Abstract
Background
The live smallpox and Bacillus Calmette-Guérin (BCG) vaccinations have been associated with better adult survival in both Guinea-Bissau and Denmark. In Guinea-Bissau, human immunodeficiency virus (HIV)-1 became an important cause of death after smallpox vaccination was phased out globally in 1980. We hypothesised that smallpox and BCG vaccinations were associated with a lower prevalence of HIV-1 infection, and we tested this hypothesis in both Guinea-Bissau and Denmark.
Methods
We conducted 2 studies: (1) a cross-sectional study of HIV infection and vaccination scars in Guinea-Bissau including 1751 individuals and (2) a case-base study with a background population of 46239 individuals in Denmark. In Guinea-Bissau, HIV-1 transmission was almost exclusively sexually transmitted. In Denmark, we excluded intravenous drug users. Data were analyzed using logistic regression.
Results
Bacillus Calmette-Guérin and/or smallpox vaccination compared with neither of these vaccines was associated with an adjusted odds ratio (aOR) for HIV-1 of 0.62 (95% confidence interval [CI], 0.36–1.07) in Guinea-Bissau and 0.70 (95% CI, 0.43–1.15) in Denmark. We combined the results from both settings in a meta-analysis (aOR = 0.66; 95% CI, 0.46–0.96). Data from Guinea-Bissau indicated a stronger effect of multiple smallpox vaccination scars (aOR = 0.27; 95% CI, 0.10–0.75) as follows: women, aOR = 0.18 (95% CI, 0.05–0.64); men, aOR = 0.52 (95% CI, 0.12–2.33); sex-differential effect, P = .29.
Conclusions
The studies from Guinea-Bissau and Denmark, 2 very different settings, both suggest that the BCG and smallpox vaccines could be associated with a decreased risk of sexually transmitted HIV-1. It might be informative to pursue this observation and explore possible protective mechanisms as part of the search for an HIV-1 vaccine.
Keywords: BCG vaccine, heterologous immunity, HIV-1, nonspecific effects of vaccines, smallpox vaccine
In 1980, the world ceased to use the live-attenuated smallpox vaccine [1]. Previous studies did not investigate whether stopping smallpox vaccine affected general health. However, subsequent studies have shown that live vaccines may have beneficial nonspecific or heterologous effects (NSEs), which may be mediated via training of the innate immune system and provide cross-protection against unrelated pathogens [2, 3].
With respect to potential NSEs of smallpox vaccine, 2 studies in Guinea-Bissau showed lower mortality among individuals with smallpox vaccination scars compared with individuals with no vaccination scars 20–25 years after smallpox vaccination stopped [4, 5]. The beneficial association increased with the number of smallpox vaccination scars and was most pronounced in women. In Denmark, register-based studies showed that smallpox vaccination was associated with reduced incidence of all-cause infectious disease hospitalization [6], and smallpox vaccination as well as Bacillus Calmette-Guérin (BCG) vaccinations were associated with lower risk of death due to natural causes [7].
Human immunodeficiency virus (HIV)-1 was first registered in Guinea-Bissau in the late 1980s after smallpox vaccination had stopped [1]. Human immunodeficiency virus-1 subsequently became a common cause of adult death. With the failure to produce an effective HIV vaccine, and with the knowledge that smallpox vaccine reduced all-cause mortality, we hypothesized that having been smallpox vaccinated was associated with protection against contracting HIV-1 infection. Bacillus Calmette-Guérin may have similar NSEs [7], and, in Denmark, smallpox vaccine and BGC vaccine were phased out at the same time, and effects were difficult to disentangle. Therefore, we examined whether BCG and smallpox vaccine were associated with a lower prevalence of HIV-1 in Guinea-Bissau and Denmark.
METHODS
Our research comprises a cross-sectional study from Guinea-Bissau and a case-based study from Denmark.
The Cross-Sectional Study From Guinea-Bissau
The Bandim Health Project maintains a health and demographic surveillance system in Guinea-Bissau. This includes registration of all pregnancies and childbirths as well as a full census of the total study population every 2 or 3 years. The cohort from Guinea-Bissau was surveyed in an HIV prevalence survey (2004–2006) followed by a vaccination scar survey (2005–2007).
The “HIV prevalence survey” was conducted in 3 districts between May 2004 and December 2006. The study included 384 randomly selected houses (10% sample of houses), and all individuals aged 15 years or older in these houses were interviewed [8]. There was a parituclar focus on older individuals; therefore, the survey also included individuals aged 50 years or older who lived in houses that were not included in the random sample of houses. In total, 79% of eligible individuals had HIV status examined [8]. Similar to previous surveys, the proportion of HIV-tested individuals was higher among women (85%) than men (71%) [8]. Antiretroviral treatment was not generally available in Bissau when the survey was conducted. The National Laboratory in Guinea-Bissau used Enzygnost HIV-1/HIV-2 Plus ELISA (Behring, Marburg, Germany) to screen for HIV-1 and HIV-2 infection. Confirmation of positive sera was performed using Capillus HIV-1/HIV-2 (Cambridge Biotech Limited, Galway, Ireland) and Immunocomb II HIV-1 and -2 BiSpot RST (Orgenics, Yavne, Israel) [8]. We analyzed HIV-1-positive tests.
The “vaccination scar survey” was conducted between January 2005 and May 2007. Three field workers examined vaccination scars and interviewed study participants. The field workers examined upper arms for scars and registered up to 5 scars. Scars were classified as BCG, smallpox vaccination, or uncertain based on size, color, and general appearance of the scar. The circumference of the scar was marked with a pen: transparent adhesive tape was placed over the marking and then transferred to the questionnaire.
The questionnaires from the HIV survey and the vaccination scar survey were double entered, and scar sizes were assessed by the same data entry clerk. The scar size was calculated as the mean of the sum of the diameters. Sixty-two (3.5%) individuals had a difference in birth date of more than 5 years in the 2 surveys, and this was mostly among the elderly. We considered the scar survey to be most correct and retrieved year of birth from this source. The scar survey questionnaire included background information on year of birth, sex, field worker, place of birth, schooling, religion, ethnicity, employment (private sector, state sector, or military services), travels abroad, and living outside Guinea-Bissau before 1980. Census information about quality of house (zinc roof, electricity, indoor toilet) and ownership of television was used to define a socioeconomic status variable (0–4 items).
The Case-Based Study From Denmark
The case-based study from Denmark used a background cohort of all children born in 1965–1976 and attending school in Copenhagen. This cohort experienced the phase out of BCG and smallpox vaccinations from the Danish vaccination program (vaccination policies are explained elsewhere [7]). School health examination records were archived in the Copenhagen Municipal Archives with personal identification numbers (ID) and information on anthropometry, social and health conditions, and vaccinations. The ID enabled us to link individuals to national vital and health registers. We sampled all HIV cases in the entire background cohort (Figure 1), as identified from the Danish National Patient Registry (DNPR) using the following International Classification of Diseases (ICD) codes: 07983 (ICD-8, 1983–1993) and B20-B24 (ICD-10 from 1994 onwards). More than 99.7% of Danish HIV cases are HIV-1, and the Danish healthcare system provides free HIV treatment [9]. Vaccination information and potential confounders were digitalized from the school health records for all cases and compared with already digitalized information from a subcohort comprising 13% of the background cohort. Vital information (ie, date of birth, death, and emmigration) came from the Danish Civil Registration System.
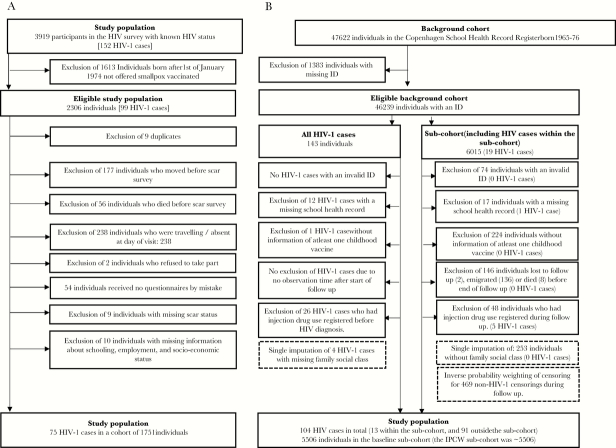
Figure 1.
Flow chart of the study population in (A) Guinea-Bissau and (B) Denmark.
Sexually Transmitted Human Immunodeficiency Virus-1
The route of transmission may be important to understand whether BCG and smallpox vaccination have effects against HIV-1 infection. Human immunodeficiency virus-1 is almost exclusively sexually transmitted in Guinea-Bissau: injection drug use is virtually nonexistent in Guinea-Bissau [10], and blood transfusions have been screened for HIV since 1987 [8]. In Denmark, blood transfusions was screened for HIV since 1986, and injection drug use is a common cause of HIV-1 [9]. For similarity, in the Danish data, we excluded individuals if they were registered with diagnoses indicating injection drug use in DNPR during follow up.
Statistical Analyses
For both studies, we present the potential risk factors for being smallpox and BCG vaccinated as prevalence ratios (PRs) adjusted for sex and year of birth using Poisson regression with robust variance estimates [11]. Logistic regression models were used to estimate the odds ratio (OR) for the association between vaccination scar status and HIV-1 infection. Assuming a causal structure, we identified potential confounders in the fully adjusted models, which included year of birth in 10-year intervals, sex, field worker, place of birth, schooling, religion, ethnicity, employment, socioeconomic status, traveled abroad, and lived abroad (Guinea-Bissau) or family social class, sex, immigration, and year of birth (Denmark). We accounted for house clustering in the study from Guinea-Bissau and for the sampling approach related to sex and year of birth for the Danish study with robust variance estimates [6]. For the Danish study, we calculated the association in adjusted OR (aOR) by applying a case-base design [12]. Missing information about family social class was single imputed for 4 HIV cases and 256 non-HIV cases to prevent bias occurring from restricting the cohort to individuals with full information. Entry was at 15 years of age or January 1, 1983 (when HIV/acquired immune deficiency syndrome was part of ICD-8), whichever occurred last. End of follow up was the last update of the DNPR (December 31, 2014). Individuals were censored if they had ever been registered with injection drug use, emigrated, died, or had unknown whereabouts according to the Danish Civil Registration System during follow up and before HIV-1 registration. We used (1) inverse probability-of-censoring weighting (IPCW) based on potential common causes to exposure and (2) censoring among controls to handle non-HIV censoring before end of follow up [13]. The results from both studies were combined in random effect meta-estimates, interpreted as an average of different true effects between studies [14].
Previous studies have shown that the beneficial effect of smallpox vaccination on mortality increased with the number of smallpox vaccination scars [15]; therefore, we tested the effect of multiple smallpox vaccination scars in the Guinea-Bissau study. Information on the number of smallpox vaccines was not available in the Danish data.
We conducted several analyses to test the robustness of the conclusions. At the exploratory stage, P values should not be “corrected” for multiple testing [16]. In our complementary analysis of Guinean and Danish data, we used a stabilized inverse probability of treatment weighted model (sIPTW) to give association measures closer to a randomized controlled trial in a similar population (see additional information in Supplementary Analysis 1) [13]. As a supplementary analysis to the Danish study, we conducted the commonly reported hazard ratio using a Cox proportional hazards model for case-cohorts with age as the underlying time scale to estimate the hazard ratio (HR) of HIV-1 by BCG and smallpox vaccination status. The proportional hazards assumption was tested with Schoenfeld residuals. All statistical analyses were conducted using Stata 14.1 (StataCorp LP, College Station, TX).
Ethics
The HIV survey and the vaccination scar survey in Guinea-Bissau were approved by the Ministry of Health’s Ethics Committee in Guinea-Bissau, and the Danish Central Scientific Ethics Committee gave its consultative approval. All participants were counseled and provided informed verbal consent before HIV testing [8]. The field workers conducting the surveys were not aware of the HIV status of the participants. The Danish Data Protection Agency approved the Danish study: registry-based research does not require approval by an ethics committee in Denmark.
RESULTS
The Guinea-Bissau study included 1751 individuals with 75 HIV-1 cases (Figure 1), and the analyzed study population in Denmark was derived from an eligible background cohort of 46 239 individuals, using 5506 individuals as a subcohort with 104 HIV-1 cases (Figure 1).
Guinea-Bissau
Two thousand three hundred six individuals born before January 1, 1974 were eligible for this study, because they had a probability of being smallpox vaccinated (Figure 1). On average, there was more than 1 year between the HIV survey and the subsequent scar examination, thus 1751 individuals (76% of the eligible population) were analyzed (Figure 1). The prevalence of HIV-1 was 4.3% in both the analyzed study population and the eligible population. Seventy-five of the included individuals were HIV-1 infected. The female/male prevalence ratio of HIV-1 was 1.53 (0.97–2.42), and 5% women and 3% men were infected with HIV-1. Supplementary Figure 1 shows the age distribution of HIV-1-positive individuals.
In the population included in the study, 22% (393) had no vaccination scars, 46% (808) had smallpox vaccination scars only, 11% (191) had BCG vaccination scars only, and 21% (359) had both BCG and smallpox vaccination scars. The median diameter of scars classified as smallpox scars was 15.0 mm (25–75 percentiles: 11.5–19.3) and 5.0 mm (25–75 percentiles: 4.0–6.5 mm) for BCG vaccination. Sixty-eight percent of men and 66% of women had a smallpox vaccination scar (10-year age interval adjusted PR = 0.99 [0.77–1.18]), whereas 32% of men and 31% of women had BCG vaccination scars (10-year age interval adjusted PR = 0.92 [0.78–1.09]) (Table 1). Twenty-five percent (445 of 1751) of the study population had 2 or more smallpox vaccination scars.
Table 1.
Baseline Variables by Smallpox and BCG Vaccination Scar Status in Guinea-Bissau
| n = 1751 | Smallpox Vaccination Scar | BCG Vaccination Scar | ||||||
|---|---|---|---|---|---|---|---|---|
| No Smallpox Vaccination Scars | Smallpox Vaccination Scars | aPR (95% CI)a | P Valueb | No BCG Vaccination Scars | BCG Vaccinaion Scars | aPR (95% CI)a | P Valueb | |
| n (%) | n (%) | n (%) | n (%) | |||||
| Sex | ||||||||
| Male | 218 (32%) | 469 (68%) | 1 (ref) | .81 | 464 (68%) | 223 (32%) | 1 (ref) | .35 |
| Female | 366 (34%) | 698 (66%) | 0.99 (0.88–1.11) | 737 (69%) | 327 (31%) | 0.92 (0.78–1.09) | ||
| BCG Vaccination Scar | ||||||||
| No | 393 (33%) | 808 (67%) | 1 (ref) | .76 | ||||
| Yes | 191 (35%) | 359 (65%) | 1.02 (0.90–1.16) | |||||
| Smallpox Vaccination Scar | ||||||||
| No | 393 (67%) | 191 (33%) | 1 (ref) | .65 | ||||
| Yes | 808 (69%) | 359 (31%) | 1.04 (0.86–1.26) | |||||
| Assistent | ||||||||
| A | 108 (28%) | 279 (72%) | 1 (ref) | .04 | 266 (69%) | 121 (31%) | 1 (ref) | <.01 |
| B | 424 (36%) | 739 (64%) | 0.86 (0.75–0.98) | 834 (72%) | 329 (28%) | 0.96 (0.78–1.18) | ||
| C | 52 (26%) | 149 (74%) | 1.01 (0.83–1.23) | 101 (50%) | 100 (50%) | 1.63 (1.25–2.12) | ||
| Birth Place | ||||||||
| Bissau city | 215 (39%) | 341 (61%) | 1 (ref) | .47 | 369 (66%) | 187 (34%) | 1 (ref) | .76 |
| Elsewhere | 369 (31%) | 826 (69%) | 1.05 (0.92–1.19) | 832 (70%) | 363 (30%) | 0.97 (0.81–1.16) | ||
| School level | ||||||||
| No school | 263 (33%) | 530 (67%) | 1 (ref) | .13 | 603 (76%) | 190 (24%) | 1 (ref) | <.01 |
| Primary | 136 (27%) | 377 (73%) | 1.15 (0.99–1.33) | 353 (69%) | 160 (31%) | 1.26 (1.01–1.58) | ||
| Secondary | 185 (42%) | 260 (58%) | 1.16 (0.96–1.40) | 245 (55%) | 200 (45%) | 1.51 (1.17–1.95) | ||
| Religion | ||||||||
| Animist | 125 (35%) | 232 (65%) | 1 (ref) | .30 | 274 (77%) | 83 (23%) | 1 (ref) | .10 |
| Catholic or Protestant | 195 (30%) | 464 (70%) | 1.14 (0.97–1.34) | 429 (65%) | 230 (35%) | 1.35 (1.04–1.73) | ||
| Muslim | 75 (36%) | 134 (64%) | 1.03 (0.83–1.28) | 134 (64%) | 75 (36%) | 1.37 (1.00–1.88) | ||
| Other | 189 (36%) | 337 (64%) | 1.03 (0.87–1.21) | 364 (69%) | 162 (31%) | 1.19 (0.91–1.55) | ||
| Ethnicity | ||||||||
| Papel | 280 (34%) | 542 (66%) | 1 (ref) | .04 | 575 (70%) | 247 (30%) | 1 (ref) | .81 |
| Manjaco | 52 (24%) | 165 (76%) | 1.17 (0.99–1.40) | 145 (67%) | 72 (33%) | 1.10 (0.84–1.43) | ||
| Balanta | 86 (46%) | 103 (54%) | 0.82 (0.66–1.01) | 124 (66%) | 65 (34%) | 1.10 (0.84–1.45) | ||
| Other | 166 (32%) | 357 (68%) | 1.04 (0.91–1.19) | 357 (68%) | 166 (32%) | 1.07 (0.88–1.31) | ||
| Work | ||||||||
| Unemployed | 270 (37%) | 454 (63%) | 1 (ref) | .41 | 510 (70%) | 214 (30%) | 1 (ref) | .51 |
| Employed | 228 (35%) | 416 (65%) | 1.05 (0.92–1.20) | 424 (66%) | 220 (34%) | 1.01 (0.84–1.23) | ||
| Militaty | 34 (32%) | 73 (68%) | 1.00 (0.77–1.30) | 69 (64%) | 38 (36%) | 1.23 (0.85–1.78) | ||
| State-employed | 52 (19%) | 224 (81%) | 1.16 (0.97–1.40) | 198 (72%) | 78 (28%) | 1.21 (0.90–1.64) | ||
| SES | ||||||||
| 0 | 160 (38%) | 258 (62%) | 1 (ref) | .23 | 304 (73%) | 114 (27%) | 1 (ref) | .07 |
| 1 | 317 (35%) | 593 (65%) | 1.05 (0.91–1.21) | 627 (69%) | 283 (31%) | 1.17 (0.94–1.46) | ||
| 2 | 69 (27%) | 184 (73%) | 1.16 (0.96–1.41) | 162 (64%) | 91 (36%) | 1.37 (1.04–1.80) | ||
| 3 | 25 (24%) | 80 (76%) | 1.19 (0.92–1.53) | 62 (59%) | 43 (41%) | 1.57 (1.11–2.24) | ||
| 4 | 13 (20%) | 52 (80%) | 1.31 (0.97–1.76) | 46 (71%) | 19 (29%) | 1.11 (0.68–1.80) | ||
| Travel Abroad | ||||||||
| No | 560 (34%) | 1092 (66%) | 1 (ref) | .66 | 1133 (69%) | 519 (31%) | 1 (ref) | .48 |
| Yes | 24 (24%) | 75 (76%) | 1.06 (0.83–1.34) | 68 (69%) | 31 (31%) | 1.14 (0.79–1.66) | ||
| Living Outside of Guinea-Bissau | ||||||||
| No | 547 (34%) | 1051 (66%) | 1 (ref) | .29 | 1104 (69%) | 494 (31%) | 1 (ref) | .24 |
| Yes | 37 (24%) | 116 (76%) | 1.11 (0.91–1.35) | 97 (63%) | 56 (37%) | 1.18 (0.89–1.56) | ||
Abbreviations: aPR, annual percentage rate; BCG, Bacillus Calmette-Guérin; CI, confidence interval; ref, reference group; SES, socioeconomic status.
aPrevalence ratios and 95% CIs are estimated by Poisson regression adjusted for sex and year of birth in 10-year intervals with robust estimation of variance clustered by house bWald test.
Individuals with smallpox and/or BCG vaccination scars had a lower prevalence of HIV-1 compared with individuals without smallpox and BCG vaccination scars (aOR = 0.62; 95% confidence interval [CI], 0.36–1.07) (Table 3). Multiple smallpox vaccination scars were associated with a lower prevalence of HIV-1 (aOR = 0.27; 95% CI, 0.10–0.75) compared with individuals without smallpox or BCG vaccination scars. The aOR for multiple smallpox vaccination scars was 0.18 (95% CI, 0.05–0.64) for women and 0.52 (95% CI, 0.12–2.33) for men (sex-differential effect, P = .29) (Table 4).
Table 3.
BCG and Smallpox Vaccination and HIV-1 in Guinea-Bissau and Denmark: Separate and Combined Estimates
| Smallpox/BCG Vaccinated | Guinea-Bissau (GB) | Denmark (DK) | Combined Estimatee | ||||
|---|---|---|---|---|---|---|---|
| % (HIV/All) | Adjusted ORa (95% CI) | IPCW % (HIV/Allb)c | IPCW and Adjusted ORd (95% CI) | GB Weight | DK Weight | Combined Estimate of Adjusted ORs | |
| All | |||||||
| −/− | 6.1% (24/393) | 1 (ref) | 1.6% (16/1011.9) | 1 (ref) | - | - | 1 (ref) |
| +/− | 3.3% (27/808) | 0.72 (0.39–1.33) | 1.5% (6/407.6) | 0.52 (0.17–1.60) | 77% | 23% | 0.67 (0.39–1.14) |
| −/+ | 4.7% (9/191) | 0.50 (0.23–1.10) | 1.5% (22/1511.4) | 0.70 (0.41–1.18) | 31% | 69% | 0.63 (0.41–0.98) |
| +/+ | 4.2% (15/359) | 0.59 (0.28–1.25) | 2.3% (60/2665.7) | 0.74 (0.44–1.26) | 33% | 67% | 0.69 (0.45–1.06) |
| Either or | 3.8% (51/1358) | 0.62 (0.36–1.07) | 1.9% (88/4584.7) | 0.70 (0.43–1.15) | 45% | 55% | 0.66 (0.46–0.96) |
| Women | |||||||
| −/− | 6.9% (17/247) | 1 (ref) | 1.0% (5/492.3) | 1 (ref) | - | - | 1 (ref) |
| +/− | 3.7% (18/490) | 0.64 (0.30–1.36) | 1.0% (2/202.6) | 0.53 (0.12–2.35) | 79% | 21% | 0.61 (0.31–1.21) |
| −/+ | 7.6% (9/119) | 0.73 (0.31–1.71) | 0.6% (4/697.8) | 0.45 (0.13–1.59) | 67% | 33% | 0.62 (0.31–1.26) |
| +/+ | 4.8% (10/208) | 0.56 (0.23–1.36) | 0.7% (10/1353.6) | 0.37 (0.17–0.81) | 44% | 56% | 0.43 (0.25–0.78) |
| Either or | 4.5% (37/817) | 0.63 (0.33–1.21) | 0.7% (16/2254.0) | 0.40 (0.19–0.82) | 56% | 44% | 0.52 (0.32–0.84) |
| Men | |||||||
| −/− | 4.8% (7/146) | 1 (ref) | 2.1% (11/519.5) | 1 (ref) | - | - | 1 (ref) |
| +/− | 2.8% (9/318) | 0.90 (0.32–2.51) | 2.0% (4/204.9) | 0.51 (0.12–2.07) | 66% | 34% | 0.74 (0.32–1.71) |
| −/+ | 0% (0/72) | N/A | 2.2% (18/814.0) | 0.82 (0.48–1.38) | N/A | N/A | N/A |
| +/+ | 3.3% (5/151) | 0.62 (0.16–2.37) | 3.8% (50/1311.9) | 0.92 (0.49–1.70) | 18% | 82% | 0.86 (0.49–1.51) |
| Either or | 2.6% (14/541) | 0.60 (0.23–1.55) | 3.1 (72/2330.7) | 0.84 (0.49–1.45) | 24% | 76% | 0.77 (0.48–1.24) |
Abbreviations: BCG, Bacillus Calmette-Guérin; CI, confidence interval; DK, Denmark; GB, Guinea-Bissau; HIV, human immunodeficiency virus; IPCW, inverse probability of censoring weighted; NA, nonapplicable; OR, odds ratio; ref, reference group.
aLogistic regression adjusted for year of birth in 10-year intervals, sex, field worker, place of birth, schooling, religion, ethnicity, employment, socioeconomic status, traveled abroad, and lived abroad and stratified by house with robust variance.
bNon-HIV cases are weighted by the inverse probability of being censored during follow up.
cNote the percentages cannot be interpreted as the prevalence of HIV-1, because only a subcohort of the background cohort is included in the denominator, whereas all HIV-1 cases in the background cohort are included in the numerator (an estimation of the prevalence is given in Supplementary Table 1). Because the cohort was sampled by birth year and vaccination coverage decreases with time, the percentage of HIV cases cannot directly be compared among vaccination groups.
dLogistic regression adjusted for family social class, sex, immigration, year of birth and weighted by the inverse probability of being censored during follow-up and with robust variance clustered by birth year and sex.
eThe combined adjusted odds ratios from Denmark and Guinea-Bissau were combined in random effect meta-estimates.
Table 4.
Number of Smallpox Vaccination Scars and HIV-1, Guinea-Bissau
| (N = 1751a) | All | Women | Men | ||||||
|---|---|---|---|---|---|---|---|---|---|
| % (HIV-1/All) | Crude ORb (95% CI) |
Adjusted ORc (95% CI) |
% (HIV-1/All) | Crude ORb (95% CI) |
Adjusted ORc (95% CI) |
% (HIV-1/All) | Crude ORb (95% CI) |
Adjusted ORc (95% CI) |
|
| Multiple Smallpox Vaccination Scar/One Smallpox Vaccination Scar /BCG Vaccination Scars | |||||||||
| −/−/− | 6.1% (24/393) | 1 (ref) | 1 (ref) | 6.9% (17/247) | 1 (ref) | 1 (ref) | 4.8% (7/146) | 1 (ref) | 1 (ref) |
| −/−/+ | 4.7% (9/191) | 0.59 (0.28–1.23) | 0.51 (0.23–1.12) | 7.6% (9/119) | 0.79 (0.35–1.80) | 0.75 (0.32–1.75) | 0.0% (0/72) | N/A | N/A |
| −/+/NAd | 5.0% (36/722) | 0.98 (0.56–1.72) | 0.83 (0.47–1.48) | 5.8% (25/433) | 0.96 (0.49–1.86) | 0.81 (0.40–1.62) | 3.8% (11/289) | 1.02 (0.39–2.64) | 0.87 (0.33–2.30) |
| +/+/NAe | 1.4% (6/445) | 0.33 (0.12–0.87) | 0.27 (0.10–0.75) | 1.1% (3/265) | 0.23 (0.07–0.80) | 0.18 (0.05–0.64) | 1.7% (3/180) | 0.55 (0.13–2.39) | 0.52 (0.12–2.33) |
Abbreviations: BCG, Bacillus Calmette-Guérin; CI, confidence interval; HIV, human immunodeficiency virus; NA, nonapplicable; OR, odds ratio; ref, reference group.
aIndividuals with a smallpox vaccination scar but with uncertain number of smallpox vaccination scars (n = 8) were set to a single smallpox vaccination scar.
bLogistic regression adjusted by year of birth in 10-year intervals and stratified by house with robust variance.
cLogistic regression adjusted for year of birth in 10-year intervals, sex, field worker, place of birth, schooling, religion, ethnicity, employment, socioeconomic status, traveled abroad, and lived abroad and stratified by house with robust variance.
dThe adjusted OR is 0.71 (0.33–1.53) for individuals without BCG vaccination and 0.92 (0.49–1.73) for individuals with BCG vaccination.
eThe adjusted OR is 0.25 (0.05–1.30) for individuals without BCG vaccination and 0.29 (0.09–0.87) for individuals with BCG vaccination.
Denmark
The eligible background cohort of 46 239 individuals had 143 HIV-1 cases, and the 13% subsample was equivalent to 6015 individuals. We excluded 39 (27%) HIV-1 cases and 509 (8%) individuals in the subcohort for reasons indicated in Figure 1. Hence, 104 HIV-1 cases were analyzed (21 women, 83 men) along with a subcohort of 5506 of individuals. Among these, 18% (996) had no vaccination, 7% (402) had smallpox vaccination only, 27% (1491) had BCG vaccination only, and 48% (2617) had both BCG and smallpox vaccination (Table 2). Smallpox vaccinated individuals were more likely to be BCG vaccinated, and those having all other childhood vaccinations was positively associated with both smallpox and BCG vaccination (Table 2). The prespecified potential confounders, immigrants and family social class, were associated with BCG and smallpox vaccination, and more women had smallpox vaccines than men (Table 2).
Table 2.
Baseline Variables by Smallpox and BCG Vaccination Status Among the Baseline Subcohort in Denmark
| n = 5506 | Smallpox Vaccination | BCG Vaccination | ||||||
|---|---|---|---|---|---|---|---|---|
| No Smallpox Vaccination | Smallpox Vaccination | aPR (95% CI)a | P Valueb | No BCG Vaccination | BCG Vaccinaion | aPR (95% CI)a | P Valueb | |
| n (%) | n (%) | n (%) | n (%) | |||||
| Sex | ||||||||
| Men | 1306 (47%) | 1471 (53%) | 1 (ref) | 0.07 | 708 (25.5%) | 2069 (74.5%) | 1 (ref) | .96 |
| Women | 1181 (43.3%) | 1548 (56.7%) | 1.07 (0.99–1.15) | 690 (25.3%) | 2039 (74.7%) | 1 (0.94–1.06) | ||
| BCG | ||||||||
| No | 996 (71.2%) | 402 (28.8%) | 1 (ref) | <.01 | ||||
| Yes | 1491 (36.3%) | 2617 (63.7%) | 1.22 (1.1–1.36) | |||||
| Smallpox Vaccination | ||||||||
| No | 996 (40%) | 1491 (60%) | 1 (ref) | <.01 | ||||
| Yes | 402 (13.3%) | 2617 (86.7%) | 1.16 (1.06–1.26) | |||||
| Other Childhood Vaccinations but Smallpox and BCG Registered | ||||||||
| All other recommended | 1789 (46.9%) | 2025 (53.1%) | 1 (ref) | <.01 | 922 (24.2%) | 2892 (75.8%) | 1 (ref) | <.01 |
| Some other recommended | 626 (41.1%) | 897 (58.9%) | 0.87 (0.81–0.95) | 440 (28.9%) | 1083 (71.1%) | 0.87 (0.81–0.94) | ||
| None other | 72 (42.6%) | 97 (57.4%) | 0.79 (0.64–0.97) | 36 (21.3%) | 133 (78.7%) | 0.93 (0.78–1.11) | ||
| Eczema | ||||||||
| No or missing | 2289 (44.2%) | 2890 (55.8%) | 1 (ref) | .15 | 1284 (24.8%) | 3895 (75.2%) | 1 (ref) | .27 |
| Yes | 198 (60.6%) | 129 (39.4%) | 0.88 (0.74–1.05) | 114 (34.9%) | 213 (65.1%) | 0.93 (0.81–1.06) | ||
| Immigration | ||||||||
| No | 2331 (44.2%) | 2938 (55.8%) | 1 (ref) | <.01 | 1298 (24.6%) | 3971 (75.4%) | 1 (ref) | .01 |
| Yes | 156 (65.8%) | 81 (34.2%) | 0.69 (0.56–0.87) | 100 (42.2%) | 137 (57.8%) | 0.81 (0.68–0.96) | ||
| Family Social Class | ||||||||
| I | 196 (51.9%) | 182 (48.1%) | 1 (ref) | .18 | 113 (29.9%) | 265 (70.1%) | 1 (ref) | .33 |
| II | 310 (47.5%) | 343 (52.5%) | 0.98 (0.82–1.18) | 138 (21.1%) | 515 (78.9%) | 1.07 (0.92–1.24) | ||
| III | 368 (42%) | 509 (58%) | 0.97 (0.82–1.14) | 201 (22.9%) | 676 (77.1%) | 1.02 (0.89–1.18) | ||
| IV | 772 (41.6%) | 1084 (58.4%) | 0.95 (0.81–1.11) | 418 (22.5%) | 1438 (77.5%) | 1.03 (0.9–1.17) | ||
| V | 486 (41.8%) | 676 (58.2%) | 0.91 (0.77–1.07) | 307 (26.4%) | 855 (73.6%) | 0.96 (0.83–1.1) | ||
| Unclassifiable | 178 (54.4%) | 149 (45.6%) | 0.78 (0.63–0.97) | 100 (30.6%) | 227 (69.4%) | 0.93 (0.78–1.12) | ||
| Missing (imputed for analysis) | 177 (70%) | 76 (30%) | Not calculated | 121 (47.8%) | 132 (52.2%) | Not calculated | ||
| Siblings | ||||||||
| None | 382 (48.6%) | 404 (51.4%) | 1 (ref) | .33 | 224 (28.5%) | 562 (71.5%) | 1 (ref) | .07 |
| One | 1178 (44.2%) | 1488 (55.8%) | 1.03 (0.92–1.15) | 603 (22.6%) | 2063 (77.4%) | 1.05 (0.96–1.15) | ||
| Two or more | 809 (44.4%) | 1012 (55.6%) | 0.97 (0.86–1.09) | 503 (27.6%) | 1318 (72.4%) | 0.97 (0.88–1.07) | ||
| Missing (imputed for analysis) | 118 (50.6%) | 115 (49.4%) | Not calculated | 68 (29.2%) | 165 (70.8%) | Not calculated | ||
| Daycare Before School | ||||||||
| No | 422 (35.3%) | 773 (64.7%) | 1 (ref) | .71 | 293 (24.5%) | 902 (75.5%) | 1 (ref) | .12 |
| Yes | 1854 (50.6%) | 1807 (49.4%) | 0.98 (0.9–1.07) | 947 (25.9%) | 2714 (74.1%) | 1.06 (0.98–1.15) | ||
| Missing (imputed for analysis) | 211 (32.5%) | 439 (67.5%) | Not calculated | 158 (24.3%) | 492 (75.7%) | Not calculated | ||
Abbreviations: aPR, annual percentage rate; BCG, Bacillus Calmette-Guérin; CI, confidence interval; ref, reference group.
aPrevalence ratios and 95% CIs are estimated by Poisson regression adjusted for sex and year of birth.
bWald test.
During follow up, 9% of individuals were censored in the subcohort (emigration, 6%; death, 2%; unknown whereabouts, 0.3%). To correct the study population for non-HIV-positive individuals leaving the study before end of follow up, we used inverse probability of censoring weights, which resulted in a weighted subcohort of ~5506. Bacillus Calmette-Guérin and/or smallpox vaccination was associated with an IPCW aOR of 0.70 (95% CI, 0.43–1.15) for having HIV-1 compared with neither of these (Table 3).
Combined Estimate
The combined meta-estimate from both study locations for BCG and/or smallpox scars/vaccinations compared with none of these vaccines gave an aOR of 0.66 (95% CI, 0.46–0.96) (Table 3). The estimate for women was an aOR of 0.52 (95% CI, 0.32–0.84); the estimate for men was an aOR of 0.77 (95% CI, 0.48–1.24) (sex-differential effect, P = .26).
Sensitivity Analyses
We tested for an association with any smallpox and BCG vaccination scar versus none of these in the alternative analyses using inverse probability of treatment weights and found similar results in both Guinea-Bissau and Denmark (Supplementary Table 1). The Cox proportional hazard estimate in the Danish study indicated a similar association to the IPCW logistic regression, but the result should be interpreted cautiously because the test for proportional hazards was 0.05 (Supplementary Table 1).
DISCUSSION
Our studies from 2 very different settings suggest that smallpox and/or BCG vaccinations were associated with a lower prevalence of HIV-1 when combining the evidence from Guinea-Bissau and Denmark. The pattern was consistent across all combinations of smallpox and BCG vaccinations, but estimates from comparison groups with smaller samples had high uncertainty. The indication that multiple smallpox vaccination scars were associated with an additionally lower aOR of being HIV-1 infected compared with individuals without any vaccinationscars in the study from Guinea-Bissau, where this information was available, suggest a biological dose response relationship. Although not statistically significant, the association tended to be stronger for women than for men; the beneficial effect of smallpox vaccination on adult mortality in Guinea-Bissau was also stronger for women than for men [4, 5].
Strengths and Limitations
Because there is no central register of smallpox vaccinations in Guinea-Bissau, we relied on reading smallpox and BCG vaccination scars to determine vaccination status. In a small validation study in the capital, 90% (62 of 69) of Bandim residents with a smallpox vaccination identified in the city register had a smallpox vaccination scar, which was identified by field workers in the study area; a few minor scars were missed [4]. Misclassified smallpox vaccination scars can lead to conservative estimates. Unfortunately, we cannot check whether the individuals censored between the HIV survey and the subsequent vaccination scar survey differed with regard to confounders relevant to the scar survey. As in 2 previous scar surveys, there was some variation in how fieldworkers measured the size of the scar [4, 5]; however, the fieldworkers conducting the scar survey were not involved in the HIV survey.
The main concerns in the Guinean study are the potential common causes for “not” having smallpox and BCG vaccination scars and to acquire HIV-1. We adjusted for 11 potential confounders that we think cover social mechanisms for vaccination and HIV-1, but we cannot exclude residual confounding. The study population was examined for vaccination scars in 2005–2007 and in relation to the HIV-survey cohort in 2004–2006; therefore, only a selected population survived until then. If HIV-1-infected individuals with several smallpox vaccination scars died more rapidly before follow-up than HIV-1-infected individuals without scars, then this could explain the estimate we found. However, we know from previous follow-up studies that individuals with several smallpox vaccination scars have significantly better survival rates [4]. The study population included a higher proportion of elderly patients due to the sampling approach, but this would not affect the estimates (assuming an age-homogeneous effect) because all analyses were stratified by 10-year age intervals.
For the Danish study, both BCG and smallpox vaccinations were given long before the individuals contracted HIV-1, which ensures temporality. We cannot be certain that all the included HIV-1 cases were related to sexual transmission because injection drug use is underreported in Danish health registries.
We used the complementary sIPTW analyses, which supported the main conclusion. The 2 studies differ on several conditions: the measurement of vaccination (health record vs scar read), the study population’s characteristics, climate, HIV-1 infection pressure, competing diseases, and social interactions. We interpret the consistent pattern across such different studies to suggest an underlying causal effect. Our random effects meta-analysis estimate should not be interpreted as an effect of 1 well defined intervention, but rather a combination of effects occurring across different settings, where one would a priori expect different effects.
Consistency With Other Studies
When the smallpox vaccination was first introduced, several reports suggested that it prevented more than smallpox infection [17]. Very few formal studies of associations between smallpox vaccination and (non-smallpox) mortality and morbidity have been conducted, but they have consistently found that smallpox vaccination is associated with improved general health. In high-income countries, smallpox vaccination was associated with fewer infectious disease hospital admissions [6] and less risk of asthma [18], multiple sclerosis [19], and some cancers [20–24]. In Denmark, having documented BCG and smallpox vaccinations at school was associated with reduced mortality by 46% (range, 19%–64%) in up to 40 years after vaccination [7].
Increasing evidence suggests that the nonspecific effects of BCG vaccination are more pronounced if BCG-vaccinated individuals develop a BCG scar [25–29]. Smallpox and BCG vaccination status were assessed by scar status in the study from Guinea-Bissau, whereas it was assessed by recorded vaccination dates in the Danish study. This might have influenced the lower point estimate for HIV-1 by smallpox and/or BCG vaccination (scars) in Guinea-Bissau of 0.62 compared with 0.70 in Denmark (due to low power, we cannot rule out that this result could be due to chance) (Table 3).
In 2 studies from Guinea-Bissau, smallpox vaccination scars were associated with better survival [4, 5], a beneficial association that also increased with the number of vaccination scars, as we observed in the present study. Multiple studies indicate a beneficial boosting effect on nonspecific outcomes from revaccination with the same live vaccines [15], corroborating our finding that multiple smallpox vaccination scars were associated with an additionally lower prevalence ratio of HIV-1 in our study in Guinea-Bissau (Table 4). Because smallpox and BCG vaccination may have different mechanisms for a potential reduced risk of HIV-1, several different live vaccines could be expected to be associated with a lower risk of HIV-1, compared with having received 1 live vaccine. However, we did not observe a lower effect by having received both smallpox and BCG vaccination compared with 1 of the 2 in either the study from Guinea-Bissau or Denmark (Table 3).
Possible Biological Mechanisms
The nonspecific effects of vaccines challenge our current understanding of the immune system, which considers only the pathogen-specific effects of vaccination. The immunological mechanisms underlying nonspecific effects are still largely unknown, but new evidence is emerging. In brief, nonspecific effects may occur via effects on the innate immune system, the adaptive immune system, or both [2]. With respect to the innate immune system, BCG may induce epigenetic modifications of innate immune cells, leading to long-lasting innate immune memory [3]. Very recent in vitro studies indicate that smallpox vaccine induces similar “innate training”, which increases cytokine responses of monocytes to unrelated stimuli. There are possible benefits associated with this immune activation; however, it was recently observed that BCG was associated with increased simian immunodeficiency virus (SIV) acquisition in infant macaques, leading to speculation that the BCG-induced immune activation could have facilitated SIV infection [30].
For the adaptive immune system, cross-reactive epitopes can lead to the formation of cross-reactive T-memory cells, which can induce long-lasting heterologous T cell-mediated immunity [31]. The vaccinia virus used for the smallpox vaccination shows homologs of the HIV-1 envelope glycoprotein, suggesting a potential for cross-reactive immunity [32]. We know that several viruses interact with and inhibit HIV-1 replication and sometimes alter the course of infection and prolong survival of HIV-1 infected individuals [33–36].
Pathogens typically invade the host through epithelial interfaces with the environment. Intriguingly, both smallpox and BCG vaccination are given directly in the skin. In animal models, administering smallpox vaccination via skin scarification increased immune response and survival upon subsequent challenge compared with other modes of administration [37]. It has been shown in mice that localized vaccinia virus skin infection generates long-lived nonrecirculating CD8+ skin resident T-memory cells that resided within the entire skin and protected against reinfection [38]. Thus, although the mechanism is unknown, memory acquired at the site of vaccination can spread throughout the entire epithelial surface, which creates a “shield” against reinfection. We speculate that innate training by epigenetic modifications observed in monocytes also takes place at the level of epithelial progenitor cells, leading to direct alterations in the first-line epithelial defense system.
In order for HIV-1 to establish a primary infection, activation of the chemokine receptor CCR5 is important [39]. Therefore, it is interesting note that vaccinia virus may downregulate the CCR5 receptor on the surface of the T cell [40, 41]. In an ex vivo tonsillar tissue model, vaccinia virus depleted CCR5 T cells, significantly inhibiting the replication of the HIV-1 variant R5 [42]. Further immunological studies have supported a role for CCR5 in establishing smallpox infection [43]. The CCR5-delta-32 deletion confers resistance to HIV-1 by preventing expression of the receptor on the cell surface. In a recent study, based on modeling, researchers argued that the high frequency of the CCR5-delta-32 deletion allele in Europe is consistent with a role of smallpox epidemics; the large European smallpox epidemics leads to selective survival advantages of individuals with the deletion allele [44].
Thus, it is plausible that smallpox vaccination may alter the expression of CCR5 on cell surfaces and/or the production of CCR5-specific ligands, and this may interfere with establishing HIV infection. Corroborating this notion, a recent US study found that cells from smallpox vaccinated individuals had an up to 5-fold reduction in CCR5 tropic HIV-1 replication in vitro [40, 41].
CONCLUSIONS
In conclusion, there is some immunological evidence to support that intradermally administrated smallpox vaccination can provide cross-protection against HIV-1 infection. None of the above immunological studies reported effects by sex, but it is well known that males and females respond immunologically different to vaccines [45], and it seems plausible that an enhanced epithelial protection would be particularly protective against vaginally acquired HIV-1 infection. This could explain why smallpox vaccination might be more associated with protection against HIV-1 in women than in men.
Combining the evidence from Guinea-Bissau and Denmark, BCG and/or smallpox vaccinations were associated with less sexually transmitted HIV-1. The studies were based on a few HIV-1 cases; however, the results are consistent with immunological studies that showed reduced HIV-1 replication in cells from smallpox vaccinated individuals. So far, these results suggest that the mechanisms linking BCG and smallpox vaccines with protection against HIV-1 warrant further investigation. Hopefully, immunologists will investigate additional mechanisms for how smallpox and BCG vaccinations could possibly prevent HIV-1, and why such an effect might be stronger for women than men. The findings, if confirmed, could have major implications for future interventions against HIV-1, and thus future prospective studies are urgently warranted.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
We thank Professor Thorkild I. A. Sørensen (Department of Clinical Epidemiology at Bispebjerg and Frederiksberg Hospital) for access to data and valuable comments. We are grateful to Dr. Joachim Knop (Department of Clinical Epidemiology at Bispebjerg and Frederiksberg Hospital) for advice on registration of injection drug use in the Danish National Patient Register.
Author contributions. A. Ri., M. V., and P. A. are guarantors of the study. M. L. J., A. Ri., M. V., Z. J. d., A. E. R., A. Ro., C. S. B., and P. A. contributed to the study design and collection of data from Guinea-Bissau; M. V., A. Ri., M. L. J., and P. A. analyzed and interpreted the data; M. V., A. Ri., M. L. J., C. S. B., and P. A. drafted this part of the manuscript. M. V., S. S., A. E. R., and A. Ri. supervised the data entry of the study from Denmark; M. V. and A. Ri. analyzed data and drafted this part of the manuscript. All authors made significant contributions to interpretation of the data and the final version of the manuscript. All authors declare that they had access to the data, accepted the final version of the article, and approved the submission of the final draft.
Disclaimer. The co-first authors and senior author affirm the following: this manuscript is an honest, accurate, and transparent account of the study being reported; no important aspects of the study have been omitted; and any discrepancies from the study as planned (and, if relevant, registered) have been explained. The funding agencies had no role in the study design, data collection, data analysis, data interpretation or in the preparation, review, or approval of the manuscript.
Financial support. A. Ri. received an unrestricted Faculty of Health Sciences scholarship from University of Southern Denmark. M. V. received an unrestricted PhD grant from the Danish Graduate School in Public Health Science and University of Copenhagen, Lundbeck Foundation (R34-A3862) and Dagmar Marshalls Foundation. S. S. received a grant from the Danish Council for Independent Research (DFF- 4183-00316). C. S. B. received funding from a European Research Council Starting Grant (ERC-2009-StG-243149). P. A. received a research professorship grant from the Novo Nordisk Foundation. Research Center for Vitamins and Vaccines (CVIVA) is funded by the Danish National Research Foundation (DNRF108). The Bandim Health Project has received support from the Danish International Development Agency (DANIDA) and European Union FP7 support for OPTIMUNISE (Grant Health-F3-2011-261375).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Strassburg MA. The global eradication of smallpox. Am J Infect Control 1982; 10:53–9. [DOI] [PubMed] [Google Scholar]
- 2. Benn CS, Netea MG, Selin LK, Aaby P. A small jab—a big effect: nonspecific immunomodulation by vaccines. Trends Immunol 2013; 34:431–9. [DOI] [PubMed] [Google Scholar]
- 3. Netea MG, Joosten LA, Latz E et al. . Trained immunity: a program of innate immune memory in health and disease. Science 2016; 352:aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aaby P, Gustafson P, Roth A et al. . Vaccinia scars associated with better survival for adults. An observational study from Guinea-Bissau. Vaccine 2006; 24:5718–25. [DOI] [PubMed] [Google Scholar]
- 5. Jensen ML, Dave S, Schim van der Loeff M et al. . Vaccinia scars associated with improved survival among adults in rural Guinea-Bissau. PLoS One 2006; 1:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sørup S, Villumsen M, Ravn H et al. . Smallpox vaccination and all-cause infectious disease hospitalization: a Danish register-based cohort study. Int J Epidemiol 2011; 40:955–63. [DOI] [PubMed] [Google Scholar]
- 7. Rieckmann A, Villumsen M, Sorup S et al. . Vaccinations against smallpox and tuberculosis are associated with better long-term survival: a Danish case-cohort study 1971–2010. Int J Epidemiol 2017; 46:695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. da Silva ZJ, Oliveira I, Andersen A et al. . Changes in prevalence and incidence of HIV-1, HIV-2 and dual infections in urban areas of Bissau, Guinea-Bissau: is HIV-2 disappearing? AIDS 2008; 22:1195–202. [DOI] [PubMed] [Google Scholar]
- 9. Lohse N, Hansen AB, Jensen-Fangel S et al. . Demographics of HIV-1 infection in Denmark: results from the Danish HIV Cohort Study. Scand J Infect Dis 2005; 37:338–43. [DOI] [PubMed] [Google Scholar]
- 10. Månsson F. HIV-1, HIV-2, and other Sexually Transmitted Infections in Guinea-Bissau, West Africa:Lund University; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol 2003; 3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pearce N. What does the odds ratio estimate in a case-control study? Int J Epidemiol 1993; 22:1189–92. [DOI] [PubMed] [Google Scholar]
- 13. Hernán MA, Robins JM.. Causal Inference. Boca Raton: Chapman & Hall/CRC, forthcoming; 2017. [Google Scholar]
- 14. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods 2010; 1:97–111. [DOI] [PubMed] [Google Scholar]
- 15. Benn CS, Fisker AB, Whittle HC, Aaby P. Revaccination with live attenuated vaccines confer additional beneficial nonspecific effects on overall survival: a review. EBioMedicine 2016; 10:312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990; 1(1):43–6. [PubMed] [Google Scholar]
- 17. Mayr A. Taking advantage of the positive side-effects of smallpox vaccination. J Vet Med B Infect Dis Vet Public Health 2004; 51:199–201. [DOI] [PubMed] [Google Scholar]
- 18. Bager P, Westergaard T, Rostgaard K et al. . Smallpox vaccination and risk of allergy and asthma. J Allergy Clin Immunol 2003; 111:1227–31. [DOI] [PubMed] [Google Scholar]
- 19. Kurtzke JF, Hyllested K, Arbuckle JD et al. . Multiple sclerosis in the Faroe Islands. 7. Results of a case control questionnaire with multiple controls. Acta Neurol Scand 1997; 96:149–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krone B, Kölmel KF, Henz BM, Grange JM. Protection against melanoma by vaccination with Bacille Calmette-Guerin (BCG) and/or vaccinia: an epidemiology-based hypothesis on the nature of a melanoma risk factor and its immunological control. Eur J Cancer 2005; 41:104–17. [DOI] [PubMed] [Google Scholar]
- 21. Pfahlberg A, Kölmel KF, Grange JM et al. . Inverse association between melanoma and previous vaccinations against tuberculosis and smallpox: results of the FEBIM study. J Invest Dermatol 2002; 119:570–5. [DOI] [PubMed] [Google Scholar]
- 22. Grufferman S, Wang HH, DeLong ER et al. . Environmental factors in the etiology of rhabdomyosarcoma in childhood. J Natl Cancer Inst 1982; 68:107–13. [PubMed] [Google Scholar]
- 23. Lankes HA, Fought AJ, Evens AM et al. . Vaccination history and risk of non-Hodgkin lymphoma: a population-based, case-control study. Cancer Causes Control 2009; 20:517–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tavani A, La Vecchia C, Franceschi S et al. . Medical history and risk of Hodgkin’s and non-Hodgkin’s lymphomas. Eur J Cancer Prev 2000; 9:59–64. [DOI] [PubMed] [Google Scholar]
- 25. Garly ML, Martins CL, Balé C et al. . BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa. A non-specific beneficial effect of BCG? Vaccine 2003; 21:2782–90. [DOI] [PubMed] [Google Scholar]
- 26. Roth A, Sodemann M, Jensen H et al. . Tuberculin reaction, BCG scar, and lower female mortality. Epidemiology 2006; 17:562–8. [DOI] [PubMed] [Google Scholar]
- 27. Roth A, Gustafson P, Nhaga A et al. . BCG vaccination scar associated with better childhood survival in Guinea-Bissau. Int J Epidemiol 2005; 34:540–7. [DOI] [PubMed] [Google Scholar]
- 28. Timmermann CA, Biering-Sørensen S, Aaby P et al. . Tuberculin reaction and BCG scar: association with infant mortality. Trop Med Int Health 2015; 20:1733–44. [DOI] [PubMed] [Google Scholar]
- 29. Storgaard L, Rodrigues A, Martins C et al. . Development of BCG scar and subsequent morbidity and mortality in Rural Guinea-Bissau. Clin Infect Dis 2015; 61:950–9. [DOI] [PubMed] [Google Scholar]
- 30. Derrick SC. Trained immunity and susceptibility to HIV. Clin Vaccine Immunol 2017; 24:e00509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Welsh RM, Che JW, Brehm MA, Selin LK. Heterologous immunity between viruses. Immunol Rev 2010; 235:244–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carter CJ. Vaccinia and other viruses with available vaccines show marked homology with the HIV-1 envelope glycoprotein: the prospect of using existing vaccines to stem the AIDS pandemic. Immunopharmacol Immunotoxicol 2012; 34:222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moss WJ, Ryon JJ, Monze M et al. . Suppression of human immunodeficiency virus replication during acute measles. J Infect Dis 2002; 185:1035–42. [DOI] [PubMed] [Google Scholar]
- 34. Watt G, Kantipong P, de Souza M et al. . HIV-1 suppression during acute scrub-typhus infection. Lancet 2000; 356:475–9. [DOI] [PubMed] [Google Scholar]
- 35. Watt G, Kantipong P, Jongsakul K. Decrease in human immunodeficiency virus type 1 load during acute dengue fever. Clin Infect Dis 2003; 36:1067–9. [DOI] [PubMed] [Google Scholar]
- 36. Esbjörnsson J, Månsson F, Kvist A et al. . Inhibition of HIV-1 disease progression by contemporaneous HIV-2 infection. N Engl J Med 2012; 367:224–32. [DOI] [PubMed] [Google Scholar]
- 37. Liu L, Zhong Q, Tian T et al. . Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med 2010; 16:224–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jiang X, Clark RA, Liu L et al. . Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature 2012; 483:227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weber J, Piontkivska H, Quiñones-Mateu ME. HIV type 1 tropism and inhibitors of viral entry: clinical implications. AIDS Rev 2006; 8:60–77. [PubMed] [Google Scholar]
- 40. Rahbar R, Murooka TT, Hinek AA et al. . Vaccinia virus activation of CCR5 invokes tyrosine phosphorylation signaling events that support virus replication. J Virol 2006; 80:7245–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weinstein RS, Weinstein MM, Alibek K et al. . Significantly reduced CCR5-tropic HIV-1 replication in vitro in cells from subjects previously immunized with Vaccinia Virus. BMC Immunol 2010; 11:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vanpouille C, Biancotto A, Lisco A, Brichacek B. Interactions between human immunodeficiency virus type 1 and vaccinia virus in human lymphoid tissue ex vivo. J Virol 2007; 81:12458–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rahbar R, Murooka TT, Fish EN. Role for CCR5 in dissemination of vaccinia virus in vivo. J Virol 2009; 83:2226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Galvani AP, Slatkin M. Evaluating plague and smallpox as historical selective pressures for the CCR5-Delta 32 HIV-resistance allele. Proc Natl Acad Sci U S A 2003; 100:15276–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Flanagan KL, Klein SL, Skakkebaek NE et al. . Sex differences in the vaccine-specific and non-targeted effects of vaccines. Vaccine 2011; 29:2349–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.