Abstract
Background
Sickle cell anemia (SCA) is considered a major risk factor for renal complications. The main goal of this study was to determine the frequency of macroalbuminuria and microalbuminuria in Congolese children <18 years of age suffering from Sickle cell anemia and to identify associated factors.
Methods
The cross-sectional study was completed in 150 hemoglobin-SS children (77 boys and 73 girls). Microalbuminuria was defined by a urine albumin:creatinine ratio of 30–299 mg/g.
Results
The mean age of this group was 8.8 ± 4.3 years (range 2–18). Microalbuminuria was found in 27 children (18%). In multivariate logistic regression, only age emerged as a determinant of microalbuminuria odds ratio 1.11 (95% confidence interval 1.00–1.22); P = 0.042].
Conclusions
In our series, only age was a major determinant of the occurrence of microalbuminuria. These results confirm the need for early screening of microalbuminuria in Congolese children suffering from Sickle cell anemia in a context where access to renal and bone marrow transplant is nonexistent.
Keywords: Africa, children, microalbuminuria, risk factors, sickle cell anemia
Introduction
Sickle cell anemia (SCA) is an autosomal recessive genetic disease characterized by the presence of an abnormal hemoglobin (Hb), hemoglobin S (Hb-S). The disease is characterized by vaso-occlusive, hemolytic crises and organ damage [1]. Renal involvement is one of the chronic complications and a major factor of early death [2, 3]. Previous studies reported that renal complications occur in 5–18% of sickle cell children and adolescents and >9% of deaths in young adults were due to complications related to kidney disease [3–5].
Sickle cell nephropathy (SCN) is clinically characterized by glomerular disease, with the appearance of significant proteinuria preceded by a microalbuminuria and progressing gradually to chronic kidney failure [4–6]. The pathogenesis of SCN includes chronic hypoxia, ischemia, low pH of the renal medulla and oxidative stress, decreased nitric oxide bioavailability, endothelial dysfunction, membranoproliferative glomerulonephritis and focal segmental glomerulosclerosis, and genetic factors such as MYH9 and APOL1 have been involved in the development of proteinuria [4, 7–10]. The progression to end-stage renal disease is at least nearly uniformly accompanied by proteinuria in SCA [4–6].
Microalbuminuria, which occurs in the subclinical phase of SCN, appears in the first decade of life and precedes the appearance of massive and persistent proteinuria. This microalbuminuria has been identified as an early marker for glomerular dysfunction [4, 11]. The reported prevalence of microalbuminuria varies across studies in Western countries, sub-Saharan countries and India [12–16].
Despite the high prevalence and incidence of the disease, sub-Saharan Africa remains one of the geographical areas from which there is limited information about SCN [17–19]. The Democratic Republic of Congo (DRC) is the country second most affected by SCA in Africa. A recent population-based study estimated the incidence to be between 40 000 and 50 000 newborns per year [20]. Tshilolo et al. [21] further showed that Congolese sickle cell patients had low levels of fetal Hb (HbF) and F cells. This situation is considered to be a major risk factor associated with renal complications [4, 22]. Aloni et al. [23] recently reported that among the pediatric population suffering from SCA, 30% had glomerular hyperfiltration and 2% had macroproteinuria. To our knowledge, no information about microalbuminuria in Congolese patients suffering from SCA has been published to date.
Here, we conducted a cross-sectional study among children suffering from SCA. The main goal of this study was to determine the prevalence of microalbuminuria and to identify associated factors. Our ultimate goal was to develop a basis for the implementation of effective preventive interventions. In this report, the prevalence of microalbuminuria and associated factors were estimated in Kinshasa, the capital of the DRC, and the results compared with other reports, especially those concerning African populations.
Materials and methods
Study participants
The cross-sectional study was completed between August 2011 and March 2012 in four health institutions located in Kinshasa, the largest city and capital of the DRC. Kinshasa is administratively divided in to four districts (Lukunga, Mont Amba, Funa and Tshangu). One health institution was selected from each district. These hospitals provide most of the paediatric beds for sickle cell patients. The four paediatric health facilities selected were the University Hospital of Kinshasa (Mont Amba), the Centre de Santé de Mbinza/Delveaux (Lukunga), the Centre Hospitalier de Kingasani (Tshangu) and the Sickle Cell Center of Yolo (Funa). SCA children 2–18 years of age who were attending outpatient clinics at the four health facilities were consecutively enrolled during the period of the study.
Data and sample collection
The following formula was used to estimate the minimum size of the study population: n = Z2pq/d2, where n = sample size, Z = confidence level at 95% (1.96), p = proportion of the target population with microalbuminuria, q = proportion of the target population without the characteristic of the study population (0.89) and d = degree of accuracy (0.05). The prevalence of 11% found recently among Nigerian children was the reference value for this study [18]. The minimum sample size was estimated at 150 children. In this study, 157 children were recruited in total.
All patients were free of pain for at least 15 days and had not been hospitalized or transfused for at least 100 days before the study. Children with prior known proteinuria, hypertension, diabetes, human immunodeficiency virus (HIV), hepatitis C virus and renal and/or cardiovascular diseases were excluded. Children undergoing hydroxyurea (HU) therapy were also excluded. HU increases the levels of HbF and inhibits polymerization by reducing the Hb-S concentration [24]. Thus HU could delay kidney damage by prevention of the development of overt nephropathy or delaying the progression of sickle cell disease nephropathy, as reported by previous studies [24, 25].
All children were examined. The following clinical and laboratory information was collected and analyzed: (i) demographic characteristics, (ii) the number of transfusions, (iii) blood pressure, (iv) creatinine, (v) proteinuria and (vi) microalbuminuria. We used hospital-based data for the number of transfusions and additional information recorded from sickle cell patients, including different sickle cell crises and organ complications that had occurred in the past. Seated blood pressure was measured twice on the left arm after 5 min of rest using a calibrated aneroid sphygmomanometer for paediatric patients (WelchAllyn, Hechingen, Germany) at the heart level. The blood pressure categories were normal, hypertension and hypotension and were defined according to age- and height-specific percentiles, as appropriate [26]. Blood samples were collected from all subjects. Sickle cell screening was performed using the isoelectric focusing technique with the Multiphor II apparatus (GE Healthcare, Buckinghamshire, UK).
Five milliliters of venous blood were drawn from each study participant into an Ethylenediaminetetraacetic acid tube, used to determine hematologic parameters. Hematologic parameters were performed using a Sysmex XS-1000i automated haematology analyzer (Sysmex, Corporation, IL, United States of America).
Three milliliters of blood were drawn into ethylenediaminetetraacetic acid (EDTA) tubes by venipuncture. The plasma, once separated from the blood by centrifugation, was used for estimation of creatinine. No dietary restrictions were imposed. This test was performed in the Clinical Biochemistry Laboratory of the University Hospital of Kinshasa. Creatinine estimation was performed by the enzymatic method with a Cobas C111 apparatus (Roche Instrument Center, Rotkreuz, Switzerland).
For estimated glomerular filtration rate (eGFR) calculation we used the new Q(height)-eGFR equation, which is considered to be an excellent screening tool for kidney disease in 1–25-year-old children, adolescents and young adults: eGFR = 107.3/(SCr/Q), where SCr is serum creatinine (in mg/dL) and with Q = 3.94 – 13.4 × L + 17.6 × L2 – 9.84 × L3 + 2.04 × L4 (L = height in meters) [27]. Hyperfiltration was defined as an eGFR <140 mL/min/1.73 m2 [28].
A morning fresh urine sample was collected in the laboratory from each participant. Urine was directly treated after collection. Standard urine dipstick screening for macroalbuminuria was performed using Combur 10 tests (Roche Diagnostics, Mannheim, Germany). Urine culture was systematically performed for infection or guided by the existence of urinary symptoms. Children were considered to have macroalbuminuria if three consecutive urinalyses were at least 1+ positive.
Urine samples with negative dipstick proteinuria were tested for their urinary albumin excretion rate, which was expressed as the urinary albumin:creatinine ratio using the immuno-turbidimetric method with DCA Bayer 2000R reagent (Siemens Healthcare Diagnostics, Victoria, NSW, Australia). Microalbuminuria was defined as a urine albumin:creatinine ratio of 30–299 mg/g.
For this study, 157 children were recruited, of which 7 were excluded: 1 for the presence of anti-HIV antibodies, 2 for hematuria and 4 for urinary tract infection.
Ethical approval
For every child participant, written informed consent was obtained from parents or legal guardians on behalf of the child. This consent form and the study were reviewed and approved by the National Ethical Committee of the Public Health School of the University of Kinshasa, Kinshasa, DRC, in compliance with the principles of the Helsinki Declaration II. Pseudonyms are used for the children to ensure confidentiality.
Data analysis
The validated data were entered into a computer using EPI info version 6.0 (Centers for Disease Control, Atlanta, Georgia, USA). The analysis was performed using Stata version 14.1 (StataCorp, College Station, Texas, United States of America). Continuous variables were expressed as mean ± SD or median [interquartile range (IQR)] and categorical variables as relative frequency in percent. Patient characteristics were presented by comparing those with and without microalbuminuria. The Levene test was used to check the homogeneity of variance. Continuous variables were compared using the Student’s t-test, the two-sample t-test with unequal variances or the Mann–Whitney U-test as appropriate. For categorical variables, we used the chi-square of Fisher’s exact tests as appropriate. A logarithmic transformation was applied to normalize the distribution of the albumin:creatinine ratio. The associations between age and albumin:creatinine and age versus estimated Glomerular Filtration Rate were measured by the Pearson correlation coefficient and visualized by dispersion patterns.
Logistic regression analysis was used to identify baseline characteristics associated with microalbuminuria. The odds ratio (OR) was estimated for the factors that have a significant effect. P-values <0.05 were considered statistically significant.
Results
A total of 150 patients (77 boys and 73 girls) were analyzed. Their mean age was 8.8 ± 4.3 years. Four patients presented with macroproteinuria. Microalbuminuria was detected in 27/146 (18.5%) sickle cell children.
Table 1 shows the clinical and biological characteristics of the sickle cell children in this study. The mean age was significantly higher in sickle cell children with microalbuminuria than in those without microalbuminuria (10.5 ± 4.3 versus 8.4 ± 4.3 years; P = 0.023). The mean level of albuminuria among patients with microalbuminuria was 78.85 ± 51.29 mg/g compared with 13.80 ± 6.12 mg/g among patients without microalbuminuria. A borderline difference in white blood cell number was observed between the two groups (P = 0.048). The mean of eGFR tended to be higher in sickle cell children with microalbuminuria compared with those without microalbuminuria. However, a statistically significant difference was not observed. Other variables considered did not significantly differ between the two groups (Table 1).
Table 1.
Patient characteristics as a function of microalbuminuria
| Variables | All (n = 150) | No microalbuminuria (n = 123) | Microalbuminuria (n = 27) | P-value |
|---|---|---|---|---|
| Age (years) | 8.8 ± 4.3 | 8.4 ± 4.3 | 10.5 ± 4.3 | 0.023 |
| Gender, boys (%) | 51.3 | 53.7 | 40.7 | 0.224 |
| Number of transfusions | 1.5 (1.0–3.0) | 2.0 (0.0–4.0) | 1.0 (1.0–3.0) | 0.925 |
| BMI (kg/m2) | 14.9 ± 2.3 | 14.8 ± 2.2 | 15.3 ± 2.5 | 0.348 |
| Blood pressure (%) | 0.253 | |||
| No hypertension | 91.3 | 92.7 | 85.2 | |
| Hypertension | 8.7 | 7.3 | 14.8 | |
| Hemoglobin (g/dL) | 7.2 ± 1.1 | 7.2 ± 1.1 | 7.5 ± 1.2 | 0.115 |
| White blood cells (×10³/μL) | 11.9 ± 3.5 | 12.1 ± 3.6 | 10.7 ± 2.7 | 0.048 |
| Serum creatinine (mg/dL) | 0.4 (0.3–0.5) | 0.4 (0.3–0.5) | 0.3 (0.3–0.6) | 0.771 |
| eGFR (mL/min/1.73m2) | 123 ± 51 | 119 ± 47 | 139 ± 65 | 0.071 |
Data are expressed as mean ± SD, median (IQR) or percentage.
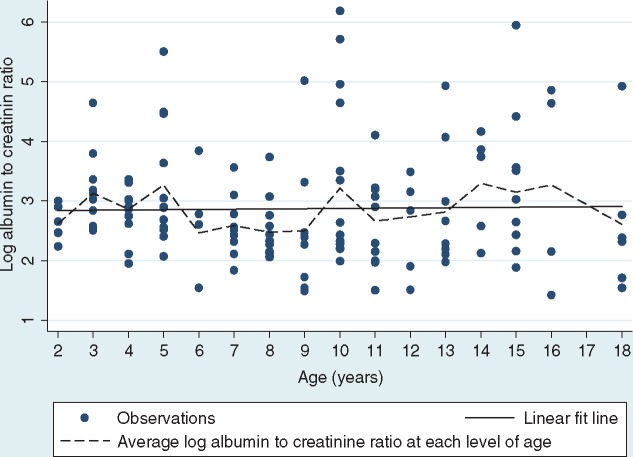
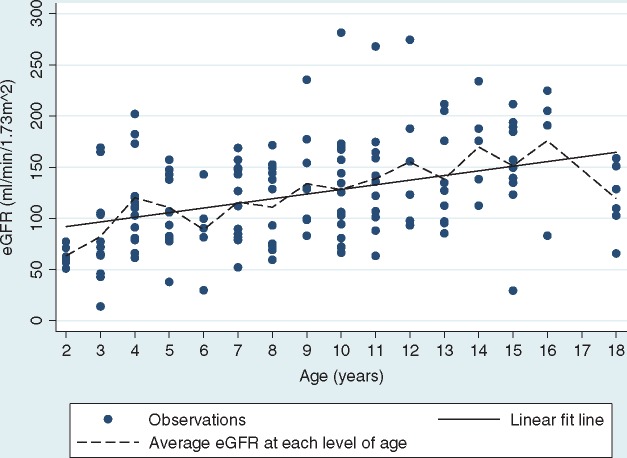
No significant relationship was found between age and the log albumin:creatinine ratio (Pearson r = 0.02; P = 0.814) (Figure 1). On the other hand, a positive association was observed between eGFR and age (Pearson r = 0.39; P < 0.001) (Figure 2).
Fig. 1.
Relationship between logarithm of albumin:creatinine ratio and age.
Fig. 2.
Relationship between eGFR and age.
Table 2 presents an analysis of the determinants of microalbuminuria. In univariate analysis, the age of the children and the number of white blood cells were significantly associated with microalbuminuria.
Table 2.
Determinants of microalbuminuria
| Variables | Simple logistic regression |
Multivariate logistic regression |
||
|---|---|---|---|---|
| Crude OR (95% CI) | P-value | Adjusted OR (95% CI) | P-value | |
| Age | 1.12 (1.01–1.23) | 0.026 | 1.11 (1.00–1.22) | 0.042 |
| Gender, boys versus girls | 0.59 (0.26–1.38) | 0.227 | – | – |
| BMI | 1.08 (0.89–1.29) | 0.490 | – | – |
| Hypertension, yes versus no | 2.20 (0.63–7.77) | 0.219 | – | – |
| Hemoglobin | 1.36 (0.93–1.98) | 0.116 | – | – |
| White blood cells | 0.86 (0.75–0.99) | 0.050 | 0.88 (0.76–1.01) | 0.078 |
| eGFR | 1.01 (0.99–1.02) | 0.075 | – | |
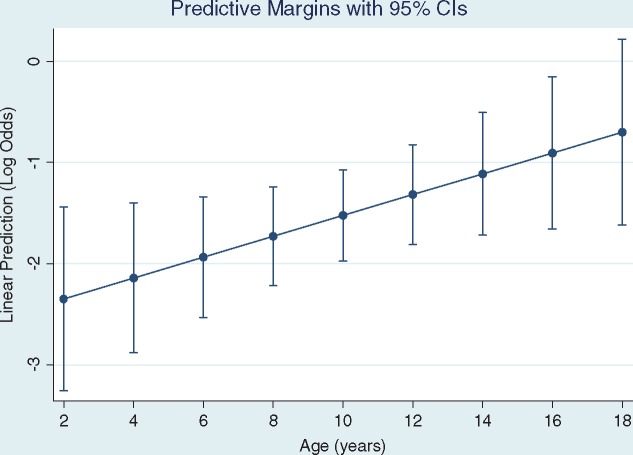
A multiple logistic regression model was developed to adjust the age and number of white blood cells that were significantly associated with microalbuminuria in simple analysis. In this model, only age emerged as an independent determinant of microalbuminuria. The rating of microalbuminuria increased by 1.11 with each year of age. This relationship is clearly shown in Figure 3, which shows a linear increase in log10 of microalbuminuria as a function of age. We tested the quadratic term of age in this model but it was not significant.
Fig. 3.
Log10 of microalbuminuria by age.
Discussion
To our knowledge, no previous reports have analyzed renal dysfunction and microalbuminuria in children suffering from SCA in the DRC. In the present report, the prevalence and determinants of microalbuminuria in sickle cell patients are assessed in this part of the world for the first time.
The prevalence of microalbuminuria was 18.5% in this cohort. Similar results have been reported in several studies worldwide with frequencies ranging from 18 to 23% [11–15, 18, 29–33], but lower than the 27 and 28% reported in a cohort of patients from Cameroon, Côte d'Ivoire, Mali and Senegal [34] and in Uganda [19]. However, the low frequency of microalbuminuria in the present study can probably be associated with environmental and genetics factors, as well as the age of the study population, as presented in Table 3. Due to the absence of a national registry in the DRC, further studies are required to confirm and identify the genetic and environmental factors contributing to the low prevalence of microalbuminuria in the Congolese sickle cell pediatric population.
Table 3.
Results of the literature review of microalbuminuria in sickle cell paediatric African series
| Source | Our study | Imuetinyan et al. [18] | Mawanda et al. [19] | Ranque et al. [34] | Ranque et al. [34] | Ranque et al. [34] | Ranque et al. [34] |
| Country | Kinshasa, DRC | Nigeria (16) | Kampala, Uganda (17) | Yaoundé, Cameroon (30) | Abidjan, Ivory Coast (30)a | Bamako, Mali (30)a | Dakar, Senegal (30)a |
| Nature of the study | Cross-sectional | Cross-sectional and descriptive | Cross-sectional | Cross-sectional | Cross-sectional | Cross-sectional | Cross-sectional |
| Study period | August 2011 and March 2012 | November 2006– February 2007 | November 2007 and April 2008 | February 2011– December 2013 | February 2011– December 2013 | February 2011– December 2013 | February 2011– December 2013 |
| Number of patients | 150 | 69 | 305 | 503 | 337 | 404 | 668 |
| Frequency, male (%) | 49 | 61 | 48 | 50 | 44 | 43 | 46 |
| Age (years) | Median 8 (IQR 2–18) | 8.8 (±4.7) | 9.7 (±4.9) | Median 13 (IQR 7–21) | Median 15 (IQR 10–23) | Median 17 (IQR 11–24) | Median 15 (IQR 10–22) |
| Prevalence of microalbuminuria (%) | 18.5 | 20.3 | 28.2 | 39 | 16 | 38 | 23 |
Cohort including Sβ0 patients.
In our study, the mean age of the sickle cell children with microalbuminuria was significantly higher compared with children without microalbuminuria (10.5 ± 4.2 years). Furthermore, the proportion of microalbuminuria in sickle cell children >9 years of age was 66.7%. Our findings corroborate those of a previous study, which reported 62% of microalbuminuria among children >10 years of age in the USA [29] .
The probability of having microalbuminuria increased with age (P = 0.009) in our cohort. This trend has been previously reported by others. Wigfall et al. [30] reported a prevalence of 6.2% among children of pediatric age, 10% among adolescents and >17% in adults. McPherson Yee et al. [35] similarly reported a prevalence of 6.9% among patients from 2 to 6 years of age, 20.0% for those from 7 to 12 years of age and 35.7% from 13 to 19 years of age {OR 1.43 [95% confidence interval (CI) 1.20–1.71]; P < 0.0001}. Age is a factor that is well-known to be associated with the risk of kidney damage in sickle cell patients [4]. Glomerular obsolescence and a decline in renal vascular flow are counted among the factors that explain the involvement of age in the progression of renal disease [4, 36]. Puberty is also an important factor in this process, as from the onset of puberty there is increased expression of the Insulin-like growth factor 1 (IGF-1) cytokine and growth hormone [37].
In the present report, the mean occurrence of microalbuminuria was around ∼4 years of age. Hence, in our cohort, the mean age of occurrence of microalbuminuria was earlier compared with the average of 7 years of age in the medical literature [12, 13].
In this study, the youngest child with microalbuminuria was 2 years of age. In their series, Marsenic et al. reported the presence of microalbuminuria in children of 2 and 4 years of age [29, 38]. However, Dharnidharka et al. [12] and McBurney et al. [13] found no microalbuminuria in children <7 years of age in the USA. Our findings suggest that microalbuminuria may occur very early in sickle cell children in the DRC. This early achievement of glomerular function could be explained by the presence of related factors including genetic predisposition, the low levels of HbF and F cells in Congolese patients, environmental factors and the quality of care and lifestyle in a developing country [20, 39]. These factors have not been studied in this report.
In this investigation, the occurrence of microalbuminuria tended to be higher in girls (23%) than in boys (13.9%). Similar observations have been reported in previous studies [12, 18, 32]. Nevertheless, there was no statistically significant relationship between microalbuminuria and gender in these reports. In studies where females have been suggested to be more likely to develop microalbuminuria, the girls had a more precocious puberty than boys. This puberty could play a determining role in the development of microalbuminuria due to increased expression of IGF-1 [37].
Systolic blood pressure was significantly higher in the SCA children with microalbuminuria (100 mmHg) than in SCA children without microalbuminuria (90 mmHg). In contrast, the difference between the two groups was not significant in the results previously reported in Nigeria [18]. The differences between these two reports from African countries presumably arise from differences in environmental and genetic factors that influence blood pressure in sickle cell populations and strongly suggest that more research in African countries would provide valuable insights into the determinants of blood pressure. Concerning the potential relationship between systolic blood pressure and microalbuminuria, it has previously been suggested that relative systemic hypertension defines a category of relative systemic hypertension in sickle cell patients that is associated with the risk of chronic kidney disease [40]. Bartolucci et al. [41] have further shown that the baseline–follow-up albumin:creatinine ratio decline is strongly associated with decreases in systolic blood pressure. In addition, decreased albuminuria under HU is associated with a reduction of systolic blood pressure between baseline and follow-up levels.
In multivariate analysis, age emerged as the major determinant of microalbuminuria. Similar results have been previously reported in the literature [15, 19, 29, 32, 42]. However, the occurrence of microalbuminuria is associated with other factors such as lower hemoglobin concentration, higher mean corpuscular volume, higher leukocyte count, hypertension and acute chest syndrome, stroke and cholelithiasis in the medical literature [19, 30, 33, 43]. The differences in the factors associated with microalbuminuria in these studies presumably arise from differences in environmental and genetic factors. Wigfall et al. [30] and others conducted their studies at centers that use a lot of HU and likely had patients with high HbF. Ranque et al. [34] included two main phenotype groups [SS and Sβ0; SC and Sβ+] in their cohort. These differences suggest that more research in developing countries would provide valuable insights into the pathogenesis of microalbuminuria in children with SCA.
Strengths and weaknesses of the study
The present study has some limitations. These include its hospital-based cross-sectional design, which limits its potential to make conclusions. One additional limitation is that biological measurements were performed once during the study. Another is the use of the Schwartz equation to estimate GFR, as well as the lack of hemolysis markers known to alter renal hemodynamics. Despite these limitations, these data provide insights into the relationship between microalbuminuria and SCA. These data, if properly used for advocacy as well as for developing future strategies for the prevention and management of SCN, will be of great importance for countries with a high prevalence of the disease. Further investigation, including the analysis of HbF levels and genetic data, should yield interesting results.
Conclusion
This study allowed us to improve our knowledge about the renal complications in sickle cell children living in Central Africa. The present report shows that the prevalence of proteinuria increased from 2 to 19% using the dipstick method and the determination of microalbuminuria. The prevalence of microalbuminuria was lower than reported in eastern and western African countries. Age emerged as a unique factor associated with microalbuminuria in SCA children in the DRC.
Conflicts of interest statement
None declared.
Acknowledgements
The authors thank the patients who participated in this study. We thank all our colleagues involved in the collection of samples, including all the nurses and technicians of the Sickle Cell Center Centre de Médecine Mixte et d'Anémie SS, for the support given for the present study.
References
- 1. Quinn CT. Sickle cell disease in childhood: from newborn screening through transition to adult medical care. Pediatr Clin North Am 2013; 60: 1363–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Powars DR, Chan LS, Hiti A. et al. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005; 84: 363–376 [DOI] [PubMed] [Google Scholar]
- 3. Powars DR, Elliott-Mills DD, Chan L. et al. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med 1991; 115: 614–620 [DOI] [PubMed] [Google Scholar]
- 4. Scheinman JI. Sickle cell disease and the kidney. Nat Clin Pract Nephrol 2009; 5: 78–88 [DOI] [PubMed] [Google Scholar]
- 5. Guasch A, Navarrete J, Nass K. et al. Glomerular involvement in adults with sickle cell hemoglobinopathies: prevalence and clinical correlates of progressive renal failure. J Am Soc Nephrol 2006; 17: 2228–2235 [DOI] [PubMed] [Google Scholar]
- 6. Guasch A, Cua M, Mitch WE.. Early detection and the course of glomerular injury in patients with sickle cell anemia. Kidney Int 1996; 49: 786–791 [DOI] [PubMed] [Google Scholar]
- 7. Ataga KI, Derebail VK, Archer DR.. The glomerulopathy of sickle cell disease. Am J Hematol 2014; 89: 907–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Emokpae MA, Uadia PO.. Association of oxidative stress markers with atherogenic index of plasma in adult sickle cell nephropathy. Anemia 2012; 2012: 767501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Falk RJ, Scheinman J, Phillips G. et al. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med 1992; 326: 910–915 [DOI] [PubMed] [Google Scholar]
- 10. Ashley-Koch AE, Okocha EC, Garrett ME. et al. MYH9 and APOL1 are both associated with sickle cell disease nephropathy. Br J Haematol 2011; 155: 386–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Datta V, Ayengar JR, Karpate S. et al. Microalbuminuria as a predictor of early glomerular injury in children with sickle cell disease. Indian J Pediatr 2003; 70: 307–309 [DOI] [PubMed] [Google Scholar]
- 12. Dharnidharka VR, Dabbagh S, Atiyeh B. et al. Prevalence of microalbuminuria in children with sickle cell disease. Pediatr Nephrol 1998; 12: 475–478 [DOI] [PubMed] [Google Scholar]
- 13. McBurney PG, Hanevold CD, Hernandez CM. et al. Risk factors for microalbuminuria in children with sickle cell anemia. J Pediatr Hematol Oncol 2002; 24: 473–477 [DOI] [PubMed] [Google Scholar]
- 14. McKie KT, Hanevold CD, Hernandez C. et al. Prevalence, prevention, and treatment of microalbuminuria and proteinuria in children with sickle cell disease. J Pediatr Hematol Oncol 2007; 29: 140–144 [DOI] [PubMed] [Google Scholar]
- 15. Alvarez O, Montane B, Lopez G. et al. Early blood transfusions protect against microalbuminuria in children with sickle cell disease. Pediatr Blood Cancer 2006; 47: 71–76 [DOI] [PubMed] [Google Scholar]
- 16. Becker AM, Goldberg JH, Henson M. et al. Blood pressure abnormalities in children with sickle cell anemia. Pediatr Blood Cancer 2014; 61: 518–522 [DOI] [PubMed] [Google Scholar]
- 17. Iwalokun BA, Iwalokun SO, Hodonu SO. et al. Evaluation of microalbuminuria in relation to asymptomatic bacteruria in Nigerian patients with sickle cell anemia. Saudi J Kidney Dis Transpl 2012; 23: 1320–1330 [DOI] [PubMed] [Google Scholar]
- 18. Imuetinyan BA, Okoeguale MI, Egberue GO.. Microalbuminuria in children with sickle cell anemia. Saudi J Kidney Dis Transpl 2011; 22: 733–738 [PubMed] [Google Scholar]
- 19. Mawanda M, Senkusu JM, Odiit A. et al. Micro-albuminuria in Ugandan children with sickle cell anaemia: a cross-sectional study. Ann Trop Paediatr 2011; 31: 115–121 [DOI] [PubMed] [Google Scholar]
- 20. Piel FB, Hay SI, Gupta S. et al. Global burden of sickle cell anaemia in children under five, 2010–2050: modelling based on demographics, excess mortality, and interventions. PLoS Med 2013; 10: e1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tshilolo L, Summa V, Gregorj C. et al. Foetal haemoglobin, erythrocytes containing foetal haemoglobin, and hematological features in congolese patients with sickle cell anaemia. Anemia 2012; 2012: 105349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rusanova I, Escames G, Cossio G. et al. Oxidative stress status, clinical outcome, and β-globin gene cluster haplotypes in pediatric patients with sickle cell disease. Eur J Haematol 2010; 85: 529–537 [DOI] [PubMed] [Google Scholar]
- 23. Aloni MN, Ngiyulu RM, Gini-Ehungu JL. et al. Renal function in children suffering from sickle cell disease: challenge of early detection in highly resource-scarce settings. PLoS One 2014; 9: e96561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aygun B, Mortier NA, Smeltzer MP. et al. Hydroxyurea treatment decreases glomerular hyperfiltration in children with sickle cell anemia. Am J Hematol 2013; 88: 116–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laurin LP, Nachman PH, Desai PC. et al. Hydroxyurea is associated with lower prevalence of albuminuria in adults with sickle cell disease. Nephrol Dial Transplant 2014; 29: 1211–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Heart, Lung, and Blood Institute, 2005
- 27. Hoste L, Dubourg L, Selistre L. et al. A new equation to estimate the glomerular filtration rate in children, adolescents and young adults. Nephrol Dial Transplant 2014; 29: 1082–1091 [DOI] [PubMed] [Google Scholar]
- 28. Piepsz A, Tondeur M, Ham H.. Revisiting normal 51Cr-ethylenediaminetetraacetic acid clearance values in children. Eur J Nucl Med Mol Imaging 2006; 33: 1477–1478 [DOI] [PubMed] [Google Scholar]
- 29. Marsenic O, Couloures KG, Wiley JM.. Proteinuria in children with sickle cell disease. Nephrol Dial Transplant 2008; 23: 715–720 [DOI] [PubMed] [Google Scholar]
- 30. Wigfall DR, Ware RE, Burchinal MR. et al. Prevalence and clinical correlates of glomerulopathy in children with sickle cell disease. J Pediatr 2000; 136: 749–753 [PubMed] [Google Scholar]
- 31. Bayazit AK, Noyan A, Aldudak B. et al. Renal function in children with sickle cell anemia. Clin Nephrol 2002; 57: 127–130 [DOI] [PubMed] [Google Scholar]
- 32. King L, MooSang M, Miller M. et al. Prevalence and predictors of microalbuminuria in Jamaican children with sickle cell disease. Arch Dis Child 2011; 96: 1135–1139 [DOI] [PubMed] [Google Scholar]
- 33. Becton LJ, Kalpatthi RV, Rackoff E. et al. Prevalence and clinical correlates of microalbuminuria in children with sickle cell disease. Pediatr Nephrol 2010; 25: 1505–1511 [DOI] [PubMed] [Google Scholar]
- 34. Ranque B, Menet A, Diop IB. et al. Early renal damage in patients with sickle cell disease in sub-Saharan Africa: a multinational, prospective, cross-sectional study. Lancet Haematol 2014; 1: e64–e73 [DOI] [PubMed] [Google Scholar]
- 35. McPherson Yee M, Jabbar SF. et al. Chronic kidney disease and albuminuria in children with sickle cell disease. Clin J Am Soc Nephrol 2011; 6: 2628–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nath KA, Hebbel RP.. Sickle cell disease: renal manifestations and mechanisms. Nat Rev Nephrol 2015; 11: 161–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bangstad HJ, Dahl-Jørgensen K, Kjaersgaard P. et al. Urinary albumin excretion rate and puberty in non-diabetic children and adolescents. Acta Paediatr 1993; 82: 857–862 [DOI] [PubMed] [Google Scholar]
- 38. Osei-Yeboah CT, Rodrigues O.. Renal status of children with sickle cell disease in Accra, Ghana. Ghana Med J. 2011; 45: 155–160 [PMC free article] [PubMed] [Google Scholar]
- 39. Cochat P, Mourani C, Exantus J. et al. Pediatric nephrology in developing countries. Med Trop (Mars) 2009; 69: 543–547 [PubMed] [Google Scholar]
- 40. Gordeuk VR, Sachdev V, Taylor JG. et al. Relative systemic hypertension in patients with sickle cell disease is associated with risk of pulmonary hypertension and renal insufficiency. Am J Hematol 2008; 83: 15–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bartolucci P, Habibi A, Stehlé T. et al. Six months of hydroxyurea reduces albuminuria in patients with sickle cell disease. J Am Soc Nephrol 2016; 27: 1847–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Osei-Yeboah CT, Rodrigues O.. Renal status of children with sickle cell disease in Accra, Ghana. Ghana Med J 2011; 45: 155–160 [PMC free article] [PubMed] [Google Scholar]
- 43. Abo-Zenah H, Moharram M, El Nahas AM.. Cardiorenal risk prevalence in sickle cell hemoglobinopathy. Nephron Clin Pract 2009; 112: c98–c106 [DOI] [PubMed] [Google Scholar]