SUMMARY
Long-term outcomes and updated clinical efficacy and safety data were evaluated for newly-diagnosed multiple myeloma patients treated on a phase II study of bortezomib and pegylated liposomal doxorubicin (PegLD). Out of 61 patients, the overall response rate was 57% and the near-complete/complete response rate was 7%. Patients aged ≥65 years old had a higher incidence of treatment-related ≥Grade 3 non-haematological toxicity (80% vs 51%, P = 0.020). Median overall survival was 5.6 years and negatively impacted by the presence of International Staging System stage III disease, underscoring the need for novel treatment strategies for this group of patients.
Keywords: Multiple myeloma, proteasome inhibitors, anthracyclines, international staging system
INTRODUCTION
A phase I study of pegylated liposomal doxorubicin (PegLD) and bortezomib in haematological malignancies identified the maximum tolerated dose of the regimen and demonstrated notable activity in patients with multiple myeloma (Orlowski et al, 2005). A subsequent phase III study comparing single agent bortezomib to PegLD/bortezomib in bortezomib-naïve patients with relapsed multiple myeloma yielded a median time to progression (TTP) of 6.5 and 9.3 months (P = 0.000004), respectively (Orlowski et al, 2007). Given the compelling pre-clinical and phase I clinical data supporting the combination of PegLD/bortezomib, the Cancer And Leukemia Group B (CALGB, now part of the Alliance for Clinical Trials in Oncology) sought to evaluate the efficacy and safety of bortezomib and PegLD in patients with newly -diagnosed multiple myeloma in a single arm phase II multicentre study. We have previously presented the short-term safety and efficacy data of this combination (Orlowski et al, 2006). With long-term follow-up, we performed a post hoc analysis of clinical variables that impact outcomes of patients prospectively treated with an upfront, bortezomib-based regimen. An equal distribution of younger and older patients on this study provided an opportunity to evaluate the impact of age on survival and treatment tolerability. Here we report long-term results of the only study of the PegLD and bortezomib doublet in the frontline setting.
METHODS
Please refer to supplemental data for details regarding patient eligibility, study design and treatment, and assessment of efficacy and safety (Data S1).
Statistical Analysis
With a planned enrolment of 50 evaluable patients, this single stage study had 0.9 power and a type I error rate of 0.06 to test whether the regimen could induce a near-complete response / complete response (nCR/CR) rate greater than 0.10 under the null hypothesis that the nCR/CR rate with PegLD and bortezomib is ≤10% versus the alternate hypothesis of ≥25%.
Best achieved response rates were assessed as the proportion of patients achieving each type of confirmed response, and exact 95% confidence intervals (CIs) for the true rate were calculated, assuming a binomial distribution for each response category. Survival functions for overall survival (OS), progression-free survival (PFS), event-free survival (EFS), duration of response (DOR) and time to response (TTR) were estimated using the Kaplan–Meier method (Kaplan & Meier, 1958). The differences between survival distributions with respect to age, dichotomized at 65 years, and other baseline clinical characteristics were evaluated using the log-rank test. Hazard ratios and their 95% CIs were estimated using a Cox regression model (Cox, 1972). Differences in baseline clinical characteristics between those responding to therapy and those with unresponsive disease were evaluated using the score statistic from logistic regression models. Cumulative incidence with competing risks was used to assess potential differences between age groups when factoring in the following competing risks: death, progression, receipt of non-protocol therapy and stem cell transplant (Fine and Gray, 1999).
Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center (Durham, NC). Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies. Statistical analyses were performed by CALGB (Alliance) statisticians using SAS 9.2 (SAS Institute, Inc., Cary, NC) and R package version 2.15.1 (http://www.R-project.org/) and were based on the study database frozen on 7 April 2014. The cmprsk extension package (version 2.2-2) was used for the purpose of estimation and inference for the cumulative incidence curves (Gray, 2011). P values ≤0.05 were considered statistically significant
RESULTS
Efficacy
,A total of 63 patients with newly diagnosed, symptomatic multiple myeloma enrolled on the study between 15 June 2004 and 25 October 2005. Information regarding patient disposition, therapy received and baseline demographics are provided as supplementary data (Data S1, Table S1).
Sixty-one patients were evaluable for response. The overall response rate (ORR) was 57% (95% CI 44 – 70%) and nCR/CR rate 7% (95% CI 2 – 16%) (Table I). The median TTR was 1.2 months (95% CI 1.1–1.5 months) and median DOR 17.5 months (95% CI 9.7 – 25.7 months).
Table I.
Response data with pegylated liposomal doxorubicin and bortezomib
| Best Response | N | % | 95% CI |
|---|---|---|---|
| Modified EBMT Criteria | |||
| Overall Response | 35 | 57 | 44 – 70 |
| Complete Response* | 3 | 5 | 1 – 14 |
| Near Complete Response* | 1 | 2 | 0 – 9 |
| Partial Response | 31 | 51 | 38 – 64 |
| Near Complete Response + Complete Response | 4 | 7 | 2 – 16 |
| Minimal Response | 6 | 10 | 4 – 20 |
| Stable Disease | 15 | 25 | 15 – 37 |
| Progressive Disease | 1 | 2 | 0 – 9 |
| Not Evaluable | 4 | 7 | 2 – 16 |
| IMWG Criteria | |||
| Overall Response | 35 | 57 | 44 – 70 |
| Complete Response | 3 | 5 | 1 – 14 |
| Very Good Partial Response | 13 | 21 | 12 – 34 |
| Partial Response | 19 | 31 | 20 – 44 |
| Median Time to Response, months (95% CI) | 1.2 (1.1 – 1.5) | ||
All near complete and complete responses were seen in the first 50 evaluable patients.
Abbreviations: EBMT=European Group for Blood and Marrow Transplant (Blade et al, 1998); IMWG=International Myeloma Working Group (Rajkumar et al, 2011)
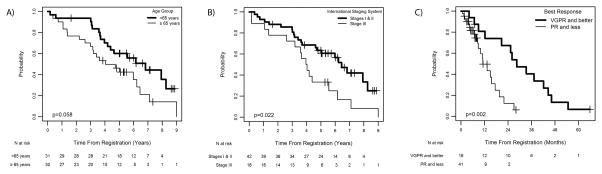
Median follow-up was 6.6 years; to date, 47 patients have had disease progression and 39 have died. Data on PFS and EFS are provided as supplementary data (Data S1, Figure S1). The median OS was 5.6 years (95% CI 4.0 – 6.8 years) and was not significantly different when stratified by gender, race, Durie-Salmon stage or renal function. The median OS was 6.8 years for those aged <65 years (95% CI 4.2 – 8.3 years) and 4.0 years for those ≥65 years of age (95% CI 3.0 – 6.2 years, P = 0.058) (Figure 1A). Those with International Staging System (ISS) III disease had a median OS of 4.0 years (95% CI 2.6 – 5.6 years) compared to 6.4 years for stage I or II disease (95% CI 4.6 – 8.3 years, P = 0.022)(Figure 1B). Among the variables age, gender, ISS stage, renal function, platelets and haemoglobin, only ISS stage was statistically significant, where those with ISS stage III disease had a hazard of death that was 2.13 times greater than those with ISS stage I and II disease (p=0.026) (Table S2). OS was longer for patients achieving ≥very good partial response (VGPR), with a median OS of 8.3 years (95% CI 6.2 – 8.3 years) compared with 4.2 years (95% CI 3.2 – 6.4 years) for those with < VGPR (Figure 1C, P = 0.048).
Figure 1.
Overall survival by (A) age <65years vs. ≥65 years, (B) International Staging System score and (C) response to therapy (<VGPR vs. ≥VGPR, for patients treated with pegylated liposomal doxorubicin and bortezomib. VGPR, very good partial response; PR, partial response.
Safety
Sixty-one patients were evaluable for safety. Therapy-related ≥Grade 3 haematological and non-haematological adverse events were seen in 44% and 66% of patients, respectively (Table S3). Grade 3 and higher treatment-related non-haematological adverse events were seen more often in older patients, occurring in 80% compared with 51% for those aged <65 years (P = 0.020). Thirty per cent of those aged ≥65 years had to stop protocol treatment due to adverse events, in contrast to 19% of those <65 years old. Additional data on commonly experienced adverse events, treatment delays, dose reductions and stem cell mobilization and engraftment are provided as supplementary data (Data S1, Table S4).
DISCUSSION
The nCR/CR rate with PegLD/bortezomib was lower than expected given the fact that nCR/CR rates of 13% – 36% were seen in phase I and III studies of the combination in patients with relapsed or refractory multiple myeloma (Orlowski et al, 2005, Orlowski et al, 2007). However, in line with our results, the addition of PegLD to bortezomib did not improve the nCR/CR rate compared with bortezomib for relapsed multiple myeloma patients treated on a phase III study (13% vs 10%) (Orlowski et al, 2007), and the nCR/CR rate of bortezomib monotherapy in the frontline setting was only 9% (Richardson et al, 2009). In contrast, a phase II study evaluating PegLD/bortezomib in combination with dexamethasone demonstrated an ORR of 85.0% and nCR/CR rate of 37.5% after 6 cycles (Jakubowiak et al, 2009), highlighting the efficacy of dexamethasone in newly diagnosed patients. Nonetheless, the ORR in our study was notable for a corticosteroid-free regimen, recognizing that such regimens have not been rigorously evaluated as frontline therapy. As such, PegLD/bortezomib could represent a potential therapeutic option for newly diagnosed patients who have more significant contraindications to the use of corticosteroid-containing therapy
Patients with ISS stage III disease had a decreased median OS compared to those with ISS stage I and II disease, reaffirming the prognostic value of the ISS for patients treated with this regimen. Our results are consistent with previous retrospective analyses demonstrating the prognostic relevance of the ISS for those treated with novel therapies (Kastritis et al, 2009; Srivastava et al, 2013). Nonetheless, the value of the ISS will require on-going re-validation with the emergence of newer combinations.
Age has been shown to be a predictor of survival in multiple myeloma, but much of these data were derived from patients treated prior to the availability of bortezomib (Avet-Loiseau et al, 2013, Turesson et al, 2010). In our study, there was a strong trend toward inferior outcomes for those aged ≥65 years. Although we were not able to evaluate differences in the incidence of high-risk cytogenetic abnormalities in older and younger patients treated on this study given the small number of patients with cytogenetically-confirmed high-risk disease, the proportion of patients with ISS stage III disease was evenly distributed. A potentially more compelling reason for the disparity in outcomes is that older patients do not tolerate therapy as well, leading to more dose reductions and delays and earlier discontinuation of treatment. Notably, patients aged ≥65 years on our study experienced more treatment-related ≥Grade 3 non-haematological toxicity, and 30% developed Grade 3 fatigue, which may have led to more dose modifications and treatment discontinuations.
In conclusion, PegLD/bortezomib therapy in newly diagnosed multiple myeloma did not meet the nCR/CR rate specified in the protocol and was associated with increased adverse events in older patients. Overall survival was reflective of the therapeutic advances made in multiple myeloma over the last 10 years but negatively impacted by the presence of ISS stage III disease. Last, our results reveal potential differences in treatment tolerability and overall survival for older patients treated with PegLD/bortezomib. A better understanding of the impact of age on treatment tolerability and survival with novel induction regimens will be critical if we are to further improve outcomes for this patient population.
Supplementary Material
Acknowledgments
Funding:
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821, U10CA180882, CA31946, CA33601, CA47559, CA101140, and CA41287. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The following institutions participated in this study:
Cancer Centers of the Carolinas, Greenville, SC, Jeffrey K. Giguere, M.D., supported by CA29165
Christiana Care Health Services, Inc. CCOP, Wilmington, DE, Stephen Grubbs, M.D., supported by CA45418
Dartmouth Medical School-Norris Cotton Cancer Center, Lebanon, NH, Konstantin Dragnev, M.D., supported by CA04326
Duke University Medical Center, Durham, NC, Jeffrey Crawford, M.D., supported by CA47577
Georgetown University Medical Center, Washington, DC, Bruce Cheson, M.D., supported by CA77597
Kansas City Community Clinical Oncology Program CCOP, Kansas City, MO, Rakesh Gaur, M.D.
Missouri Baptist Medical Center, St. Louis, MO, Alan P. Lyss, M.D.
Mount Sinai Medical Center, Miami, FL, Michael A. Schwartz, M.D., supported by CA45564
Mount Sinai School of Medicine, New York, NY, Lewis R. Silverman, M.D., supported by CA04457
Roswell Park Cancer Institute, Buffalo, NY, Ellis Levine, M.D., supported by CA59518
Southeast Cancer Control Consortium Inc. CCOP, Goldsboro, NC, James N. Atkins, M.D., supported by CA45808
University of Chicago, Chicago, IL, Hedy L. Kindler, M.D., supported by CA41287
University of Minnesota, Minneapolis, MN, Bruce A. Peterson, M.D., supported by CA16450
University of North Carolina at Chapel Hill, Chapel Hill, NC, Thomas C. Shea, M.D., supported by CA47559
University of Vermont, Burlington, VT, Steven M. Grunberg, M.D., supported by CA77406
Wake Forest University School of Medicine, Winston-Salem, NC, David D. Hurd, M.D., supported by CA03927
Walter Reed Army Medical Center, Washington, DC, David C. Van Echo, M.D., supported by CA26806
Washington University School of Medicine, St. Louis, MO, Nancy Bartlett, M.D., supported by CA77440
Footnotes
Author contributions:
PV conducted the research, reviewed, verified and analysed the data and drafted the manuscript. RO designed the study, conducted the research, reviewed and verified the data. PW and A C-K made substantial contributions to the conduct of the research. FM, BS and SG performed statistical analysis of the data. EB reviewed the data and facilitated the resolution of study-related data queries. CB oversaw the cytogenetic analysis of bone marrow samples submitted to CALGB / Alliance. RL conducted the research and contributed to the study design. All authors critically reviewed the manuscript and approved of the final submitted version.
References
- Avet-Loiseau H, Durie BG, Cavo M, Attal M, Gutierrez N, Haessler J, Goldschmidt H, Hajek R, Lee JH, Sezer O, Barlogie B, Crowley J, Fonseca R, Testoni N, Ross F, Rajkumar SV, Sonneveld P, Lahuerta J, Moreau P, Morgan G International Myeloma Working G. Combining fluorescent in situ hybridization data with ISS staging improves risk assessment in myeloma: an International Myeloma Working Group collaborative project. Leukemia. 2013;27:711–717. doi: 10.1038/leu.2012.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, Gertz M, Giralt S, Jagannath S, Vesole D. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression Models and Life-Tables. J Royal Stat Soc Series B (Methodological) 1972;34(2):187–220s. [Google Scholar]
- Fine JP, Gray RJ. A proportional hazards model for the sub-distribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- Gray RJ. R package version 2.2-2. 2011. cmprsk: Subdistribution Analysis of Competing Risks. [Google Scholar]
- Jakubowiak AJ, Kendall T, Al-Zoubi A, Khaled Y, Mineishi S, Ahmed A, Campagnaro E, Brozo C, Braun T, Talpaz M, Kaminski MS. Phase II trial of combination therapy with bortezomib, pegylated liposomal doxorubicin, and dexamethasone in patients with newly diagnosed myeloma. J Clin Oncol. 2009;27:5015–5022. doi: 10.1200/JCO.2008.19.5370. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. J AmStat Assoc. 1958;53(282):457–481. [Google Scholar]
- Kastritis E, Zervas K, Symeonidis A, Terpos E, Delimbassi S, Anagnostopoulos N, Michali E, Zomas A, Katodritou E, Gika D, Pouli A, Christoulas D, Roussou M, Kartasis Z, Economopoulos T, Dimopoulos MA. Improved survival of patients with multiple myeloma after the introduction of novel agents and the applicability of the International Staging System (ISS): an analysis of the Greek Myeloma Study Group (GMSG) Leukemia. 2009;23:1152–1157. doi: 10.1038/leu.2008.402. [DOI] [PubMed] [Google Scholar]
- Orlowski RZ, Voorhees PM, Garcia RA, Hall MD, Kudrik FJ, Allred T, Johri AR, Jones PE, Ivanova A, Van Deventer HW, Gabriel DA, Shea TC, Mitchell BS, Adams J, Esseltine DL, Trehu EG, Green M, Lehman MJ, Natoli S, Collins JM, Lindley CM, Dees EC. Phase 1 trial of the proteasome inhibitor bortezomib and pegylated liposomal doxorubicin in patients with advanced hematologic malignancies. Blood. 2005;105:3058–3065. doi: 10.1182/blood-2004-07-2911. [DOI] [PubMed] [Google Scholar]
- Orlowski RZ, Peterson BL, Sanford B, Chanan-Khan AA, Zehngebot LM, Watson PR, Caligiuri MA, Larson R the Cancer Leukemia Group B. Bortezomib and Pegylated Liposomal Doxorubicin as Induction Therapy for Adult Patients with Symptomatic Multiple Myeloma: Cancer and Leukemia Group B Study 10301. Blood (ASH Annual Meeting Abstracts) 2006;108:797. [Google Scholar]
- Orlowski RZ, Nagler A, Sonneveld P, Blade J, Hajek R, Spencer A, San Miguel J, Robak T, Dmoszynska A, Horvath N, Spicka I, Sutherland HJ, Suvorov AN, Zhuang SH, Parekh T, Xiu L, Yuan Z, Rackoff W, Harousseau JL. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, Blade J, Richardson P, Orlowski R, Siegel D, Jagannath S, Facon T, Avet-Loiseau H, Lonial S, Palumbo A, Zonder J, Ludwig H, Vesole D, Sezer O, Munshi NC, San Miguel J International Myeloma Workshop Consensus P. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–4695. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PG, Xie W, Mitsiades C, Chanan-Khan AA, Lonial S, Hassoun H, Avigan DE, Oaklander AL, Kuter DJ, Wen PY, Kesari S, Briemberg HR, Schlossman RL, Munshi NC, Heffner LT, Doss D, Esseltine DL, Weller E, Anderson KC, Amato AA. Single-agent bortezomib in previously untreated multiple myeloma: efficacy, characterization of peripheral neuropathy, and molecular correlations with response and neuropathy. J Clin Oncol. 2009;27:3518–3525. doi: 10.1200/JCO.2008.18.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava G, Rana V, Lacy MQ, Buadi FK, Hayman SR, Dispenzieri A, Gertz MA, Dingli D, Zeldenrust S, Russell S, McCurdy A, Kapoor P, Kyle R, Rajkumar SV, Kumar S. Long-term outcome with lenalidomide and dexamethasone therapy for newly diagnosed multiple myeloma. Leukemia. 2013;27:2062–2066. doi: 10.1038/leu.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turesson I, Velez R, Kristinsson SY, Landgren O. Patterns of improved survival in patients with multiple myeloma in the twenty-first century: a population-based study. J Clin Oncol. 2010;28:830–834. doi: 10.1200/JCO.2009.25.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.