Abstract
Background
Increased locomotor activity in response to the same stimulus is an index of behavioral sensitization observed in preclinical models of drug addiction and compulsive behaviors. Repeated administration of quinpirole, a D2/D3 dopamine agonist, induces locomotor sensitization. This effect is potentiated and accelerated by co-administration of U69593, a kappa opioid receptor agonist. The mechanism underlying kappa opioid receptor potentiation of quinpirole-induced locomotor sensitization remains to be elucidated.
Methods
Immunofluorescence anatomical studies were undertaken in mice brain slices and rat presynaptic synaptosomes to reveal kappa opioid receptor and D2R pre- and postsynaptic colocalization in the nucleus accumbens. Tonic and phasic dopamine release in the nucleus accumbens of rats repeatedly treated with U69593 and quinpirole was assessed by microdialysis and fast scan cyclic voltammetry.
Results
Anatomical data show that kappa opioid receptor and D2R colocalize postsynaptically in medium spiny neurons of the nucleus accumbens and the highest presynaptic colocalization occurs on the same dopamine terminals. Significantly reduced dopamine levels were observed in quinpirole, and U69593-quinpirole treated rats, explaining sensitization of D2R. Presynaptic inhibition induced by kappa opioid receptor and D2R of electrically evoked dopamine release was faster in U69593-quinpirole compared with quinpirole-repeatedly treated rats.
Conclusions
Pre- and postsynaptic colocalization of kappa opioid receptor and D2R supports a role for kappa opioid receptor potentiating both the D2R inhibitory autoreceptor function and the inhibitory action of D2R on efferent medium spiny neurons. Kappa opioid receptor co-activation accelerates D2R sensitization by contributing to decrease dopamine release in the nucleus accumbens.
Keywords: dopamine, quinpirole, locomotor sensitization, kappa opioid receptor, U69593
Significance Statement
Repeated activation of dopamine D2 receptors (D2R) induces compulsive behavior and locomotor sensitization, features observed in experimental models of addiction and obsessive-compulsive disorder. The concomitant activation of kappa opioid receptors (KOR) potentiates these features, pointing to a functional crosstalk between D2R and KOR. The results showed that less dopamine in the nucleus accumbens of the brain is associated with locomotor sensitization. D2R function is enhanced by KOR coactivation and anatomic data indicate that the crosstalk occurs presynaptically in dopamine terminals and postsynaptically in a subpopulation of efferent neurons of the nucleus accumbens. These data help to understand how alterations in dopamine neurotransmission underlie behavioral impairments.
Introduction
Dopamine mesolimbic circuit connecting the ventral tegmental area (VTA) with the nucleus accumbens (NAc) is a key modulator of goal-directed behaviors. Dopamine and glutamate terminals synapse on 2 populations of efferent GABAergic medium spiny neurons (MSNs) of the NAc (Sesack and Pickel, 1990, 1992). One MSN population, the direct pathway, expresses preferentially D1 receptors and the other, the indirect pathway, D2 receptors (D2Rs) (Bertran-Gonzalez et al., 2008). The integration of incoming neurochemical signals on MSN controls goal-directed behaviors (Goto and Grace, 2008). Imbalances of dopamine neurotransmission modify goal-direct behaviors, and its chronic impairment underlies several neuropsychiatric diseases. In this regard, repeated administration of psychostimulants, which increase extracellular dopamine in the NAc, results in locomotor sensitization and compulsive motivation for the abused drug (Pierce and Kalivas, 1997). Interestingly, the repeated activation of only D2R is sufficient to induce locomotor sensitization and compulsive behaviors (Szechtman et al., 1993, 1998; Triana del Rio et al., 2011), being suggested as an animal model for obsessive-compulsive disorder (Dvorkin et al., 2006; Stuchlik et al., 2016).
D2R-induced locomotor sensitization is accelerated and potentiated by the concomitant administration of kappa opioid receptor (KOR) agonists (Perreault et al., 2006, 2007a, 2007b). The fact that KOR activation increases locomotor sensitization and compulsive behaviors is surprising given the bulk of data pointing to KOR limiting the reinforcing properties of drugs of abuse. However, increasing information supports the idea that chronic activation of KOR would rather have effects that go in the same direction as that of drugs of abuse, contributing to enhance sensitized behaviors (Wee and Koob, 2010). Anatomical and molecular bases of KOR enhancing D2R-induced sensitization are unknown. We hypothesize that KOR coactivation enhances the neuroplastic changes underlying D2R-induced locomotor sensitization. Our goals were to study the colocalization of KOR and D2R at pre- and postsynaptic levels in the NAc and the neurochemical modifications that accompany KOR enhancement of D2R-induced locomotor sensitization. Currently, it is known that D2R are present presynaptically in glutamate and dopamine terminals in the NAc and postsynaptically on dendritic spines of MSN (Sesack et al., 1994). The acute activation of presynaptic D2R inhibits dopamine release and locomotor activity. However, high doses of agonists stimulate postsynaptic D2R inducing locomotor activity by inhibiting the indirect pathway. We and others have shown that the locomotor sensitization induced by the repeated activation of postsynaptic D2R is associated with a significant reduction of extracellular dopamine in the NAc (Koeltzow et al., 2003; Escobar et al., 2015). Like D2R, acute KOR activation decreases extracellular dopamine in the NAc and locomotor activity (Di Chiara and Imperato, 1988), and KOR knockout mice have increased extracellular dopamine (Chefer et al., 2005), indicating that KOR tonically inhibits dopamine release. Supporting a direct role regulating dopamine extracellular levels in the NAc, electronic microscopy data showed that KOR are present on dopamine terminals in this nucleus (Svingos et al., 2001). Also, electrophysiological studies indicate that the soma of dopamine neurons projecting to the NAc lack KOR (Margolis et al., 2006). Increasing data suggest a functional crosstalk between KOR and D2R regulating dopamine in the NAc. For instance, the repeated activation of KOR decreases D2R presynaptic inhibitory control over stimulated dopamine release in the NAc (Acri et al., 2001; Fuentealba et al., 2006). A molecular explanation of these data relates the repeated activation of KOR to a reduction of D2R (Izenwasser et al., 1998). However, repeated concomitant activation of KOR and D2R increases the number of D2R in the high-affinity state (Perreault et al., 2007b). Altogether, the data suggest a crosstalk between KOR and D2R regulating dopamine release and locomotor activity. However, there is no anatomical information regarding KOR and D2R colocalization and their functional crosstalk in the mesolimbic system that could explain KOR potentiation of D2R-induced locomotor sensitization.
Here, we assessed the presynaptic control of tonic and phasic dopamine release in the NAc in rats repeatedly co-treated with KOR and D2R agonists and determined KOR and D2R colocalization at pre- and postsynaptic levels in the NAc. Our results show that chronic coactivation of KOR enhances D2R-induced sensitization. In this way, KOR directly enhances D2R inhibition of dopamine release in the NAc, an effect supported by anatomical data showing their colocalization on the same dopamine terminals. The anatomical data also show postsynaptic colocalization of KOR and D2R on a subpopulation of MSN, suggesting that KOR potentiation of D2R-induced locomotor sensitization arises by a postsynaptic crosstalk between these receptors in the indirect efferent pathway of the NAc.
Materials and Methods
Animals
Male Sprague-Dawley rats (270–300 g) were obtained from the animal care facility of the Faculty of Chemical and Pharmaceutical Sciences of Universidad de Chile. Rats were housed in pairs in a temperature-controlled colony room (25°C) and maintained under a 12-hour- light/-dark cycle (lights on at 8:00 am) with food and water available ad libitum. Following arrival, rats were allowed to acclimate to the colony room for 1 week. Rats were used for locomotor sensitization, microdialysis, FSCV, and synaptosomal preparation.
Male C57BL/6 mice were obtained from the animal care facility of Pontificia Universidad Católica de Chile, where they were maintained 4 to 5 mice per cage under a 12-/12-hour inverted light/dark cycle (lights on at 10:00 pm) and constant temperature (24°C) with food and water available ad libitum. Mice were used for tissue immunofluorescence experiments.
All procedures were conducted to reduce the number of animals and their level of pain and discomfort. The experimental protocols were approved by the Bioethical Committee of the Faculty of Biological Sciences of the Pontificia Universidad Católica de Chile.
Reagents
Quinpirole (QNP) hydrochloride (Sigma-Aldrich) was dissolved in physiological saline (NaCl 0.9%). U69593 (Sigma-Aldrich) was dissolved in 20% propylene glycol in sterile water (vehicle). nor-Binaltorphirmine (Nor-BNI, Abcam Biochemicals) was dissolved in physiological saline.
Schedule of Locomotor Sensitization
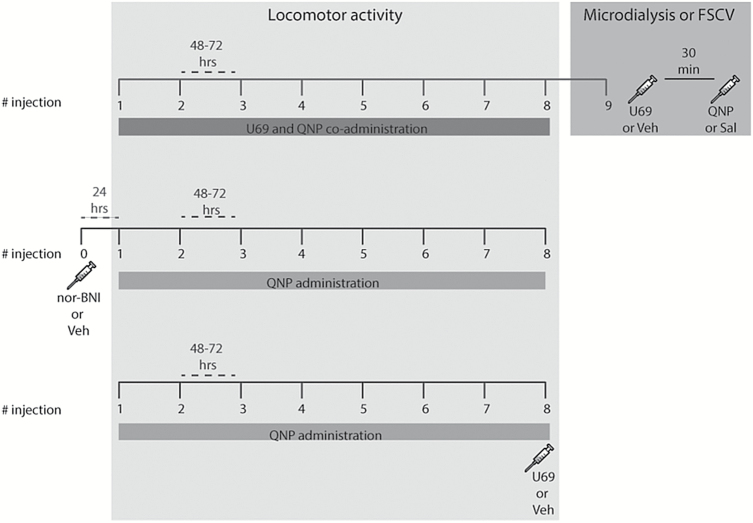
After 5 days of handling, all rats received one s.c. saline injection for 2 consecutive days and were placed in the locomotor activity chamber to habituate to novelty and reduce injection-induced stress. Twenty-four hours later, rats received an s.c. injection of a mix of U69593 (0.3 mg/kg), a KOR agonist, and QNP (0.5 mg/kg) or an equivalent volume of vehicle and saline. Four experimental groups were obtained: U69593-QNP, U69593-Saline, Vehicle-QNP, and Vehicle-Saline. Another group of rats received QNP repeatedly and, on the last injection, they were co-administered with U69593 (QNP-U69 acute) or Vehicle (QNP-Veh acute) (Figure 1). A different group of rats received a single i.p. injection of the long-lasting KOR antagonist nor-BNI, 24 hours before the beginning of repeated injection with U69593 and QNP. Rats were injected on a twice-weekly schedule until 8 injections were delivered, as previously described (Perreault et al., 2006) (Figure 1). Immediately after each injection, rats were transferred individually to the locomotor activity chamber, and horizontal locomotor activity was quantified for 60 minutes as we have described (Fuentealba et al., 2006).
Figure 1.
Schedule of U69593 and quinpirole (QNP) administration. Upper panel, rats were co-administered with U69593 or an equivalent volume of vehicle and with QNP or an equivalent volume of saline; injections were made every 48 to 72 hours until 8 injections were completed. Forty-eight hours later, rats were anesthetized and subjected to microdialysis or fast scan cyclic voltammetry (FSCV), where they received a ninth injection of U69593 or vehicle and 30 minutes later another injection of QNP or saline. Middle panel, rats were pretreated with nor-binaltorphirmine (nor-BNI) or vehicle. Twenty-four hours later, they received repeated injections of QNP every 48 to 72 hours until 8 injections were completed. Bottom panel, rats were repeatedly treated with QNP every 48 to 72 hours; on the last QNP injection, an acute injection of U69593 or vehicle was administered.
NAc Synaptosomal Preparation, Immunofluorescence, and Quantification
Synaptosomes devoid of postsynaptic density, immunofluorescence assay, image capture, and quantification were carried out as indicated in Slater et al. (2016). After decapitation, the NAc was dissected and homogenized in the following buffer 10 mM HEPES, 320 mM sucrose, and 3mM EDTA, pH 7.4. The homogenate was submitted to centrifugation (1000 g, 10 min, 4ºC). The supernatant was centrifuged at 17000 g, 20 min, 4ºC, and the obtained pellet was resuspended and placed in the top of a discontinuous Percoll gradient (PVP-silica colloid; Sigma-Aldrich), which was centrifuged at 15000 g, 20 min, 4ºC. The obtained synaptosome fraction was seeded in coverslips coated with Poly-L-Lysine (Sigma-Aldrich) and fixed with 4% paraformaldehyde/10%sucrose. Synaptosomes were permeabilized with 0.2% triton X-100 and incubated for 2 h with blocking solution (4% bovine serum albumin in phosphate buffered saline). Immunofluorescence assays were performed with the following primary antibodies: Synapsin 1 (1/500, SC-20780, Santa Cruz Biotechnology), Syntaxin 1 (1/2000, MAB 336 Millipore Corporation), PSD-95 (1/1000, 73-028, RRID:AB_10698024, UC Davis/NIH NeuroMab Facility), tyrosine hydroxylase (1/1000, 213004, Synaptic Systems), KOR (1:500, Ab10566, Abcam), KOR (1/500, sc-7494, Santa Cruz Biotechnology), D2R (1/250; 73-230, RRID:AB_2094978, UC Davis/NIH NeuroMab Facility), and D2R (1:250, sc-7522, Santa Cruz Biotechnology). Secondary antibodies were donkey anti-rabbit AlexaFluor488, donkey anti-goat AlexaFluor 594, donkey anti-mouse AlexaFluor 594, donkey anti-guinea pig AlexaFluor647 (1/500; Invitrogen Life Technologies), and donkey anti-mouse AlexaFluor 647 (1/500; Jackson Immunoresearch).
We validated anti-D2R antibody (sc-7522, Santa Cruz Biotechnology) by immunofluorescence assays in HEK293 cells transfected with a vector encoding D2R epitope tagged with mcherry to its c-terminus. Positive signal was observed only in transfected cells, which matched mcherry fluorescence (data not shown). This antibody has been previously used for immunostaining in rat midbrain (Angelucci et al., 2000) and other tissues such as spleen and thymus (Mignini et al., 2009). We validated immunofluorescence detection of KOR by using 2 antibodies: Ab10566 (Abcam) and sc-374479 (Santa Cruz Biotechnology). In our hands, KOR immune labeling in mouse brain using these antibodies was similar (data not shown).
Microdialysis Procedure
Microdialysis experiments were carried out in anesthetized rats 48 hours after the last injection (8th) (Figure 1). Rats were anesthetized with chloral hydrate (400 mg/kg) and placed in a stereotaxic apparatus (Stoelting). Anesthesia was maintained by supplemental administration of chloral hydrate as required to maintain the suppression of limb compression withdrawal reflex. Body temperature was maintained by keeping the animals over a controlled heating pad. Microdialysis was carried out as previously described (Escobar et al., 2015). A microdialysis probe 2 mm long (CMA 11; CMA Microdialysis AB) was placed primarily into the NAc core by using the following coordinates relative to Bregma: A.P.: +1.2 to 1.5 mm; M.L.: 1.4 mm; D.V.: 7.4 mm. The microdialysis probe was continuously perfused with Krebs solution at a flow rate of 2 µL/min, and samples were taken every 10 minutes. After 3 baseline levels were taken and to test the effect of KOR activation, U69593 (0.3 mg/kg) was injected 30 min before QNP (0.5 mg/kg) (Figure 1). To stimulate dopamine release, 70 mM K+ Krebs-Ringer phosphate was perfused through the microdialysis probe for 10 minutes. The same microdialysis procedure was performed in a separate group of rats that received U69593 and/or QNP for the first time during the procedure. Microdialysis samples were immediately analyzed for dopamine content as previously described (Escobar et al., 2015). Probe localization was determined using a rat brain atlas (Paxinos and Watson, 2007) on cresyl-stained brain slices. Only data resulting from correct probe placement were considered for further analysis.
Fast Scan Cyclic Voltammetry (FSCV) Procedure
FSCV experiments were carried out in urethane (1.5 g/kg)-anesthetized rats 48 hours after the last injection (8th). FSCV experiments were carried out as we have described (Escobar et al., 2015). A working electrode (glassy-carbon microelectrode) was dropped down into the NAc core by using the following coordinates AP: +1.3 to 1.5 mm; ML: 1.3 mm; DV: 6.5 mm. A stimulating electrode (Plastics One) was lowered into the VTA with the following coordinates AP: -5.2 mm; ML: 1.1 mm; DV: 7.5 mm. Electrical stimulation was delivered every 5 minutes; 3 baseline collections were conducted, and U69593 (0.15 mg/kg i.v.) or vehicle was injected. At 30 minutes later, QNP (0.25 mg/kg i.v.) or saline was injected. Data were analyzed with Demon Voltammetry and Analysis software as described (Yorgason et al., 2011). Working electrodes were calibrated using aCSF containing 3 µM dopamine. On average, the sensitivity obtained was 7 nA/µM. To assure that the electrode was collocated in the appropriate position, a histological inspection of the scar left by the widest part of the glass electrodes in the dorsal part of the coronal slice was performed. In addition, dopamine recapture slope was monitored while the electrode went from the striatum to the NAc. Since dopamine transporter expression is higher in striatum, dopamine recapture is faster in this nucleus compared with the NAc.
Immunofluorescence in Mouse Brain
C57BL/6 mice were anesthetized with a mixture of ketamine/xylazine (50/5 mg/kg, respectively) and fixed by cardiac perfusion with 2% paraformaldehyde. Immunofluorescence studies in brain slices of 40 µm were performed with the following antibodies: goat polyclonal anti-D2R antibody (1:250, sc-7522, Santa Cruz Biotechnology) and rabbit polyclonal anti-KOR antibody (1:1000, Ab10566, Abcam), followed by AlexaFluor 488-conjugated donkey anti-goat IgG antibody (1:1000, Jackson Immunoresearch) and CY3-conjugated donkey anti-rabbit IgG antibody (1:1000, Jackson Immunoresearch). Fluorescence imaging was acquired on a laser-scanning confocal microscope (Olympus, Fluoview 1000) coupled to Fluoview v6.0 software Olympus. Images were obtained with a 100× oil immersion objective.
Data Analysis and Statistics
Statistical analyses were performed using Prism 5 Graphpad software. Differences in locomotor activity and tonic and phasic dopamine release were analyzed by 2-way repeated-measures ANOVA (groupxtime) followed by Bonferroni post-test. Changes in basal dopamine levels, as in the average of phasic dopamine released, was analyzed by 1-way ANOVA. Data are reported as mean ± SEM. Values of P<.05 were considered significant.
Results
Repeated KOR Coactivation Potentiates D2R-Induced Locomotor Sensitization
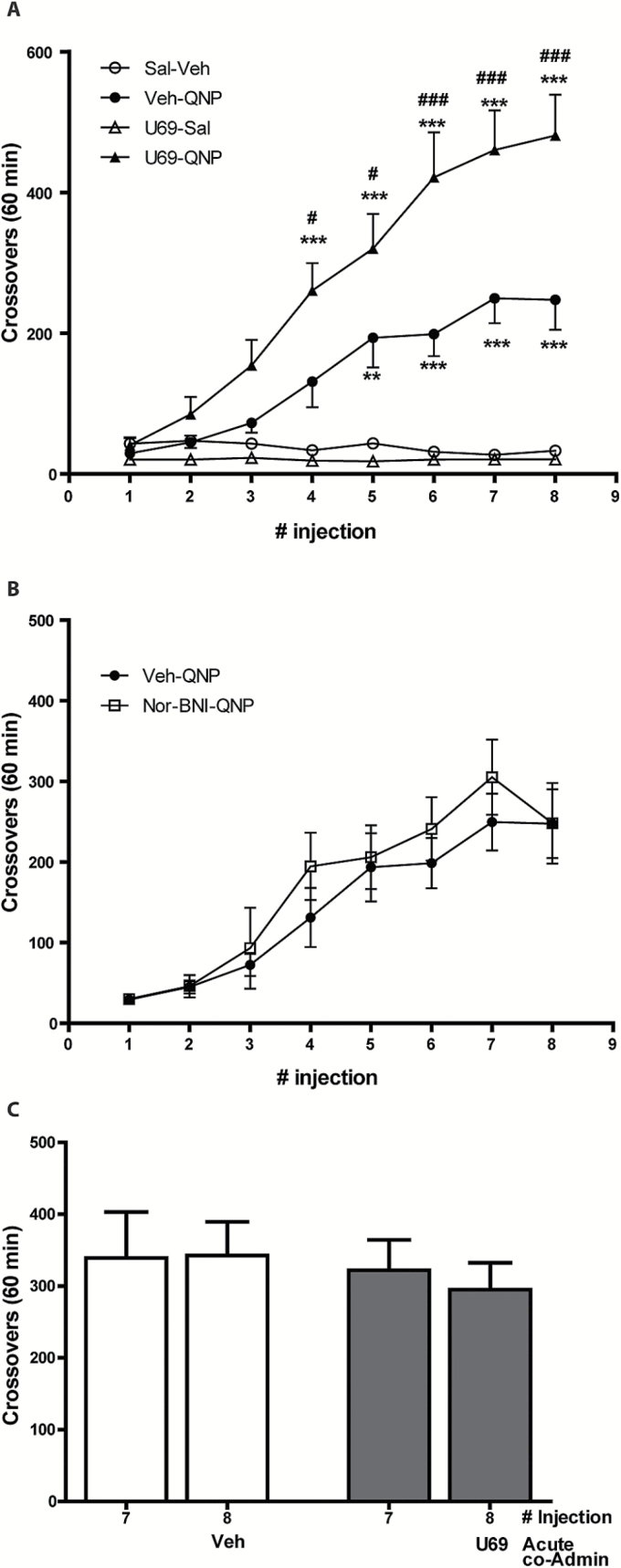
We assessed horizontal locomotor activity in 3 experimental groups. The first group of rats was repeatedly co-treated with U69593 and QNP or with either drug plus the corresponding vehicle to confirm that KOR agonists enhance D2R-induced sensitization. Two-way ANOVA revealed a significant effect of treatment group (F3,399 =137.05, P<.0001), time (F7,399 = 18.42, P<.0001), and interaction (F21,399 = 8.57, P<.0001). Bonferroni post-test indicates that the U69-QNP group displays significantly higher locomotor activity, beginning at the fourth injection, compared with Veh-QNP-treated rats (Figure 2A).
Figure 2.
Repeated kappa opioid receptor (KOR) activation potentiates quinpirole (QNP)-induced locomotor sensitization. (A) Rats were s.c. injected twice per week until 8 injections were completed with a mixture of U69593 (0.3 mg/kg) or an equivalent volume of vehicle, and with QNP (0.5 mg/kg) or an equivalent volume of saline. **P<.01; ***P<.001 compared with Veh-Sal group; #P<.05; ###P<.001 compared with Veh-QNP, according to 2-way ANOVA and Bonferroni post-test. Veh-Sal (n=12); Veh-QNP (n =14); U69-Sal (n=14); U69-QNP (n= 16). (B) Twenty-four hours before the initiation of repeated administration of QNP, rats were preinjected with the long-lasting KOR antagonist, nor-binaltorphirmine (Nor-BNI) (10 mg/kg), or Veh. Veh-QNP (n = 14); Nor-BNI-QNP (n = 6). (C) Rats were repeatedly treated with QNP (0.5 mg/kg), and on the last QNP injection rats were divided into 2 groups: one group received U69593 (0.3 mg/kg) in co-administration with QNP and the other group received an equivalent volume of vehicle in co-administration with QNP. Chronic QNP, acute Veh (n = 6); Chronic QNP, acute U69 (n = 7). Horizontal locomotor activity was quantified for 60 minutes immediately after each injection.
To assess whether endogenous KOR mediates QNP-induced locomotor sensitization, the second group of rats received a single dose of the long-lasting KOR antagonist nor-BNI (Horan et al., 1992) 24 hours before QNP treatment. Two-way ANOVA showed a significant effect of time (F1,18 = 45.44, P<.0001) but no effect of pretreatment (F 1,18 = 0.73, P=.4054) or interaction (F1,18 = 0.65, P=.65). Bonferroni post-test showed no significant differences (Figure 2B). These data eliminate a role of endogenous KOR mediating QNP-induced locomotor sensitization.
Next, we examined whether potentiation of QNP-induced locomotor sensitization requires repeated KOR activation or its acute activation is sufficient. Therefore, the third group of rats was repeatedly treated with QNP, and on the last injection (8th) they were co-injected with QNP and either U69593 or vehicle. Two-way ANOVA showed a significant effect of time (F7,88= 7.17, P<.0001) and no effect of treatment (F1,88=0.16, P=.69) or interaction (F7,88=0.62, P=.74). Bonferroni post-test showed no significant effect (Figure 2C). These data indicate that QNP-induced locomotor sensitization is potentiated by KOR only when both receptors are repeatedly co-activated, suggesting that a slow, enduring incubation of neuroplastic modification underlies KOR enhancement of D2R-induced sensitized behaviors.
Presynaptic Colocalization of KOR and D2R in Dopamine Terminals of the NAc
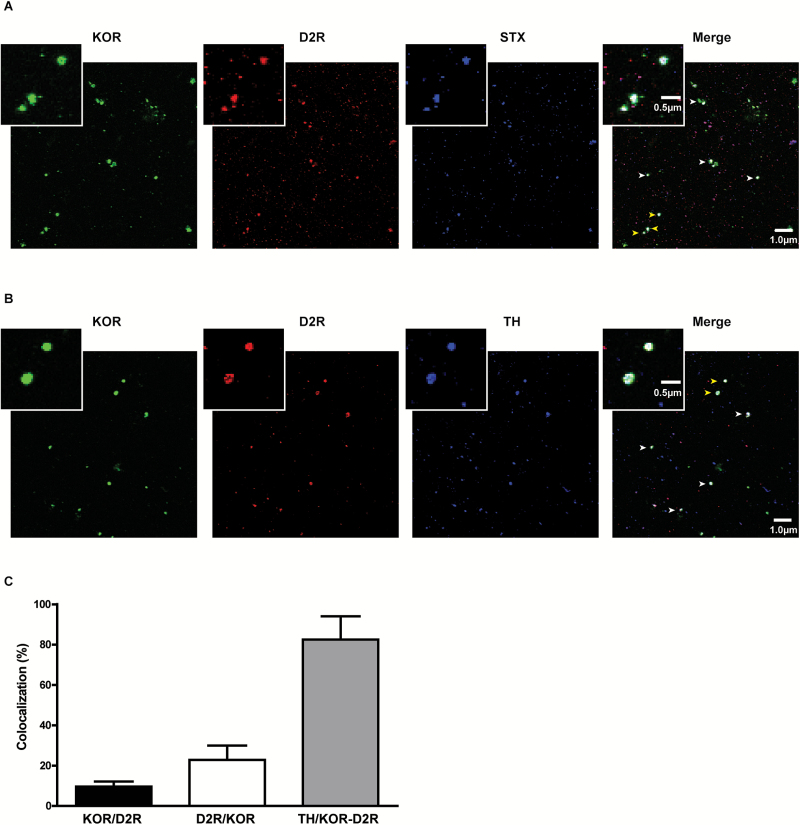
To quantify presynaptic colocalization of KOR and D2R in the NAc, we used a synaptosomal preparation devoid of postsynaptic elements (Slater et al., 2016). The ratio between PSD95 (postsynaptic marker) and Synapsin 1 (Syn) (presynaptic marker) was used in each synaptosomal preparation to control for postsynaptic contamination. Only synaptosomal preparations with PSD95/Syn <5% were considered (Rodrigues et al., 2005). KOR and D2R colocalize in presynaptic synaptosomes as shown by positive Syntaxin 1 signaling (Figure 3A). Quantitative analysis showed that 9.67 ± 2.50% of D2R-positive synaptosomes are also positive for KOR immunostaining, and 22.82 ± 7.14% of KOR positive synaptosomes are also D2R positive (Figure 3C). To investigate whether KOR and D2R colocalize in dopamine terminals, triple colocalization assays including also tyrosine hydroxylase (TH) were carried out. Most KOR-D2R double-positive synaptosomes were positive for TH immunostaining (82.53 ± 11.53%) (Figure 3B-C). These data indicate that even though there is scarce colocalization of KOR and D2R in the NAc presynaptic terminals, KOR-D2R colocalization occurs preferentially in TH-positive terminals, supporting a direct role of KOR regulating D2R autoreceptor function.
Figure 3.
Kappa opioid receptor (KOR) and dopamine D2 receptor (D2R) colocalize presynaptically in tyrosine hydroxylase (TH) terminals of the nucleus accumbens (NAc). (A) Representative 60x images of KOR and D2R colocalization in rat NAc synaptosomes positive for presynaptic marker syntaxin (STX). (B) Representative 60x images of KOR and D2R colocalization in rat NAc synaptosomes positive for the dopamine marker TH. White arrowheads indicate synaptosomes where triple label was found. Yellow arrowheads indicate magnified synaptosomes where triple label was found. (C) Quantification of colocalization of three independent experiments. Black bar = percent of D2R synaptosomes positive for KOR, white bar = percent of KOR synaptosomes positive for D2R, gray bar = percent of synaptosomes positive for both KOR and D2R that are also TH positive.
Lower Dopamine Extracellular Levels but Preserved D2R and KOR Presynaptic Inhibitory Action in the NAc of QNP- and U69593/QNP-Repeated Treated Rats
The colocalization of KOR and D2R in TH terminals of the NAc prompted us to study their functional interaction regulating dopamine. First, we carried out in vivo microdialysis to monitor tonic release that allows determining basal dopamine extracellular levels. A significant reduction of basal extracellular dopamine levels in the NAc was found for Veh-QNP- and U69-QNP-repeatedly treated rats (Table 1). The extent of the reduction was similar between QNP and U69-QNP groups, indicating that lower extracellular dopamine levels are required for D2R-induced locomotor sensitization, but not for KOR potentiating this effect.
Table 1.
Lower Basal Extracellular Dopamine Levels in the NAc of Rats Repeatedly Treated with Veh-QNP or U69-QNP
| Repeated Treatment | Dopamine (fmol/µL) |
|---|---|
| Veh-Sal | 0.86 ± 0.21 |
| Veh-QNP | 0.47 ± 0.11* |
| U69-Sal | 0.80 ± 0.21 |
| U69-QNP | 0.45 ± 0.11* |
*P<.01 compared with Veh-Sal, according to 1-way ANOVA and Bonferroni posttest.Veh-Sal n=7, U69-Sal n=4, Veh-QNP n=6, U69-QNP n=6.
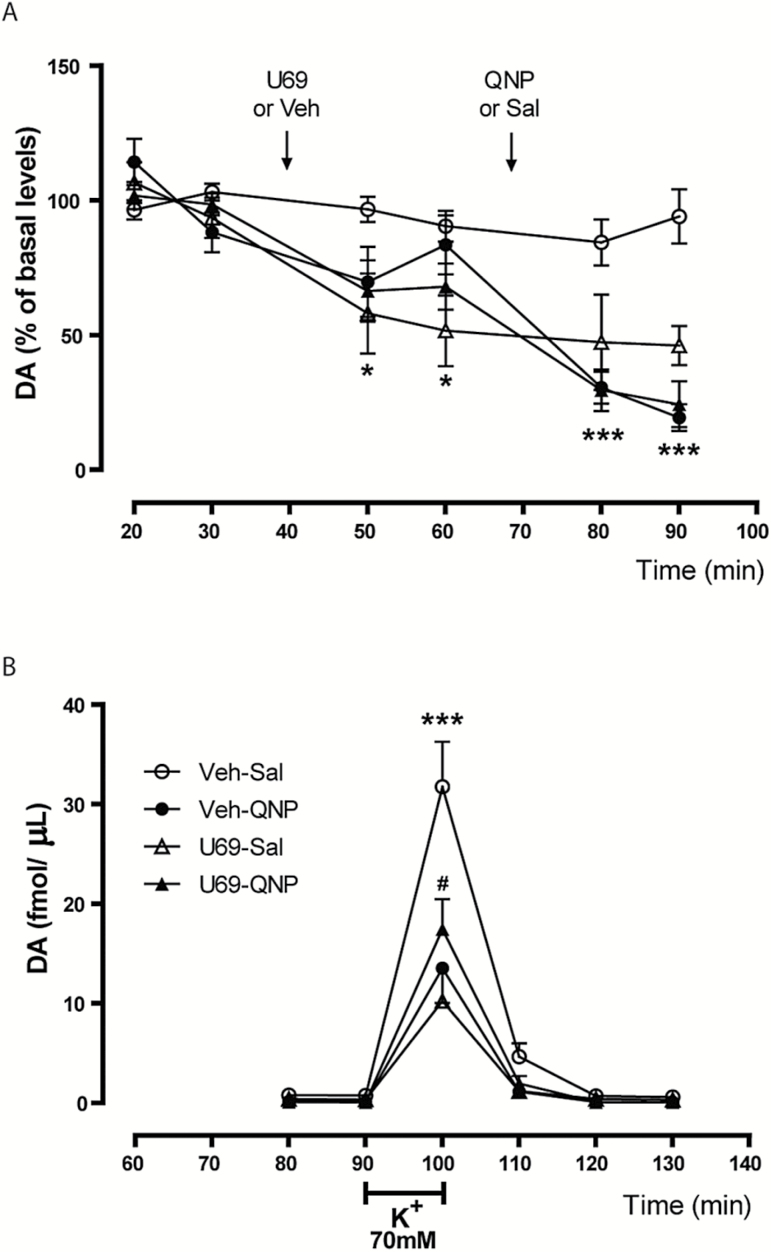
Previous behavioral work suggested that a reduction of presynaptic inhibitory D2R autoreceptor function might mediate locomotor potentiation induced by KOR activation (Perreault et al., 2006). To test this hypothesis, we studied the role of D2R-inhibiting basal and -stimulated dopamine release. Rats were injected (9th injection) with the same drugs that they received during repeated protocol while microdialysis samples of the NAc were taken. To discriminate between KOR and D2R inhibitory action, U69593 was injected first, followed 30 minutes later by QNP injection. A within-subjects 2-way ANOVA showed a significant effect of treatment group (F3,38= 11.65, P=.0001), time (F2,38 = 44.83, P<.0001), and interaction (F6,38 = 6.74, P<.0001). A Bonferroni post-test showed that there were no significant differences in QNP inhibitory effect on basal dopamine levels of U69-QNP- and Veh-QNP-repeatedly treated rats (Figure 3A). Similarly, U69593 injection decreased dopamine levels to the same extent in U69-Sal and U69-QNP-repeatedly treated rats (Figure 4A). The magnitude of the inhibitory action of U69593 and QNP in naïve rats is similar to that found for rats repeatedly treated with the drugs (supplementary Figure 1). A 70-mM K+ stimulus was used to assess KOR and D2R presynaptic inhibitory function during depolarization. A 2-way ANOVA showed a significant effect of time (F5,112 = 67.31, P<.0001), treatment (F3,112 = 9.28, P<.0001), and interaction (F15,112=5.26, P<.0001). A Bonferroni post-test showed significant differences in maximal dopamine release reached by each group. QNP and U69593 injected to Veh-QNP- and U69-Sal-repeatedly treated rats, respectively, inhibited to the same extent K+-stimulated dopamine release (Figure 4B). Rats repeatedly co-treated with U69-QNP showed no significant differences in inhibition of K+-induced dopamine release after coactivation of KOR and D2R compared with Veh-QNP-repeatedly treated rats (Figure 4B). However, the inhibitory effect was significantly lower compared with U69-Sal-repeatedly treated rats, suggesting occlusion between agonists. Altogether, these data eliminate the loss of D2R autoreceptor function as an explanation for KOR potentiation of QNP-induced sensitization. It is interesting to note that the inhibitory effects exerted by KOR and D2R did not add or synergize, indicating that these receptors share the same mechanism to inhibit dopamine release.
Figure 4.
Preserved dopamine D2 receptor (D2R) presynaptic inhibition of tonic dopamine levels after kappa opioid receptor (KOR) potentiation of quinpirole (QNP) locomotor sensitization. (A) Effect of acute U69593, QNP, or both on basal dopamine levels. Data are expressed as the percentage of basal dopamine levels *P<.05, U69-Veh and U69-QNP compared with Sal-Veh, ***P<.001 Veh-QNP and U69-QNP compared with Veh-Sal, according to 2-way ANOVA and Bonferroni posttest. (B) Temporal course of the K+-stimulated dopamine release after the activation of KOR and D2R on chronically treated rats. ***P<.001, Veh-Sal compared with U69-Sal, Veh-QNP, and U69-QNP; #P<.05, U69-QNP compared with U69-Sal, according to 2-way ANOVA and Bonferroni posttest. Veh-Sal n=7, U69-Sal n=4, Veh-QNP n=6, U69-QNP n=6.
Faster D2R Inhibition of Dopamine Release after Repeated Coactivation of KOR and D2R
Microdialysis experiments do not allow for detection of rapid changes in dopamine neurotransmission. Therefore, we used FSCV to investigate phasic dopamine release by directly stimulating VTA cell bodies to induce dopamine release in NAc terminals. Similar to microdialysis procedure, a 9th injection following the same treatment that each rat received repeatedly was carried out during the FSCV. A within-subjects 2-way ANOVA showed a significant effect of time (F20,280 = 24.62, P<.0001), treatment (F3,280 = 6.67, P=.0050), and interaction (F60,280 =3.98, P<.0001), thus indicating that U69593 and QNP injection induced a significant decrease in electrically evoked dopamine release in all groups (Figure 5B), supporting microdialysis data indicating intact KOR and D2R inhibitory presynaptic function of dopamine in the NAc, after their independent repeated activation.
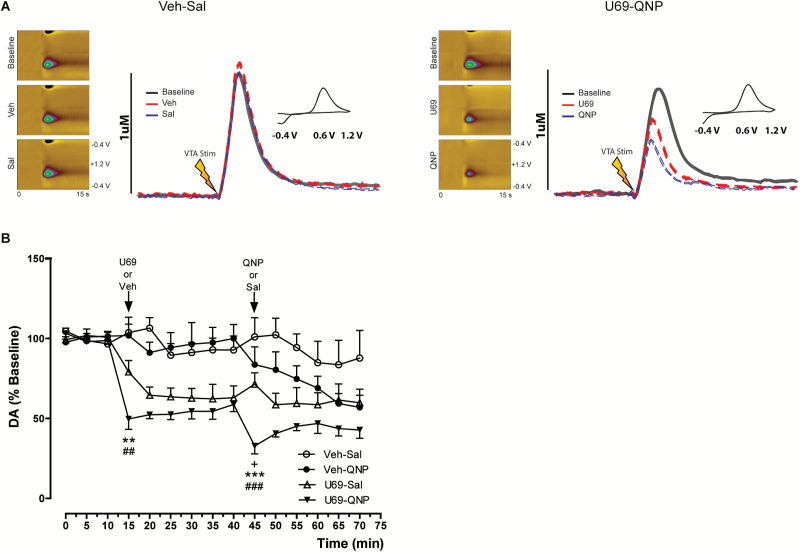
Figure 5.
Faster and higher dopamine D2 receptor (D2R) presynaptic inhibition of dopamine release in U69-quinpirole (QNP)-repeatedly treated rats. (A) Examples of dopamine release dynamics, expressed as dopamine concentration vs time in control (Veh-Sal) and U69-QNP-repeatedly treated rats. Baseline conditions (black trace) and first injection (red trace) and second injection (blue trace). Insets show cyclic voltammograms in response to applied potential, left panels represent the current generated, depicted in color, in response to the applied potential and the time of register. The oxidation of dopamine occurs at 0.6 V. (B) Time course of electrically evoked dopamine release expressed as percent of the baseline. Data correspond to mean±SEM. **P<.01; ***P<.001 for U69-Sal group; +P<.05 for Veh-QNP group; ##P<.01; ###P<.001 for U69-QNP group; significant differences compared with the third electrically evoked dopamine release (minute 10), according to 2-way ANOVA and Bonferroni post-test. Veh-Sal n= 4; U69-Sal n= 6; Veh-QNP n= 5; U69-QNP n= 6.
Unexpectedly, KOR and D2R presynaptic inhibitory function was enhanced during the first minutes in rats repeatedly treated with U69-QNP, as indicated by Bonferroni posttest. Temporal courses of electrically evoked dopamine release show that the inhibitory effect of U69593 and QNP reached their maximum faster in rats co-treated with U69593 plus QNP than single drug-treated rats (Figure 5B). Altogether, the data show that repeated KOR and D2R coactivation results in an enhanced and faster presynaptic inhibitory action of both KOR and D2R in the NAc, suggesting that these receptors are more available or have a better coupling to second messenger system after their coactivation.
KOR and D2R Colocalize Postsynaptically in MSN Neurons of the NAc
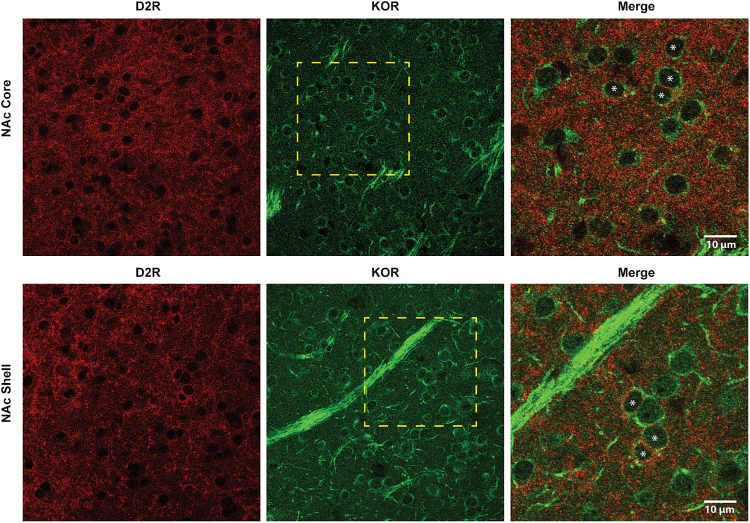
The colocalization of KOR and D2R on TH terminals supports KOR enhancing D2R presynaptic inhibitory function that associates with the acceleration of D2R-induced locomotor sensitization. However, it does not explain KOR potentiating D2R-induced locomotor activity. To assess this possibility, immunofluorescence assays were carried out in the core and shell subregions of the NAc. The experiments were performed in mice instead of rats, since the quality of immunofluorescence signals was significantly better. High magnification images of the NAc core and shell show a strong signal for KOR in D2R-positive as well as in D2R-negative MSN of the NAc (Figure 6). These anatomical data prove that KOR and D2R are in the same MSN neurons, supporting a role for KOR potentiating D2R-inducing locomotor sensitization.
Figure 6.
Kappa opioid receptor (KOR) and dopamine D2 receptor (D2R) co-localize in nucleus accumbens (NAc) cell bodies. Representative 100x images of a section of NAc core, upper panel, and NAc shell, lower panel. Yellow square represents a magnified section of the image from which a merge has been made. White asterisks represent cell bodies positive for both KOR and D2R. Brains of 3 mice were used for immunofluorescence experiments.
Discussion
Here, we replicated previous data showing that coactivation of KOR accelerates and potentiates locomotor sensitization induced by repeated administration of QNP. Our neurochemical data show that decreased extracellular dopamine levels in the NAc are associated with D2R sensitization, and KOR coactivation accelerates the inhibitory action of D2R autoreceptors. Anatomically, we found that the highest KOR and D2R presynaptic colocalization occurs in the same dopamine terminals, supporting a role for KOR in regulating D2R presynaptic function in the NAc. Also, we found high postsynaptic localization of KOR in D2R-positive MSN cell bodies of the NAc, supporting a role of KOR potentiating D2R inhibition of the indirect pathway.
D2R-induced locomotor sensitization, defined as an increased response to the same stimulus, has pre- and postsynaptic components. On one hand, we and others have shown a significant decrease of synaptic dopamine in the NAc after repeated QNP treatment (Koeltzow et al., 2003; Escobar et al., 2015). In fact, QNP-sensitized rats show decreased burst activity of VTA dopamine neurons, which may explain less synaptic dopamine in the NAc (Sesia et al., 2013). On the other hand, less dopamine induces supersensitivity of dopaminergic receptors, leading to an overinhibition of the indirect pathway of motor control with each QNP challenge. Which of these components is accelerated and potentiated with the concomitant activation of KOR receptors?
Initially, we tested the hypothesis that KOR endogenous activity could underlie locomotor sensitization induced by repeated stimulation of D2R. The results showed that KOR blockade did not prevent enhancement in horizontal locomotion induced by QNP, indicating that endogenous KOR signaling does not mediate the induction of locomotor sensitization by repeated D2R activation. Consistent with this finding, no increase in the endogenous KOR agonist, dynorphin, was observed after repeated D2R activation (Engber et al., 1992; Perreault et al., 2007a).
We then tested the hypothesis that KORs have an immediate effect enhancing locomotor sensitization induced by repeated activation of D2R. Indeed, it was reported that enhancement of QNP-induced locomotor activity starts from the first co-injection with U69593 (Perreault et al., 2006), which suggested that the KOR effect enhancing QNP-induced locomotor sensitization does not require time to develop. Two pieces of data showed that this is not the case. First, our behavioral data showed that the KOR effect is not immediate. Horizontal locomotor activity was significantly greater by the fourth injection in rats treated with U69-QNP compared with control rats. Second, no differences were found on QNP-induced locomotor activity in QNP-sensitized rats acutely injected with U69593 or vehicle. These observations indicate that KOR-dependent potentiation of QNP-sensitized locomotion requires repeated KOR coactivation.
Our behavioral and neurochemical data indicate that KOR enhancement of D2R-induced locomotor sensitization involves both pre- and postsynaptic components. To obtain a neuroanatomical substrate for the functional presynaptic interaction between KOR and D2R, we studied whether KOR and D2R are colocalized in dopamine terminals of the NAc. We found that the highest colocalization of D2R and KOR in the NAc was in synaptosomes containing TH, supporting a role of KOR regulating D2R autoreceptor function. FSCV data showed that KOR acceleration of QNP-induced locomotor sensitization is accompanied by faster inhibitory response of presynaptic D2R decreasing electrically evoked dopamine release. In conclusion, the data indicate that the acceleration of D2R-dependent locomotor sensitization induced by KOR coactivation is due to its presynaptic action reinforcing the decrement of phasic dopamine release.
The effect of KOR potentiating D2R-dependent locomotor sensitization, that is, reaching more locomotor activity, can have 2 possible explanations: recruiting more MSN efferent cells of the indirect pathway or enhancing postsynaptic D2R action on these same neurons. We reject that KOR coactivation increases postsynaptic D2R sensitization, since steady-state extracellular dopamine levels in the NAc were reduced to the same extent in U69-QNP and Veh-QNP. Therefore, to further investigate the mechanism underlying KOR potentiation of D2R-dependent locomotor sensitization, we studied colocalization of these receptors postsynaptically in the NAc. As expected, D2R is localized in some MSN cell bodies of striatum and NAc. Unexpectedly, we found large KOR-like immunolabeling in postsynaptic MSN cell bodies of the NAc. More important and supporting a role of KOR potentiating D2R-induced locomotor sensitization, a subpopulation of MSN of the NAc was positive for both KOR and D2R. It is worth mentioning that immunofluorescent assays were performed in mice instead of rats to have better quality anatomical data. Even though it is possible that the distribution of the receptors could be different in both species, behavioral and neurochemical studies indicate that mice and rats share molecular mechanisms regarding locomotor sensitization. For instance, similar to rats, mice develop locomotor sensitization induced by drugs of abuse and QNP (Thompson et al., 2010), and D2R and KOR activation decrease dopamine release in the NAc (Ehrich et al., 2014).
Altogether, we propose that potentiation of D2R-dependent sensitization by KOR coactivation is due to stabilizing D2R inhibitory action on efferent MSN of the NAc. However, we cannot exclude that dorsal striatum or other brain nuclei play important roles in KOR enhancing D2R-induced compulsive behaviors. Indeed, KOR and D2R have a similar role regulating dopamine neurotransmission in dorsal striatum and locate presinaptically in dopamine terminals and postsynaptically in MSN (data not shown), where they can also interact. Further studies are required to test the role of dorsal striatum in KOR enhancing D2R-mediated sensitization.
In conclusion, the diminution of the extracellular levels of dopamine and consequently the sensitization of the D2R seem to be key elements in the maintenance of compulsive behaviors. Our data show that repeated coactivation of KOR potentiates D2R-induced locomotor sensitization by increasing the efficacy of D2R pre- and postsynaptic inhibitory action, suggesting that repeated activation of KOR stabilizes D2R in the plasmatic membrane or the coupling to secondary messenger system. Increasing data show that KOR activation enhances motivation for drugs of abuse and mediates the effect of stress associated with chronic abuse of drugs (Koob et al., 2014). Our data provide a mechanism by which KOR modifies the action of D2R that could be the basis of lasting changes observed in compulsive behaviors.
Statement of Interest
None.
Supplementary Material
Acknowledgments
This work was supported by Fondo Nacional de Desarrollo Científico y Tecnológico (grants 1110352, 1150200 to M.E.A.). A.P.E. received Conicyt doctoral fellowship no. 21110343.
References
- Acri JB, Thompson AC, Shippenberg T (2001) Modulation of pre- and postsynaptic dopamine D2 receptor function by the selective kappa-opioid receptor agonist U69593. Synapse 39:343–350. [DOI] [PubMed] [Google Scholar]
- Angelucci F, Mathé AA, Aloe L (2000) Brain-derived neurotrophic factor and tyrosine kinase receptor TrkB in rat brain are significantly altered after haloperidol and risperidone administration. J Neurosci Res 60:783–794. [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E, Girault JA (2008) Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci 28:5671–5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefer VI, Czyzyk T, Bolan EA, Moron J, Pintar JE, Shippenberg TS (2005) Endogenous kappa-opioid receptor systems regulate mesoaccumbal dopamine dynamics and vulnerability to cocaine. J Neurosci 25:5029–5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A (1988) Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther 244:1067–1080. [PubMed] [Google Scholar]
- Dvorkin A, Perreault ML, Szechtman H (2006) Development and temporal organization of compulsive checking induced by repeated injections of the dopamine agonist quinpirole in an animal model of obsessive-compulsive disorder. Behav Brain Res 169:303–311. [DOI] [PubMed] [Google Scholar]
- Ehrich JM, Phillips PE, Chavkin C (2014) Kappa opioid receptor activation potentiates the cocaine-induced increase in evoked dopamine release recorded in vivo in the mouse nucleus accumbens. Neuropsychopharmacol 39:3036–3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engber TM, Boldry RC, Kuo S, Chase TN (1992) Dopaminergic modulation of striatal neuropeptides: differential effects of D1 and D2 receptor stimulation on somatostatin, neuropeptide Y, neurotensin, dynorphin and enkephalin. Brain Res 581:261–268. [DOI] [PubMed] [Google Scholar]
- Escobar AP, Cornejo FA, Olivares-Costa M, Gonzalez M, Fuentealba JA, Gysling K, Espana RA, Andres ME (2015) Reduced dopamine and glutamate neurotransmission in the nucleus accumbens of quinpirole-sensitized rats hints at inhibitory D2 autoreceptor function. J Neurochem 134:1081–1090. [DOI] [PubMed] [Google Scholar]
- Fuentealba JA, Gysling K, Magendzo K, Andres ME (2006) Repeated administration of the selective kappa-opioid receptor agonist U-69593 increases stimulated dopamine extracellular levels in the rat nucleus accumbens. J Neurosci Res 84:450–459. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA (2008) Limbic and cortical information processing in the nucleus accumbens. Trends Neurosci 31:552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan P, Taylor J, Yamamura HI, Porreca F (1992) Extremely long lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J Pharmacol Expe Ther 260:1237–1243. [PubMed] [Google Scholar]
- Izenwasser S, Acri JB, Kunko PM, Shippenberg T (1998) Repeated treatment with the selective kappa opioid agonist U-69593 produces a marked depletion of dopamine D2 receptors. Synapse 30:275–283. [DOI] [PubMed] [Google Scholar]
- Koeltzow TE, Austin JD, Vezina P (2003) Behavioral sensitization to quinpirole is not associated with increased nucleus accumbens dopamine overflow. Neuropharmacology 44:102–110. [DOI] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW Jr, George O (2014) Addiction as a stress surfeit disorder. Neuropharmacol 76:370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL (2006) Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc Natl Acad Sci U S A 103:2938–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignini F, Tomassoni D, Traini E, Amenta F (2009) Dopamine, vesicular transporters and dopamine receptor expression and localization in rat thymus and spleen. J Neuroimmunol 206:5–13. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2007) The rat brain in stereotaxic coordinates. San Diego: Academic Press. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Graham D, Bisnaire L, Simms J, Hayton S, Szechtman H (2006) Kappa-opioid agonist U69593 potentiates locomotor sensitization to the D2/D3 agonist quinpirole: pre- and postsynaptic mechanisms. Neuropsychopharmacology 31:1967–1981. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Graham D, Scattolon S, Wang Y, Szechtman H, Foster JA (2007a) Cotreatment with the kappa opioid agonist U69593 enhances locomotor sensitization to the D2/D3 dopamine agonist quinpirole and alters dopamine D2 receptor and prodynorphin mRNA expression in rats. Psychopharmacology (Berl) 194:485–496. [DOI] [PubMed] [Google Scholar]
- Perreault ML, Seeman P, Szechtman H (2007b) Kappa-opioid receptor stimulation quickens pathogenesis of compulsive checking in the quinpirole sensitization model of obsessive-compulsive disorder (OCD). Behav Neurosci 121:976–991. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW (1997) A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev 25:192–216. [DOI] [PubMed] [Google Scholar]
- Rodrigues RJ, Alfaro TM, Rebola N, Oliveira CR, Cunha RA (2005) Co-localization and functional interaction between adenosine A(2A) and metabotropic group 5 receptors in glutamatergic nerve terminals of the rat striatum. J Neurochem 92:433–441. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Aoki C, Pickel VM (1994) Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci 14:88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM (1990) In the rat medial nucleus accumbens, hippocampal and catecholaminergic terminals converge on spiny neurons and are in apposition to each other. Brain Res 527:266–279. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM (1992) Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol 320:145–160. [DOI] [PubMed] [Google Scholar]
- Sesia T, Bizup B, Grace AA (2013) Evaluation of animal models of obsessive-compulsive disorder: correlation with phasic dopamine neuron activity. Int J Neuropsychopharmacol 16:1295–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater PG, Noches V, Gysling K (2016) Corticotropin-releasing factor type-2 receptor and corticotropin-releasing factor-binding protein coexist in rat ventral tegmental area nerve terminals originated in the lateral hypothalamic area. Eur J Neurosci 43:220–229. [DOI] [PubMed] [Google Scholar]
- Stuchlik A, Radostová D, Hatalova H, Vales K, Nekovarova T, Koprivova J, Svoboda J, Horacek J (2016) Validity of quinpirole sensitization rat model of OCD: linking evidence from animal and clinical studies. Front Behav Neurosci 26:10–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svingos AL, Chavkin C, Colago EE, Pickel VM (2001) Major coexpression of kappa-opioid receptors and the dopamine transporter in nucleus accumbens axonal profiles. Synapse 42:185–192. [DOI] [PubMed] [Google Scholar]
- Szechtman H, Talangbayan H, Eilam D (1993) Environmental and behavioral components of sensitization induced by the dopamine agonist quinpirole. Behav Pharmacol 4:405–410. [PubMed] [Google Scholar]
- Szechtman H, Sulis W, Eilam D (1998) Quinpirole induces compulsive checking behavior in rats: a potential animal model of obsessive-compulsive disorder (OCD). Behav Neurosci 112:1475–1485. [DOI] [PubMed] [Google Scholar]
- Thompson D, Martini L, Whistler JL (2010) Altered ratio of D1 and D2 dopamine receptors in mouse striatum is associated with behavioral sensitization to cocaine. PLoS One 5:e11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triana-Del Rio R, Montero-Domínguez F, Cibrian-Llanderal T, Tecamachaltzi-Silvaran MB, Garcia LI, Manzo J, Hernandez ME, Coria-Avila GA (2011) Same-sex cohabitation under the effects of quinpirole induces a conditioned socio-sexual partner preference in males, but not in female rats. Pharmacol Biochem Behav 4:604–613. [DOI] [PubMed] [Google Scholar]
- Wee S, Koob GF (2010) The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacol 210:121–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Jones SR (2011) Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods 202:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.