10.1007/s00439-005-0036-6. [DOI] [PubMed] [Google Scholar]
- 91.Porcu E, Medici M, Pistis G, Volpato CB, Wilson SG, Cappola AR, et al. A meta-analysis of thyroid-related traits reveals novel loci and gender-specific differences in the regulation of thyroid function. PLoS Genet. 2013;9:e1003266. doi: 10.1371/journal.pgen.1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Malinowski JR, Denny JC, Bielinski SJ, Basford MA, Bradford Y, Peissig PL, et al. Genetic variants associated with serum thyroid stimulating hormone (tsh) levels in european americans and african americans from the emerge network. PLoS One. 2014;9:e111301. doi: 10.1371/journal.pone.0111301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weinstein LS, Yu S, Warner DR, Liu J. Endocrine manifestations of stimulatory g protein alpha-subunit mutations and the role of genomic imprinting. Endocr Rev. 2001;22:675–705. doi: 10.1210/edrv.22.5.0439. [DOI] [PubMed] [Google Scholar]
- 94.Devriendt K, Vanhole C, Matthijs G, de Zegher F. Deletion of thyroid transcription factor-1 gene in an infant with neonatal thyroid dysfunction and respiratory failure. N Engl J Med. 1998;338:1317–8. doi: 10.1056/NEJM199804303381817. [DOI] [PubMed] [Google Scholar]
- 95.Krude H, Schutz B, Biebermann H, von Moers A, Schnabel D, Neitzel H, et al. Choreoathetosis, hypothyroidism, and pulmonary alterations due to human nkx2–1 haploinsufficiency. The Journal of clinical investigation. 2002;109:475–80. doi: 10.1172/JCI14341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pohlenz J, Dumitrescu A, Zundel D, Martine U, Schonberger W, Koo E, et al. Partial deficiency of thyroid transcription factor 1 produces predominantly neurological defects in humans and mice. The Journal of clinical investigation. 2002;109:469–73. doi: 10.1172/JCI14192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Veneziano L, Parkinson MH, Mantuano E, Frontali M, Bhatia KP, Giunti P. A novel de novo mutation of the titf1/nkx2–1 gene causing ataxia, benign hereditary chorea, hypothyroidism and a pituitary mass in a uk family and review of the literature. Cerebellum. 2014;13:588–95. doi: 10.1007/s12311-014-0570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Carre A, Szinnai G, Castanet M, Sura-Trueba S, Tron E, Broutin-L’Hermite I, et al. Five new ttf1/nkx2. 1 mutations in brain-lung-thyroid syndrome: Rescue by pax8 synergism in one case. Hum Mol Genet. 2009;18:2266–76. doi: 10.1093/hmg/ddp162. [DOI] [PubMed] [Google Scholar]
- 99.Maruo Y, Nagasaki K, Matsui K, Mimura Y, Mori A, Fukami M, et al. Natural course of congenital hypothyroidism by dual oxidase 2 mutations from the neonatal period through puberty. Eur J Endocrinol. 2016;174:453–63. doi: 10.1530/EJE-15-0959. [DOI] [PubMed] [Google Scholar]
- 100.Fu C, Luo S, Zhang S, Wang J, Zheng H, Yang Q, et al. Next-generation sequencing analysis of duox2 in 192 chinese subclinical congenital hypothyroidism (sch) and ch patients. Clinica chimica acta; international journal of clinical chemistry. 2016;458:30–4. doi: 10.1016/j.cca.2016.04.019. [DOI] [PubMed] [Google Scholar]
- 101.Park KJ, Park HK, Kim YJ, Lee KR, Park JH, Park JH, et al. Duox2 mutations are frequently associated with congenital hypothyroidism in the korean population. Ann Lab Med. 2016;36:145–53. doi: 10.3343/alm.2016.36.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Srichomkwun P, Takamatsu J, Nickerson DA, Bamshad MJ, Chong JX, Refetoff S. Duox2 gene mutation manifesting as resistance to thyrotropin phenotype. Thyroid : official journal of the American Thyroid Association. 2017;27:129–31. doi: 10.1089/thy.2016.0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grasberger H. Defects of thyroidal hydrogen peroxide generation in congenital hypothyroidism. Mol Cell Endocrinol. 2010;322:99–106. doi: 10.1016/j.mce.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 104.Ladsous M, Vlaeminck-Guillem V, Dumur V, Vincent C, Dubrulle F, Dhaenens CM, et al. Analysis of the thyroid phenotype in 42 patients with pendred syndrome and nonsyndromic enlargement of the vestibular aqueduct. Thyroid : official journal of the American Thyroid Association. 2014;24:639–48. doi: 10.1089/thy.2013.0164. [DOI] [PubMed] [Google Scholar]
- *105.Senou M, Khalifa C, Thimmesch M, Jouret F, Devuyst O, Col V, et al. A coherent organization of differentiation proteins is required to maintain an appropriate thyroid function in the pendred thyroid. J Clin Endocrinol Metab. 2010;95:4021–30. doi: 10.1210/jc.2010-0228. [DOI] [PubMed] [Google Scholar]
- *106.Tenenbaum-Rakover Y, Almashanu S, Hess O, Admoni O, Hag-Dahood Mahameed A, Schwartz N, et al. Long-term outcome of loss-of-function mutations in thyrotropin receptor gene. Thyroid : official journal of the American Thyroid Association. 2015;25:292–9. doi: 10.1089/thy.2014.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mizuno H, Kanda K, Sugiyama Y, Imamine H, Ito T, Kato I, et al. Longitudinal evaluation of patients with a homozygous r450h mutation of the tsh receptor gene. Horm Res. 2009;71:318–23. doi: 10.1159/000223415. [DOI] [PubMed] [Google Scholar]
- 108.Poleev A, Okladnova O, Musti AM, Schneider S, Royer-Pokora B, Plachov D. Determination of functional domains of the human transcription factor pax8 responsible for its nuclear localization and transactivating potential. Eur J Biochem. 1997;247:860–9. doi: 10.1111/j.1432-1033.1997.00860.x. [DOI] [PubMed] [Google Scholar]