ABSTRACT
The haematopoietic niche is contributed to by bone marrow-resident mesenchymal stromal cells (BM-MSCs) and subverted by prostate cancer cells. To study mechanisms by which BM-MSCs and prostate cancer cells may interact, we assessed the migration, invasion, adhesion and proliferation of bone-derived prostate cancer cells (PC-3) in co-culture with pluripotent human BM-MSCs. We observed a strong adhesive, migratory and invasive phenotype of PC-3 cells with BM- MSC-co-culture and set out to isolate and characterize the bioactive principle. Initial studies indicated that chemotaxis was secondary to a protein residing in the >100kDa fraction. Size-exclusion chromatography (SEC) recovered peak activity in a high-molecular weight fraction containing thrombospondin-1 (TSP1). While TSP1 immunodepletion decreased activity, put-back with purified TSP1 did not reproduce bioactivity. Further purification of the TSP1-containing high-molecular weight fraction of the BM-MSC secretome with heparin-affinity chromatography recovered bioactivity with highly restricted bands on polyacrylamide gel electrophoresis, determined by mass spectroscopy to be proteolytic fragments of fibronectin (FN). Put-back experiments with full-length FN permitted adhesion but failed to induce migration. Monospecific antibodies to FN blocked adhesion. Proteolytic cleavage of FN generated FN fragments which now induced migration. Neutralizing monoclonal antibodies to FN receptors α5 and β1 integrins, and α5 knockdown specifically blocked migration and adhesion. Conclusion: Fibronectin fragments (FNFr) function as matrikines driving the chemotactic affinity of prostate cancer cells via the α5β1 integrin. Taken together with the high-frequency of α5β1 expression in disseminated prostate cancer cells in bone marrow aspirates from patients, the FNFr/FN-α5β1 interaction warrants further study as a therapeutic target.
KEYWORDS: adhesion, bone marrow mesenchymal stromal cells, bone metastases, chemotaxis, fibronectin fragments, matrikine, prostate cancer
Introduction
Prostate cancer is unique among solid neoplasms for its proclivity to disseminate with great efficiency within the bone marrow microenvironment, mimicking a leukemic illness. The morbidity and mortality of prostate cancer is highly correlated with this conserved biological behavior described as the lethal phenotype of the disease.1 The specific advantages endowed by the bone marrow microenvironment for prostate cancer are therefore of great interest as they may offer avenues to effectively interdict the illness at its earliest stages of progression in the metastatic niche, long before evolutionary pressures drive the emergence of variant and resistant biological traits. Currently the only niche-directed therapeutics in prostate cancer is represented by bone-homing radioisotopes that modestly impact survival and skeletal-related events.2,3
The clinical observations that bone metastases from solid tumors from infancy to adulthood concord with the centripetal migration of hematopoiesis with aging suggest that bone metastases require subversion of the haematopoietic niche. Although endosteal, perivascular and other locations of the haematopoietic niche continue to be assessed,4 mesenchymal stromal cells and their osteoblastic derivatives have been previously implicated as architects of the haematopoietic niche.5-7 In experimental murine models prostate cancer cells can be demonstrated to compete with CD45+ haematopoietic stem cells for a niche specified by osteoblasts.8 Interactions between prostate cancer cells and human bone marrow mesenchymal stromal cells (BM-MSCs) and their osteoblast derivatives likely contribute critically to the well-recognized phenotype of osseous-dominant progression of the disease, a phenotype that cannot be replicated in animal models to date, potentially because of species-specific differences in epithelial-matrix interactions.
In order to generate candidate seed-and-soil mechanisms of disease progression in the bone marrow microenvironment, we studied the interactions of bone-derived human PC-3 prostate cancer cells and pluripotent human bone marrow derived BM-MSCs in co-culture and sought to isolate and characterize the mechanisms driving the strong migratory, invasive and adhesive behavior that was immediately observed.
Results
Human bone-derived prostate cancer cells display strong migratory affinity to human bone-derived BM-MSC in 2-chamber co-culture assays
When PC-3 prostate cancer cells are co-cultured with BM-MSCs, a strong migratory and invasive behavior is identified. This migratory and invasive behavior is conserved with the BM-MSC secretome along with a strong adhesive phenotype. By contrast, there is apparently little impact on the proliferation of the prostate cancer cells (Fig. 1).
Figure 1.
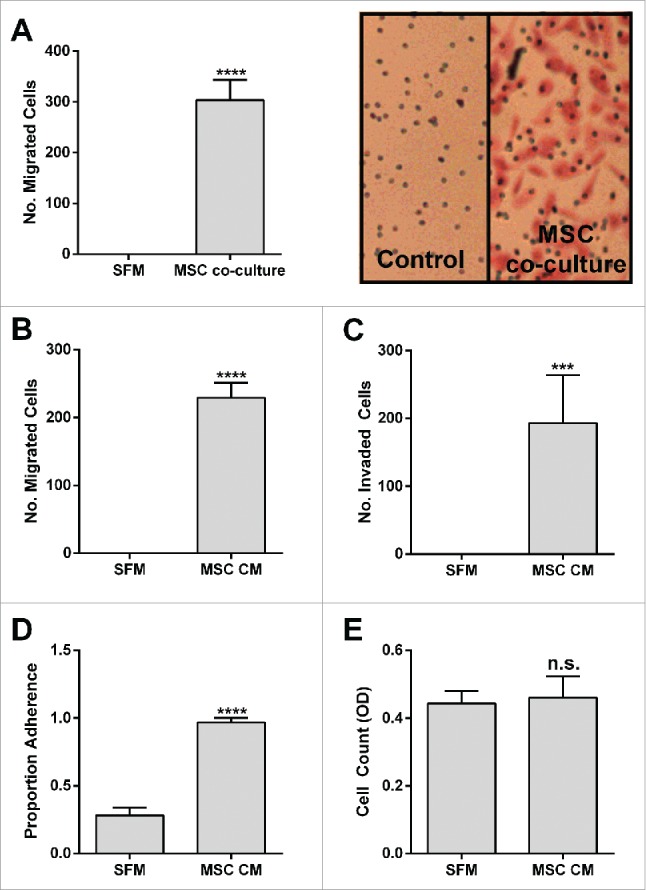
BM-MSCs promote PC-3 Migration, Invasion and Adhesion. PC-3 (A) migration toward BM-MSCs in co-culture (****p < 0.0001), (B) migration toward BM-MSC CM (****p < 0.0001), (C) invasion toward BM-MSC CM (****p < 0.001), (D) adhesion to BM-MSC CM (*****p < 0.0001), (E) cell count after 72 h culture in BM-MSC CM (n.s.). Number of migrated and invaded cells reported are mean ± SD of 5 representative fields on the membrane. Proportion adherence reported is mean ± SD (n = 3) of the ratio of adherent to input cells as determined by cell viability (MTS) assay. Cell count reported is mean ± SD (n = 3) of cell viability (MTS). SFM: serum free media, MSC CM: BM-MSC conditioned media.
Initial proteomic characterization of the BM-MSC secretome
The chemotactic and adhesive bioactivity of the BM-MSC secretome was preserved under serum-free and phenol-red free conditions without the need for protease inhibitors. Trypsinization and heat inactivation suggested that the bioactivity was mediated by a protein. Initial purification efforts with anion-exchange chromatography failed to recover activity. Size separation with spin columns showed that activity was retained by up to 100 kDa filters (Fig. 2a). In order to characterize this further, BM-MSC serum-free conditioned media was concentrated and subjected to size-exclusion chromatography (SEC) (Fig. 2b). The resultant chromatogram allowed recovery of bioactivity in a high-molecular weight peak close to 440 kDa (Fig. 2c). Polyacrylamide gel electrophoresis demonstrated restricted bands (Fig. 2d) which were analyzed by mass spectrometry and a band containing thrombospondin-1 (TSP1) was identified. TSP1 is a 420 kDa glycoprotein comprised of 3 identical polypeptide chains (140 kDa) cross-linked by disulfide bonds9 and had been previously implicated in the migratory behavior of tumor cells.10 Immunospecific depletion of TSP1 resulted in consistent loss of bioactivity, however put-back assays with purified platelet-TSP1 following immunodepletion failed to recover bioactivity (Fig. 2e).
Figure 2.

Initial proteomic characterization of BM-MSC secretome (A) BM-MSC-derived conditioned media (CM) was subjected to ultracentrifugation (50,000 × g for 3 hrs) and separately to variable molecular-weight exclusion filters (Centricon) by centrifugation. The starting CM, UC-derived supernatant (UC), and Centricon filtered (F) and retained (R) fractions were tested in a PC3 migration assay. (B) Concentrated CM (400 mL to 200 μl through a 100 kDa Centricon filter; solid line) was fractionated by size exclusion chromatography (gray line: MW standards). (C) Fractions corresponding to protein peaks in (B) were assessed for PC-3 chemotactic activity. The chemotactic activity in Fx1 and Fx2 was significantly increased relative to SFM (***p < 0.001). (D) Polypeptides from Fx1 were resolved on a 4–12% gel (Invitrogen), and silver stained (SilverQuest, Invitrogen) according to manufacturer's instructions. The bands were excised from the gel and subjected to mass spectroscopic (LC/MS/MS) analysis at Tufts Core Facility. Thrombospondin-1 (TSP-1) was identified from the 140 kDa band (arrow). (E) CM was incubated with 10 μg of α-TSP-1 antibody (IgG1κ, Invitrogen Life Technologies) or isotype control (Iso) antibody, immune complexes cleared by adding protein G-coupled agarose. TSP-1 (15 µg/ml), courtesy Jack Lawler, Beth Israel Deaconess Medical Center, was used for putback assay into TSP-1 depleted CM. Supernatants were tested in the PC-3 migration bioassay. TSP-1 immunodepletion reduced bioactivity, but TSP-1 putback did not recover activity. Number of migrated cells reported are mean ± SD of 5 representative fields on the membrane. Fil: Filtrate obtained by initial concentration of CM. SFM: serum-free medium control.
Heparin-affinity chromatography to purify the TSP1-containing BM-MSC secretome recovers bioactivity leading to identification of fibronectin fragments as the putative chemotaxin
TSP1 is known to bind to a wide range of proteins to mediate cell-cell and cell-matrix interactions.11 Reasoning that a TSP1 associated protein may be responsible for bioactivity we turned to heparin-affinity chromatography which is routinely employed to purify TSP1 from platelets.12 We found that BM-MSC bioactivity was depleted by heparin-affinity and elution of the column recovered bioactivity (Fig. 3a) allowing for further purification. Persistent, albeit depleted, bioactivity in the flowthrough compared to the input likely resulted from saturation of the heparin-affinity column.The eluate from heparin-affinity was then subjected to SEC with recovery of peak bioactivity in a discrete fraction (Fig. 3b, 3c) with a highly-restricted band on PAGE (Fig. 3d). Mass spectroscopy identified a proteolytic fragment of fibronectin (FN) of approximate mass of 140 kDa (Fig. 3d). With N-terminal Edman sequencing a starting peptide sequence (VATSE) was identified. This matches with the start of the second Type III FN repeat which is a major proteolytic site.13 Fibronectin and its fragments have been demonstrated to bind or associate with TSP1 in the matrix.14-16 Although immunoprecipitation of TSP1 with a FN antibody was demonstrable in the BM-MSC secretome, immunoprecipitation studies of FN and FN fragments with a TSP1 antibody have been inconclusive (Suppl Fig. 1). FN fragments in the BM-MSC secretome may also be purified directly by heparin affinity independent of TSP1 binding. A strong heparin affinity site in fibronectin is found in the C-terminal domain which is conserved in the bioactive FN fragments we isolated.13
Figure 3.
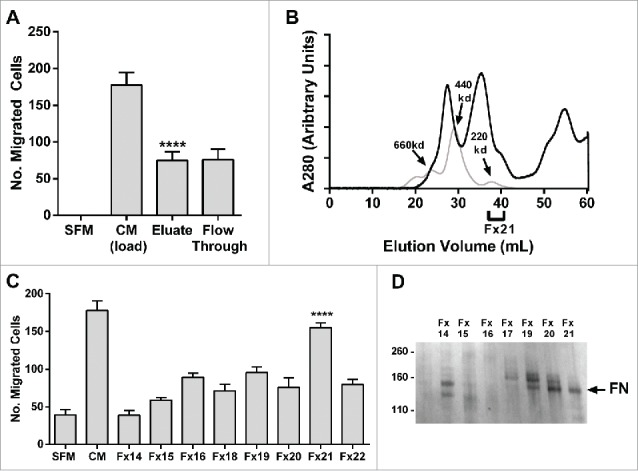
Fibronectin fragments are identified as the putative chemotaxin. (A) 500 mL of BM-MSC CM was subjected to heparin affinity chromatography (as described in methods). PC-3 cell migration assay was performed on load, eluate and flow through demonstrating that bioactivity was recovered in the eluate (****p < 0.0001 compared to SFM). (B) Concentrated heparin eluate (black line) was fractionated on size exclusion chromatography (gray line: molecular weight standards) and (C) resulting fractions were tested in a PC3 migration assay. Peak activity was found in Fx21 (****p < 0.0001). (D) Polypeptides from Fx14-21 were resolved on a 4–12% gel (Invitrogen Life Technologies), and silver stained (SilverQuest, Invitrogen) according to manufacturer's instructions. The bands were excised from the gel and subjected to mass spectroscopic (LC/MS/MS) analysis (Taplin Mass Spectrometry Facility, Harvard). A fragment of fibronectin was identified from the 140 kDa band (arrow). Number of migrated cells reported are mean ± SD of 5 representative fields on the membrane. SFM: serum-free media, CM: BM-MSC conditioned media, FN: fibronectin.
The classic fibronectin receptors a5 and β1 integrin mediate the migratory and adhesive behavior of prostate cancer cells to the BM-MSC secretome and fibronectin and its fragments
To determine the mechanism by which fibronectin fragments (FNFr) signal in prostate cancer cells, the classic fibronectin receptors a4β1 and a5β1 were first profiled in the prostate cancer cells. We found that PC-3 cells express the av, a5, a6 and β1 integrins. Monoclonal antibodies to a5 or β1but not to av, a4, a6 or β3 blocked the migration and adhesion of PC-3 cells to the BM-MSC secretome (Fig. 4a). Putback assays with FN induced both migration and adhesion and this was also inhibited by monospecific antibodies for a5 or β1 integrin (Fig. 4b). Genetic inactivation of integrin a5 (ITGA5) with shRNA also blocked migration and adhesion of PC-3 cells to BM-MSC secretome (Fig. 4c). Although adhesion was uniformly observed (Fig. 4d), one of several commercial preparations of human plasma FN failed to induce a migratory response in PC-3 cells and when this was compared to 2 other preparations used in earlier put-back assays (Fig. 4b) it was noted to lack fibronectin fragments (Fig. 4d).
Figure 4.
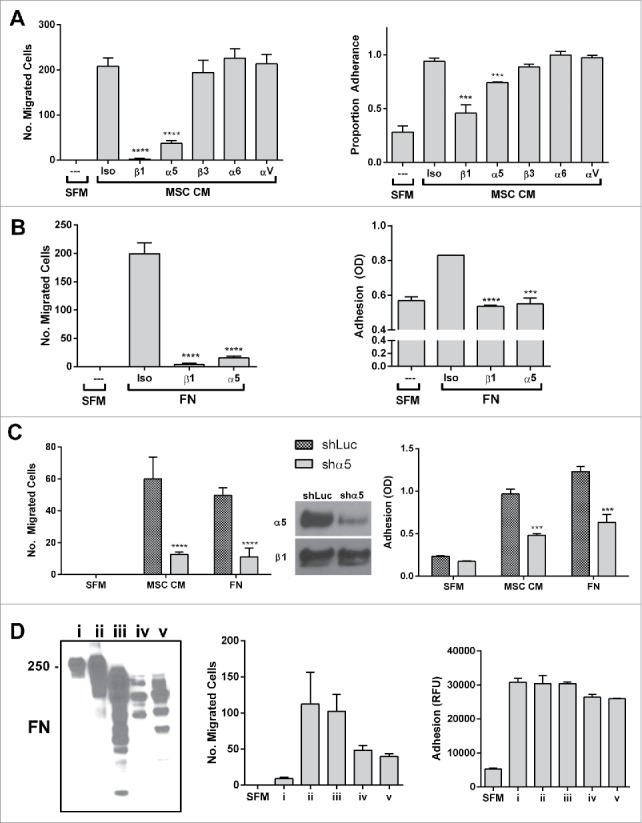
The classic fibronectin receptors α5 and β1 integrin mediate the migratory and adhesive behavior of prostate cancer cells to the BM-MSC secretome and fibronectin and its fragments. α5 or β1 integrin neutralization significantly impairs PC3 migration to (A) MSC CM (migration (left): ****p < 0.0001 and ****p < 0.0001; adhesion (right): ***p < 0.001 and ***p < 0.001 compared to isotype control) and (B) human plasma fibronectin (FN - MP Biomedicals) (migration (left): ****p < 0.0001 and ****p < 0.0001; adhesion (right): ****p < 0.0001 and ***p < 0.001 compared to isotype control), (C) Shrna knockdown of α5 significantly impairs migration (CM: ****p < 0.0001, FN: ****p < 0.0001) and adhesion (CM: ***p < 0.001, FN:***p < 0.001) to BM-MSC CM and FN. (D) Three commercial preparations of FN including FN i, which shows a restricted full length band on western, and FN ii and iii which contain fragments as well as proteolytically derived FN iv and v (30 minutes of trypsin and chymotrypsin digestion of FN i followed by quenching by PMSF) were tested in PC3 adhesion and migration assay. Although adhesion is consistently observed, only FN preparations with fragments induce migration. Integrin neutralizations were performed as described in methods. Number of migrated cells reported are mean ± SD of 5 representative fields on the membrane. Adhesion is reported as proportion adherence, mean ± SD (n = 3) of the ratio of adherent to input cells as determined by cell viability (MTS) assay, or directly as OD or RFU as determined by MTS or AlamarBlue (ThermoFisher). SFM: serum free media, MSC CM: BM-MSC conditioned media, Iso: isotype control (anti-α4), FN: fibronectin.
Fibronectin fragments and not full-length FN induce chemotaxis of prostate cancer cells
Given that the bioactivity we had purified from the BM-MSC contained only fragments of fibronectin, we hypothesized that FNFr and not intact FN was specifically required to induce a migratory response in BM-MSC secretome. Proteolytic cleavage of purified full-length FN with trypsin and chymotrypsin generated FN fragments that now induced migration (Fig. 4d). Together with the primary proteomic studies, this suggested that proteolytic fragments of FN generated by generated by BM-MSCs function as matrikines17 to specifically drive the chemotactic affinity of prostate cancer cells.
Other prostate cancer cells that express the a5 integrin also demonstrate a chemotactic and adhesive response to the BM-MSC secretome
Prostate cancer cell lines are known to vary in their integrin expression profiles. We found that additional prostate cancer cell lines (DU-145, LnCAP) that express the a5 and β1 integrins by flow cytometry also display an adhesive and migratory response to the BM-MSC secretome that is specifically blocked by monospecific antibodies to the a5 and β1 integrins (Fig. 5). Prostate cancer cell lines (MDA PCa2b, VCAP) that did not express the a5 integrin did not display adhesive or migratory responses to the BM-MSC secretome. Taken together this suggests that the expression of the a5 integrin is likely to be an integral factor in the adhesive, migratory and invasive behavior of prostate cancer cells to the human BM-MSC secretome.
Figure 5.
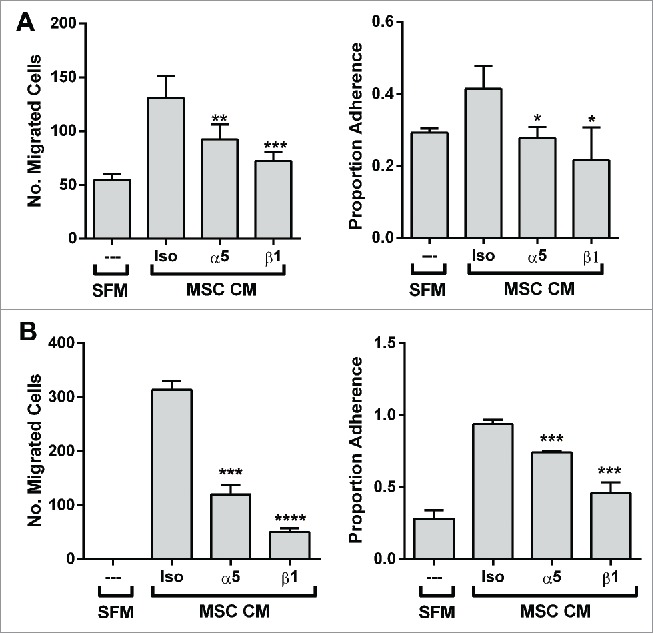
The migratory and adhesive response of other prostate cancer lines with α5-surface expression to the BM-MSC secretome is also mediated by α5 and β1 integrins. Neutralization of integrins α5 and β1 significantly impairs (A) LNCaP migration (α5: **p < 0.01, β1: ***p < 0.001) and adhesion (α5: *p < 0.05, β1: *p < 0.05) to BM-MSC CM and (B) DU-145 migration (α5: ***p < 0.001, β1: ****p < 0.0001) and adhesion (α5: ***p < 0.001, β1: ***p < 0.001) to BM-MSC CM. Integrin neutralizations were performed as described in methods. Number of migrated cells reported are mean ± SD of 5 representative fields on the membrane. Adhesion is reported as proportion adherence, mean ± SD (n = '3) of the ratio of adherent to input cells as determined by cell viability (MTS) assay. SFM: serum free media, MSC CM: BM-MSC conditioned media, Iso: isotype control (anti-α4).
Discussion
We have implicated proteolytic fragments of fibronectin as a key chemotaxin driving the strong migratory and invasive response induced in prostate cancer cells by human bone marrow derived BM-MSCs. As full-length FN induces adhesion alone, FN fragments (FNFr) may function as matrikines in the bone marrow niche. Matrikines can be defined as proteinase-generated fragments of matrix molecules that possess bioactivities not displayed by the native full-length form of the molecule.17 The virtue of increased solubility of FNFr may allow for diffusion and generation of concentration gradients compared to relatively insoluble full length FN which tends to polymerize into fibrils and aggregate with other proteins in complex assemblies in the extracellular matrix including TSP1. We find however that both FNFr- and FN-driven chemotactic and adhesive responses converge on the classic FN receptor, a5β1.
Several lines of existing evidence lend credence to the a5β1-FNFr/FN interaction as a candidate seed-and-soil principle in prostate cancer and bone metastases. Fibronectin is abundantly expressed in the bone marrow microenvironment by stromal cells.18 In studies of adhesion-molecule profiles of disseminated tumor cells recovered from bone marrow aspirates of patients with bone metastases, the a5β1 integrin was expressed in nearly 100% of disseminated tumor cells.19 Interestingly, the alternate FN receptor a4β1 (VLA-4) has been implicated in the homing of haematopoietic stem cells to the marrow-niche; neutralizing antibodies to a4β1 mobilize haematopoietic stem cells into circulation implicating FN as a functional component of the haematopoietic niche.20 There have been limited efforts targeting the a5β1 integrin in prostate cancer with particular reference to progression in the bone marrow. The a5β1monoclonal antibody volociximab has been in clinical trials but was abandoned in the absence of discernible clinical efficacy in a range of solid tumors, both as single agent and in combination with cytotoxic chemotherapy.21-24 Only a few patients with prostate cancer were included in these studies and the data are insufficient to draw conclusions.
In other studies, the avβ3 integrin has been proposed as a candidate mediator of prostate cancer progression in bone.25,26 Cilengitide, a cyclic peptide that specifically inhibits avβ3 and the avβ5 integrins was studied at 2 dose-levels in metastatic castration-resistant prostate cancer with no discernible impact on clinical outcomes or circulating tumor cells; the short half-life of the peptide following parenteral infusion may have limited target inhibition.27 A monoclonal antibody against av integrin slowed the rate of progression of bone metastases but was insufficient to prolong the overall progression-free survival in men with metastatic castration-resistant disease.28 The av integrin functions as an alternate but lower-affinity receptor for fibronectin29 and it is conceivable that these results in part represent a strategy that targets the FN-prostate cancer interaction. In our studies however, although av integrin was strongly expressed in PC-3 cells, av integrin blocking antibodies did not inhibit their migration or adhesion to the BM-MSC secretome. In disseminated tumor cells obtained from bone metastases, 100% expressed the av integrin.19 Peptide-based or small molecule strategies developed to target a5β1-integrin and fibronectin interactions have not been advanced further in the clinic.30,31
There are significant existing limitations toward assessing integrin-targeting strategies with current preclinical models of prostate cancer and bone metastases. There are no existing animal models of prostate cancer and bone metastases, engineered or xenografted, that are representative of the human phenotype of bone-homing marrow-dominant disease and that allow for reliable predictive preclinical studies. Important species-specific differences in epithelial-stromal interactions may define this modeling challenge which has constrained effective bench-to-bedside translation. A significant gap between murine and human models of prostate cancer and bone metastases exists with starkly contradictory results demonstrable between preclinical data based on mouse model data and clinical translation.32 Humanized murine models of prostate cancer bone metastases which seek to incorporate a humanized bone marrow niche such as with engineered bone scaffolds seeded with human BM-MSCs, or human fetal or hip bone grafts, or engraftment of human haematopoietic stem cells and BM-MSCs into a murine background may generate predictive preclinical models but these are cumbersome and difficult modeling strategies which are not yet well established.33-36
One strategy to demonstrate proof-of-principle implicating a candidate seed-and-soil mechanism in prostate cancer is to use the “clinic as laboratory” model to circumvent the limitations of existing animal models. For example, an early phase clinical study with a potent neutralizing antibody to a5β1 designed with a primary pharmacodynamic endpoint of preferential mobilization of a5β1-expressing circulating tumor cells into the peripheral blood of men with bone-dominant metastases could permit evidence for the role of the a5β1pathway in mediating adhesive interactions of prostate cancer cells in the bone marrow. This would be akin to studies demonstrating haematopoietic stem cell mobilization out of the bone marrow niche with CXCR4 and integrin a4 blockade.20,37 These type of studies could powerfully address our proposal that the FN-Fr/FN-a5β1 interaction represents a seed-and-soil principle that defines its unique affinity for the bone marrow niche and a potential therapeutic target relevant to the lethal phenotype of the disease.
Materials
Standard tissue culture supplies were obtained from Santa Cruz Biotechnology unless otherwise specified. Other tissue culture reagents include phosphate buffered saline (PBS, Corning 21-040-CV), Dulbecco's modified Eagle's medium (DMEM, Corning 10-013-CV), Roswell Park Memorial Institute – 1640 with L-glutamine and 25 mM Hepes (RPMI, Corning 10-041-CV) , fetal bovine serum (FBS, Gibco 10437), penicillin-streptomycin solution (P/S, Corning 30-002-Cl). Platelet purified Thrombospondin-1 (TSP1) at 125µg/ml was obtained from Jack Lawler, Beth Israel Deaconess Medical Center, Boston. Purified human plasma fibronectin (FN) preparations i, ii and iii were obtained from Sigma (F2006), R&D (1918-FN-02M) and MP Biomedicals (55913), respectively. Western blots were performed using antibodies to TSP1 (Abcam ab85762) and FN (Pierce PA1-26205).
Cell culture
BM-MSCs were obtained from the Kaplan Lab (Tufts University, Medford, MA). These plastic-adherent cells from human bone marrow aspirates (Lonza, MD) were determined to have pluripotent differentiation into osteogenic, adipogenic and chondrogenic lineages and consensus surface phenotypes by flow cytometry.38 Human prostate cancer lines PC-3, LNCaP, DU145 were obtained from ATCC (Manassas, VA). PC-3, DU145 and BM-MSC were cultured in DMEM supplemented with 10% FBS and P/S. LNCaP were cultured in RPMI supplemented with 10% FBS and P/S. All cells were cultured in a humidified 37°C, 5% CO2 incubator and subcultured by washing 3x with phosphate buffered saline (PBS, Corning 21-040-CV) and trypsinization with 0.25% trypsin, .9 mM EDTA (Life Technologies 25200).
Generation of conditioned media
BM-MSCs were allowed to reach confluence in a T75 flask, cultured as described above. Cells were washed 3x with PBS before incubation in 10 mL of serum-free culture media. Conditioned media (CM) was harvested after 24 h and gently centrifuged (300 g for 10 minutes) before being stored at 4°C. BM-MSCs were incubated for at least 48h in serum-present culture media between serum starvations.
Size separation of BM-MSC CM
BM-MSC CM was subjected to ultracentrifugation (50,000 × g for 3 hrs) and separately to variable molecular-weight exclusion filters: 3 kDa, 10 kDa, 30 kDa, 50 kDa, 100 kDa (Centricon) by centrifugation.
Cell adhesion assay
A 96 well plate was plated with 50 uL of adherent substrate in triplicate and incubated at 37°C. Non-specific binding sites were blocked by incubating the wells with 1% BSA for 30 minutes at 37°C. After washing wells 3x with PBS, 10000 cells harvested in log-phase growth were added in 100 uL of serum-free culture media to each well from a homogenous cell suspension. The plate was incubated 1 h at 37°C (6 h for LNCaP) to allow establishment of adherence profiles. To remove non-adherent cells, wells were gently washed 5x with PBS. Adherent cell counts were assayed using MTS reagent (Promega G3582). Adherence was reported either directly as optical density or as percentage adherence (normalized against number of input cells).
Cell migration and invasion assay
Cell migration and invasion assays were performed using a Boyden Chamber set up. A 24 well assay plate was prepared by adding 800 uL of chemoattractant underneath uncoated 8 micron pore transwell inserts (Corning 353097) [migration] or inserts coated with basement membrane extract (Corning 3458) [invasion] and heated to 37°C. 25,000 cells (harvested in log-phase growth) were added from a homogenous cell suspension to the top of the well to a total volume of 200 uL. The assay was stopped after 24 h at 37°C (48 h for LNCaP) by removal and washing of the membrane inserts with PBS and formalin (Fischer Sci SF100-4) fixation of the cells for 15 minutes. Following another 3x PBS wash, cells were removed from the top of the membranes using cotton-tip swabs and were stained using hematoxylin (Sigma GHS116) and eosin (Sigma HT110116) or calcein AM (ThermoFisherC1430). After drying, membranes were mounted onto microscope slides and cell counts were performed on 5 representative fields of a membrane using a 10x objective.
Cell count assay
The effect of BM-MSC-SFCM (serum-free CM) on PC-3 cell count was tested by incubating 2500 PC-3 cells (harvested in log-phase growth) in 100 uL of BM-MSC-SFCM in a 96 well format. After 72 h, viability was assessed using MTS reagent (Promega G3582). Cell count was reported directly as optical density.
Heparin affinity chromatography
BM-MSC-SFCM (500 mL) was loaded on a 1.6 × 2.5 cm heparin Sepharose column (GE Healthcare) equilibrated with 5 column volumes of buffer A (15 mM TrisHCL, 2 mM CaCl, and 0.15 M NaCl; pH 7.3), and eluted in a step with buffer B (15 mM TrisHCL, 2 mM CaCl, and 0.55 M NaCL; pH 7.3). The eluate was extensively dialyzed (Slide-A-Lyzer Dialysis Cassettes, 10 K MWCO, Pierce) against PBS and concentrated via reverse osmosis through a semipermeable membrane (10 K MWCO), to a volume of 1 mL filtered (0.22mic, Millipore).
Size exclusion chromatography
Concentrated BM-MSC-SFCM elution from heparin affinity column was loaded onto size exclusion chromatography (SEC) column HiLoad16/60 Superdex 200 (GE Healthcare) and eluted isocratically with PBS. Samples from SEC were collected using an automated fraction collector.
Immunodepletion of TSP1 and put-back assays
BM-MSC CM was incubated with 10 μg of α-TSP1 antibody (IgG1κ, Invitrogen Life Technologies) or isotype control (Iso) antibody and immune complexes cleared by adding protein G-coupled agarose. A dose-titration of TSP1 (10 ng/ml to 10 µg/ml) was employed to assess induction of bioactivity.
Proteomic identification
The fractionated protein samples from the SEC were subjected to SDS PAGE (4–12% Bis-Tris Protein Gels, ThermoFisher NP0321BOX), and silver stained (ThermoFisher SilverQuest LC6070). Bands from bioactive fractions were excised and submitted for mass spectroscopic identification (MS/MS, Taplin Mass Spectroscopy Facility, Harvard University, Boston, MA). N-terminal Edman sequencing was performed by the Tufts University Core Facility (Boston, MA).
Integrin neutralization
Blocking of surface integrins was performed by incubation 50 ug/mL of anti-integrin monoclonal antibody on ice for 20 minutes before migration and adhesion assays. Anti-β1 clone 6S5 (Millipore MAB2253Z), anti-β3 clone B3A (Millipore MAB2023Z), anti-α4 clone P1H4 (Millipore MAB16983Z), anti-α5 clone P1D6 (Millipore MAB1956Z), anti-α6 clone NKI – GoH3 (Millipore MAB1378), anti-αV clone 272–17E6 (Millipore MABT207) were all obtained azide-free from Millipore. As referenced, the neutralizing efficacy of a6 39, β3 40 and av 41 have been demonstrated.
FN proteolysis
Purified human plasma FN from Sigma (F2006) was proteolytically digested using trypsin (Life Technologies 25200) or chymotrypsin (Sigma C4129) for 30 minutes at 37°C using enzyme unit: substrate ratio of 1:50. The enzyme was quenched and inactivated by addition of 1 mM phenylmethylsulfoynyl fluoride (PMSF, Sigma P-7626).
shRNA generation and plasmid transfection
Two human ITGA5 shRNAs (shITGA5) were generated using the following primers: shIGTA5 #1, 5′- GCTACCTCTCCACAGATAACTCGAAAGTTATCTGTGGAGAGGTAGCccTTTTTG −3′ (forward) and 5′ -AATTCAAAAAggGCTACCTCTCCACAGATAACTTTCGAGTTATCTGTGGAGAGGTAGC −3′ (reverse); shIGTA5 #2, 5′- GCAGAGAGATGAAGATCTACCCGAAGGTAGATCTTCATCTCTCTGCccTTTTTG −3′ (forward) and 5′- AATTCAAAAAggGCAGAGAGATGAAGATCTACCTTCGGGTAGATCTTCATCTCTCTGC −3′ (reverse). Single strand DNA oligos were annealed and cloned into pKSU6 expression vector between MscI and EcoRI sites. Luciferase shRNA (shluc) pKSU6 expression vector was from Dr. Keyong Du (Tufts Medical Center, Boston, MA, USA). PC-3 cells were transiently transfected with shluciferase or shIGTA5 expression vector using Lipofectamine 2000 (Thermo Fisher Scientific-Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instruction. Cells were incubated at 37°C in a CO2 incubator for 36h, before proceeding with cell adhesion and migration assays.
Surface integrin expression profiling
Suspended cells were washed (PBS) and incubated with anti-integrin primary antibodies for 1 hour at room temperature and then with fluorescently-labeled secondary antibodies for 1 hour at room temperature. Fluorescence was detected using a CyAn™ ADP Analyzer (Beckman Coulter).
Coimmunoprecipitation (co-IP) assay
Co-IP of FN and TSP1 in MSC conditioned media (CM) was carried out as follows. Equal volume of freshly collected MSC-SFCM (serum-free CM) was incubated with IgG (control), or TSP1 or FN antibody in IP buffer on a rotator at 4°C overnight. Then 20 ul of Pierce™ Protein A/G Plus Agarose (Thermo Fisher Scientific, Rockford, IL, USA) were added, and the incubation continued for another 90 min at 4°C. The immunoprecipitates bound to agarose beads were washed 4 times in IP buffer, heated in 60 μl of 2.5x sample buffer, separated with use of 7.5% SDS-PAGE gels, transferred to polyvinylidene fluoride membrane (EMD Millipore, Billerica, MA, USA), and probed with TSP1 and FN antibodies. The immune complexes were detected by Pierce™ ECL Western Blotting Substrate detection system (ThermoFisher).
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We acknowledge the support of following individuals in facilitating the conduct of the study: Michael Rosenblatt MD, PhD, Rick Van Etten MD, PhD, Betty and Joe Lawrence, Simon Mendal, Bud Joyce, Will and Julie Person.
References
- [1].Loberg RD, Logothetis CJ, Keller ET, Pienta KJ. Pathogenesis and treatment of prostate cancer bone metastases: targeting the lethal phenotype. J Clin Oncol 2005; 23:8232-41; PMID:16278478; https://doi.org/ 10.1200/JCO.2005.03.0841 [DOI] [PubMed] [Google Scholar]
- [2].Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, et al.. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369:213-23; PMID:23863050; https://doi.org/ 10.1056/NEJMoa1213755 [DOI] [PubMed] [Google Scholar]
- [3].Sartor O, Coleman R, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, et al.. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol 2014; 15:738-46; PMID:24836273; https://doi.org/ 10.1016/S1470-2045(14)70183-4 [DOI] [PubMed] [Google Scholar]
- [4].Boulais PE, Frenette PS. Making sense of hematopoietic stem cell niches. Blood 2015; 125:2621-9; PMID:25762174; https://doi.org/ 10.1182/blood-2014-09-570192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, et al.. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 2003; 425:841-6; PMID:14574413; https://doi.org/ 10.1038/nature02040 [DOI] [PubMed] [Google Scholar]
- [6].Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010; 466:829-34; PMID:20703299; https://doi.org/ 10.1038/nature09262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Visnjic D, Kalajzic Z, Rowe DW, Katavic V, Lorenzo J, Aguila HL. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 2004; 103:3258-64; PMID:14726388; https://doi.org/ 10.1182/blood-2003-11-4011 [DOI] [PubMed] [Google Scholar]
- [8].Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, et al.. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Investig 2011; 121:1298-312; PMID:21436587; https://doi.org/ 10.1172/JCI43414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Margossian SS, Lawler JW, Slayter HS. Physical characterization of platelet thrombospondin. J Biol Chem 1981; 256:7495-500; PMID:7251605 [PubMed] [Google Scholar]
- [10].Tuszynski GP, Nicosia RF. The role of thrombospondin-1 in tumor progression and angiogenesis. Bioessays 1996; 18:71-6; PMID:8593167; https://doi.org/ 10.1002/bies.950180113 [DOI] [PubMed] [Google Scholar]
- [11].Resovi A, Pinessi D, Chiorino G, Taraboletti G. Current understanding of the thrombospondin-1 interactome. Matrix Biol 2014; 37:83-91; PMID:24476925; https://doi.org/ 10.1016/j.matbio.2014.01.012 [DOI] [PubMed] [Google Scholar]
- [12].Lawler J, Derick LH, Connolly JE, Chen JH, Chao FC. The structure of human platelet thrombospondin. J Biol Chem 1985; 260:3762-72; PMID:2579080 [PubMed] [Google Scholar]
- [13].Pankov R, Yamada KM. Fibronectin at a glance. J Cell Sci 2002; 115:3861-3; PMID:12244123; https://doi.org/ 10.1242/jcs.00059 [DOI] [PubMed] [Google Scholar]
- [14].Dardik R, Lahav J. Multiple domains are involved in the interaction of endothelial cell thrombospondin with fibronectin. Eur J Biochem 1989; 185:581-8; PMID:2512126; https://doi.org/ 10.1111/j.1432-1033.1989.tb15153.x [DOI] [PubMed] [Google Scholar]
- [15].Dreyfus M, Lahav J. The build-up of the thrombospondin extracellular matrix. An apparent dependence on synthesis and on preformed fibrillar matrix. Eur J Cell Biol 1988; 47:275-82; PMID:3072202 [PubMed] [Google Scholar]
- [16].Homandberg GA, Kramer-Bjerke J. Thrombospondin binds to amino-terminal fragments of plasma fibronectin. Thromb Res 1987; 48:329-35; PMID:3433257; https://doi.org/ 10.1016/0049-3848(87)90445-2 [DOI] [PubMed] [Google Scholar]
- [17].Ricard-Blum S, Salza R. Matricryptins and matrikines: biologically active fragments of the extracellular matrix. Exp Dermatol 2014; 23:457-63; PMID:24815015; https://doi.org/ 10.1111/exd.12435 [DOI] [PubMed] [Google Scholar]
- [18].Van der Velde-Zimmermann D, Verdaasdonk MA, Rademakers LH, De Weger RA, Van den Tweel JG, Joling P. Fibronectin distribution in human bone marrow stroma: matrix assembly and tumor cell adhesion via alpha5 beta1 integrin. Exp Cell Res 1997; 230:111-20; PMID:9013713; https://doi.org/ 10.1006/excr.1996.3405 [DOI] [PubMed] [Google Scholar]
- [19].Putz E, Witter K, Offner S, Stosiek P, Zippelius A, Johnson J, Zahn R, Riethmüller G, Pantel K. Phenotypic characteristics of cell lines derived from disseminated cancer cells in bone marrow of patients with solid epithelial tumors: establishment of working models for human micrometastases. Cancer Res 1999; 59:241-8; PMID:9892213 [PubMed] [Google Scholar]
- [20].Papayannopoulou T, Nakamoto B. Peripheralization of hemopoietic progenitors in primates treated with anti-VLA4 integrin. Proc Natl Acad Sci U S A 1993; 90:9374-8; PMID:7692447; https://doi.org/ 10.1073/pnas.90.20.9374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Almokadem S, Belani CP. Volociximab in cancer. Expert Opin Biol Ther 2012; 12:251-7; PMID:22192080; https://doi.org/ 10.1517/14712598.2012.646985 [DOI] [PubMed] [Google Scholar]
- [22].Bell-McGuinn KM, Matthews CM, Ho SN, et al.. A phase II, single-arm study of the anti-alpha5beta1 integrin antibody volociximab as monotherapy in patients with platinum-resistant advanced epithelial ovarian or primary peritoneal cancer. Gynecol Oncol 2011; 121:273-9; PMID:21276608; https://doi.org/ 10.1016/j.ygyno.2010.12.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Besse B, Tsao LC, Chao DT, Fang Y, Soria JC, Almokadem S, Belani CP. Phase Ib safety and pharmacokinetic study of volociximab, an anti-alpha5beta1 integrin antibody, in combination with carboplatin and paclitaxel in advanced non-small-cell lung cancer. Ann Oncol 2013; 24:90-6; PMID:22904239; https://doi.org/ 10.1093/annonc/mds281 [DOI] [PubMed] [Google Scholar]
- [24].Ricart AD, Tolcher AW, Liu G, Holen K, Schwartz G, Albertini M, Weiss G, Yazji S, Ng C, Wilding G. Volociximab, a chimeric monoclonal antibody that specifically binds alpha5beta1 integrin: a phase I, pharmacokinetic, and biological correlative study. Clin Cancer Res 2008; 14:7924-9; PMID:19047123; https://doi.org/ 10.1158/1078-0432.CCR-08-0378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cooper CR, Chay CH, Pienta KJ. The role of alpha(v)beta(3) in prostate cancer progression. Neoplasia (New York, NY) 2002; 4:191-4; https://doi.org/ 10.1038/sj.neo.7900224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sun YX, Fang M, Wang J, Cooper CR, Pienta KJ, Taichman RS. Expression and activation of alpha v beta 3 integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cells. Prostate 2007; 67:61-73; PMID:17034033; https://doi.org/ 10.1002/pros.20500 [DOI] [PubMed] [Google Scholar]
- [27].Bradley DA, Daignault S, Ryan CJ, Dipaola RS, Cooney KA, Smith DC, Small E, Mathew P, Gross ME, Stein MN, et al.. Cilengitide (EMD 121974, NSC 707544) in asymptomatic metastatic castration resistant prostate cancer patients: a randomized phase II trial by the prostate cancer clinical trials consortium. Investig New Drugs 2011; 29:1432-40; PMID:20336348; https://doi.org/ 10.1007/s10637-010-9420-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hussain M, Le Moulec S, Gimmi C, Bruns R, Straub J, Miller K. Differential Effect on Bone Lesions of Targeting Integrins: Randomized Phase II Trial of Abituzumab in Patients with Metastatic Castration-resistant Prostate Cancer. Clin Cancer Res 2016; 22(13):3192–200. [DOI] [PubMed] [Google Scholar]
- [29].Zhang Z, Morla AO, Vuori K, Bauer JS, Juliano RL, Ruoslahti E. The alpha v beta 1 integrin functions as a fibronectin receptor but does not support fibronectin matrix assembly and cell migration on fibronectin. J Cell Biol 1993; 122:235-42; PMID:8314844; https://doi.org/ 10.1083/jcb.122.1.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Veine DM, Yao H, Stafford DR, Fay KS, Livant DL. A D-amino acid containing peptide as a potent, noncovalent inhibitor of alpha5beta1 integrin in human prostate cancer invasion and lung colonization. Clin Exp Metastasis 2014; 31:379-93; PMID:24464034; https://doi.org/ 10.1007/s10585-013-9634-1 [DOI] [PubMed] [Google Scholar]
- [31].Schaffner F, Ray AM, Dontenwill M. Integrin alpha5beta1, the Fibronectin Receptor, as a Pertinent Therapeutic Target in Solid Tumors. Cancers (Basel) 2013; 5:27-47; PMID:24216697; https://doi.org/ 10.3390/cancers5010027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Rosenberg A, Mathew P. Imatinib and prostate cancer: lessons learned from targeting the platelet-derived growth factor receptor. Expert Opin Investig Drugs 2013; 22:787-94; PMID:23540855; https://doi.org/ 10.1517/13543784.2013.787409 [DOI] [PubMed] [Google Scholar]
- [33].Thibaudeau L, Holzapfel BM, Hutmacher DW. Humanized mice models for primary bone tumor and bone metastasis research. Cell cycle (Georgetown, Tex) 2015; 14:2191-2; PMID:26125650; https://doi.org/ 10.1080/15384101.2015.1062327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nemeth JA, Harb JF, Barroso U Jr., He Z, Grignon DJ, Cher ML. Severe combined immunodeficient-hu model of human prostate cancer metastasis to human bone. Cancer Res 1999; 59:1987-93; PMID:10213511 [PubMed] [Google Scholar]
- [35].Kuperwasser C, Dessain S, Bierbaum BE, et al.. A mouse model of human breast cancer metastasis to human bone. Cancer Res 2005; 65:6130-8; PMID:16024614; https://doi.org/ 10.1158/0008-5472.CAN-04-1408 [DOI] [PubMed] [Google Scholar]
- [36].Seib FP, Berry JE, Shiozawa Y, Taichman RS, Kaplan DL. Tissue engineering a surrogate niche for metastatic cancer cells. Biomaterials 2015; 51:313-9; PMID:25771021; https://doi.org/ 10.1016/j.biomaterials.2015.01.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rettig MP, Ansstas G, DiPersio JF. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia 2012; 26:34-53; PMID:21886173; https://doi.org/ 10.1038/leu.2011.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006; 8:315-7; PMID:16923606; https://doi.org/ 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- [39].Chen MS, Almeida EA, Huovila AP, Takahashi Y, Shaw LM, Mercurio AM, White JM. Evidence that distinct states of the integrin alpha6beta1 interact with laminin and an ADAM. J Cell Biol 1999; 144:549-61; PMID:9971748; https://doi.org/ 10.1083/jcb.144.3.549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kudirka JC, Panupinthu N, Tesseyman MA, Dixon SJ, Bernier SM. P2Y nucleotide receptor signaling through MAPK/ERK is regulated by extracellular matrix: involvement of beta3 integrins. J Cell Physiol 2007; 213:54-64; PMID:17620283; https://doi.org/ 10.1002/jcp.21087 [DOI] [PubMed] [Google Scholar]
- [41].Mitjans F, Sander D, Adan J, Sutter A, Martinez JM, Jäggle CS, Moyano JM, Kreysch HG, Piulats J, Goodman SL. An anti-alpha v-integrin antibody that blocks integrin function inhibits the development of a human melanoma in nude mice. J Cell Sci 1995; 108(Pt 8):2825-38; PMID:7593323 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


