In 2015, 17 million HIV-infected individuals worldwide were on antiretroviral drug therapies, which are remarkably effective in suppressing the virus. Yet, 6000 people a day became newly infected, making the quest for an effective and safe HIV vaccine a major global priority. However, developing a vaccine has been difficult for reasons related to the nature of the virus and its life cycle, including early integration into the host genome and the highly glycosylated, compact, and sequence-variable nature of the envelope (Env) “spike” that is the sole target of neutralizing antibodies (and typically associated with vaccine protection). Where are we, then, on the path to a vaccine?
From 1987 to 2013, all of the six HIV vaccine efficacy trials failed except for one. The RV144 trial in Thailand that used a viral vector prime (expressing three HIV genes, env, gag, and pro) and a boost with HIV’s glycoprotein gp120 (a constituent of the viral spike) showed a modest estimated 31.2% vaccine efficacy at 42 months (1). Antibodies to the second variable loop of gp120 as well as antibody-dependent cellular cytotoxicity (ADCC) correlated with decreased transmission risk, whereas a high immunoglobulin (Ig) A antibody response to Env (which may inhibit ADCC) correlated with increased transmission risk (1). Although the RV144 trial showed putative short-lived vaccine efficacy, it was not sufficient for vaccine deployment. Nonetheless, from RV144 (1) and studies in animal models (2), a hypothesis gained ground that ADCC and other non-neutralizing functions of Fc receptor (FcR)–bearing immune cells could contribute to protection against HIV transmission. New trials have been designed to improve RV144 vaccine efficacy by using new adjuvants and Env proteins (1). Thus, one track of vaccine development is to investigate easy-to-induce, non-neutralizing antibodies that have FcR-mediated anti-HIV effector functions in vitro for their ability to prevent HIV transmission in vivo.
Another path for vaccine development derives from observations that CD8 cytolytic T cells (CTLs) can control HIV viral load by killing HIV-infected CD4 T cells. Prime and boost regimens with conserved or mosaic HIV gene vector inserts designed to overcome viral diversity have induced considerable breath in human CTL recognition of HIV and have shown efficacy in monkey models that mimic human exposure to the virus (3). Remarkably, vaccination of macaques with simian immunodeficiency virus(SIV) gag inserted into an attenuated rhesus cytomegalovirus (rhCMV) vector cleared SIV-infected cells after initial rounds of infection in about 50% of vaccinated monkeys (4). Interestingly, attenuated rhCMV induced an extraordinary breadth of CTLs, and target cell–killing was mediated by atypical CD8 CTL recognition of antigen (4). Efforts are under way to determine whether similar immune responses can be induced in humans.
Receiving much attention is the idea of inducing broadly neutralizing antibodies (bnAbs)—those that neutralize a diversity of global HIV isolates (5, 6). This approach has been reinvigorated by the isolation of many potent bnAbs from infected individuals (7); the generation of a stable HIV Env spike (which is a trimer) (8) and the determination of its structure at high resolution (9); the description of how bnAbs interact with the trimer at the molecular level, leading to the design of new immunogens (9); the discovery of how bnAbs evolve in infected individuals (10); insight into host constraints on the induction of bnAbs (6, 11); insight into the nature of transmitted-founder (TF) viruses (6); and the development of simian-human chimeric immunodeficiency viruses (SHIVs) with TF Envs (12). Importantly, bnAbs are highly effective in protecting against retrovirus transmission when passively administered to monkeys challenged with SHIVs. A fundamental problem is the inability of current vaccines to induce high titers of bnAbs to the relatively conserved sites of vulnerability on HIV-1 Env.
The roadblocks to inducing bnAbs are multiple. The immunogen must be optimized to display the precise epitope recognized by the bnAb, requiring information at the molecular level. A soluble gp140 SOSIP trimer enabled crystallization and cryo–electron microscopy (cryo-EM) to determine its structure (9). Concordance between this structure and the cryo-EM structure of a membrane-bound trimer (9) was an important step in the evaluation of the native trimer structure. Because stabilization of the SOSIP trimer has been achieved, Envs can now be routinely made that do not expose non-neutralizing and potentially diverting epitopes. Unfortunately, immunization of rabbits and monkeys with SOSIP trimers alone did not induce bnAbs (8), indicating that additional strategies are needed. In addition, bnAb evolution has been observed only after extensive virus Env diversification (10). This suggests that multiple sequential Envs may be required to induce bnAbs through vaccination.
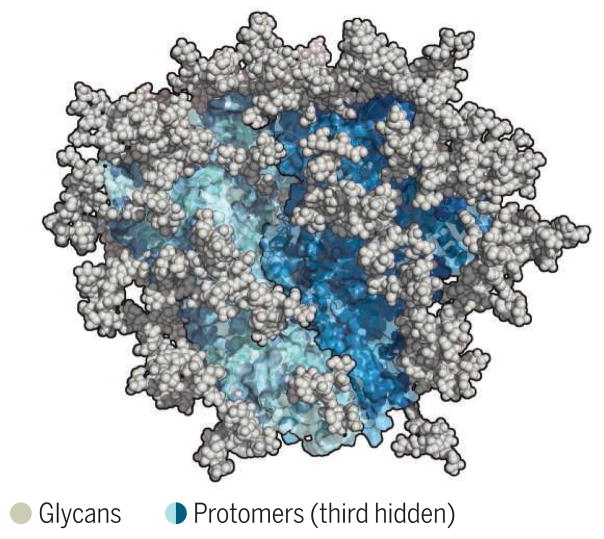
Another obstacle is that HIV Env is one of nature’s most heavily glycosylated proteins. The conserved Env sites to which bnAbs bind are heavily masked by glycans, yet most bnAbs must interact with, or at least accommodate, Env glycans (see the figure). Unfortunately, Env glycans are derived from the host, are poorly immunogenic, and can be quite heterogeneous, providing further challenges for bnAb elicitation and recognition.
Figure. The HIV envelope trimer.
The structure of a recombinant trimer has been shown to closely resemble that of the membrane-associated molecule. The trimer is the sole target of bnAbs, and most bnAbs either bind to or accommodate glycans.
To recognize epitopes, bnAbs typically have one or more unusual traits, including long heavy-chain third complementarity-determining regions (HCDR3s), high levels of somatic mutations, high frequency of insertions and deletions (indels), and reactivity with self or environmental antigens (autoreactivity or polyreactivity) (5, 6, 11). Mice engineered to express some bnAb Ig heavy-chain variable domain (VH) and light-chain variable domain (VL) genes display central tolerance (deletion), receptor editing, antibody reversion (loss of reactivity to target epitope), and peripheral anergy (self-reactive T cells become nonresponsive), all of which control bnAb development (13). Immune tolerance control of bnAbs can reduce the pool of bnAb-producing B cells capable of responding to a vaccine and may increase the propensity of bnAb B cell lineages to divert “off track” during antigen stimulation and affinity maturation. Typically, bnAb B cell lineages—even when they arise in HIV-infected individuals, and certainly in vaccination—are subdominant and therefore disfavored (11–14).
Another challenge is that germline versions of bnAbs frequently do not bind to most Env proteins, suggesting either the design of specific germline-targeting molecules or the very careful choice of a sequence of TF Env molecules (perhaps informed by antibody-virus coevolution studies) to initiate a bnAb response. Targeting naive B cell receptors (which correspond to bnAbs that the B cells produce) can indeed activate and expand bnAb precursor B cells. Moreover, Env antigens have successfully induced bnAbs in near-germline bnAb B cell receptor–expressing mice (15). What has yet to be accomplished is to induce bnAbs in outbred nonhuman primates or in human clinical trials. An additional proposal is vaccine transient immune modulation, in which vaccination occurs in concert with inhibitors of immune tolerance to allow bnAb-producing precursor B cells to survive and to activate anergic B cells in peripheral immune sites (11). To avoid systemic breaks in tolerance, specific stimulators of protective antibodies are being defined, as well as specific inhibitors of those controls that prevent the stimulation and maturation of bnAb-producing B cells.
What are the preferred targets for bnAb induction? Sites toward the “top” of the Env spike are advantageous in that they frequently trigger an antibody response in natural infection, often relatively early, and have relatively low levels of somatic hypermutation. However, such antibodies require glycan recognition, albeit to relatively homogeneous high-mannose glycans. Those bnAbs that target the CD4 binding site of the Env protein typically have high levels of somatic hypermutation but do not require direct glycan recognition. BnAbs that recognize the envelope glycoprotein 41 (gp41)–gp120 interface usually involve binding to complex heterogeneous glycans and so may be disadvantaged. Experimental approaches will be crucial in deciding the best targets.
Overall, degrees of protection from HIV, SIV, and SHIV transmission have been seen with vaccination; thus, we know that development of a protective HIV vaccine is, in principle, possible. What is not known is how studies in monkeys will translate into humans. Critical questions for HIV vaccine development include (i) What are strategies for improving vaccine efficacy seen in the RV144 trial? (ii) Can human attenuated CMV or other vectors that induce atypical CD8 T cell responses clear acute HIV infection similar to rhesus CMV vectors? (iii) What are the preferred structures, forms, and sequences of Env immunogens that are needed to induce bnAbs? (iv) How are bnAbs regulated as compared with easily induced non-neutralizing or neutralizing antibodies to very sensitive viruses? Answering these questions in the coming years should yield promising vaccine candidates to be tested in human clinical trials and bring us closer to a practical HIV vaccine.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Allergy and Infectious Diseases, the Division of AIDS, the Centers for HIV/ AIDS Vaccine Immunology–Immunogen Discovery Grants at Duke School of Medicine, Durham, NC (AI000645) and at The Scripps Research Institute, La Jolla, CA (AI 100663); and the Bill & Melinda Gates Foundation with Collaboration for AIDS Vaccine Discovery Grants OPP52282, OPP1114721, and OPP1094352 to B.F.H., and OPP1084519 to D.R.B. Many important references were not cited because of space limitations. See the supplementary materials for additional references.
Footnotes
SUPPLEMENTARY MATERIALS
References
- 1.Corey L, et al. Sci Transl Med. 2015;7:310. doi: 10.1126/scitranslmed.aac7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burton DR, Mascola J. Nat Immunol. 2015;16:571. doi: 10.1038/ni.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephenson KE, et al. Curr Opin Immunol. 2016;41:39. doi: 10.1016/j.coi.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen SG, et al. Science. 2013;340:1237874. doi: 10.1126/science.1237874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton DR, Hangartner L. Ann Rev Immunol. 2016;34:635. doi: 10.1146/annurev-immunol-041015-055515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haynes BF, et al. Cell Host Microbe. 2016;19:292. doi: 10.1016/j.chom.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCoy LE, Burton DR. Immunol Rev. 2017;275:11. doi: 10.1111/imr.12484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanders RW, Moore JP. Immunol Rev. 2017;275:161. doi: 10.1111/imr.12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ward AB, Wilson IA. Immunol Rev. 2017;275:21. doi: 10.1111/imr.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonsignori M, et al. Immunol Rev. 2017;275:145. doi: 10.1111/imr.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelsoe G, Haynes BF. Immunol Rev. 2017;275:79. doi: 10.1111/imr.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, et al. Proc Natl Acad Sci USA. 2016;113:E3413. doi: 10.1073/pnas.1606636113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verkoczy L, et al. Immunol Rev. 2017;275:89. doi: 10.1111/imr.12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Havenar-Daughton C, et al. Immunol Rev. 2017;275:49. doi: 10.1111/imr.12512. [DOI] [PubMed] [Google Scholar]
- 15.Escolano A, et al. Cell. 2016;166:1445. doi: 10.1016/j.cell.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.