Abstract
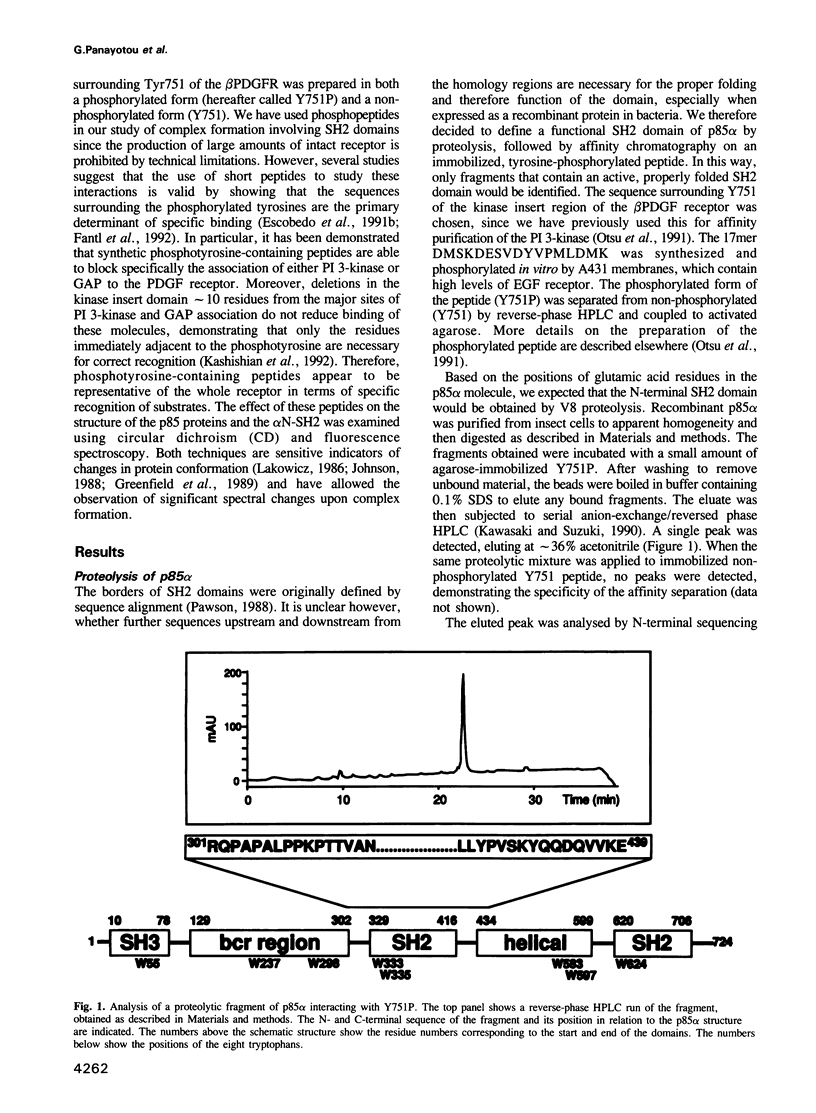
Circular dichroism and fluorescence spectroscopy were used to investigate the structure of the p85 alpha subunit of the PI 3-kinase, a closely related p85 beta protein, and a recombinant SH2 domain-containing fragment of p85 alpha. Significant spectral changes, indicative of a conformational change, were observed on formation of a complex with a 17 residue peptide containing a phosphorylated tyrosine residue. The sequence of this peptide is identical to the sequence surrounding Tyr751 in the kinase-insert region of the platelet-derived growth factor beta-receptor (beta PDGFR). The rotational correlation times measured by fluorescence anisotropy decay indicated that phosphopeptide binding changed the shape of the SH2 domain-containing fragment. The CD and fluorescence spectroscopy data support the secondary structure prediction based on sequence analysis and provide evidence for flexible linker regions between the various domains of the p85 proteins. The significance of these results for SH2 domain-containing proteins is discussed.
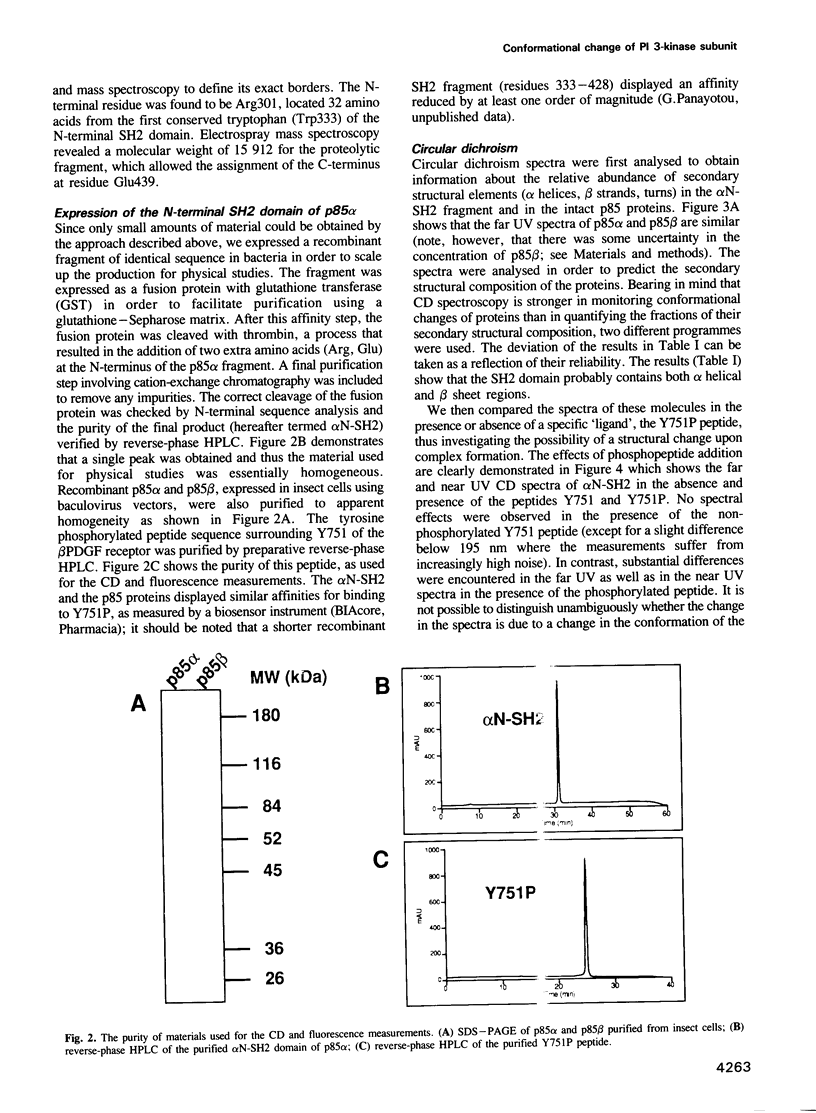
Full text
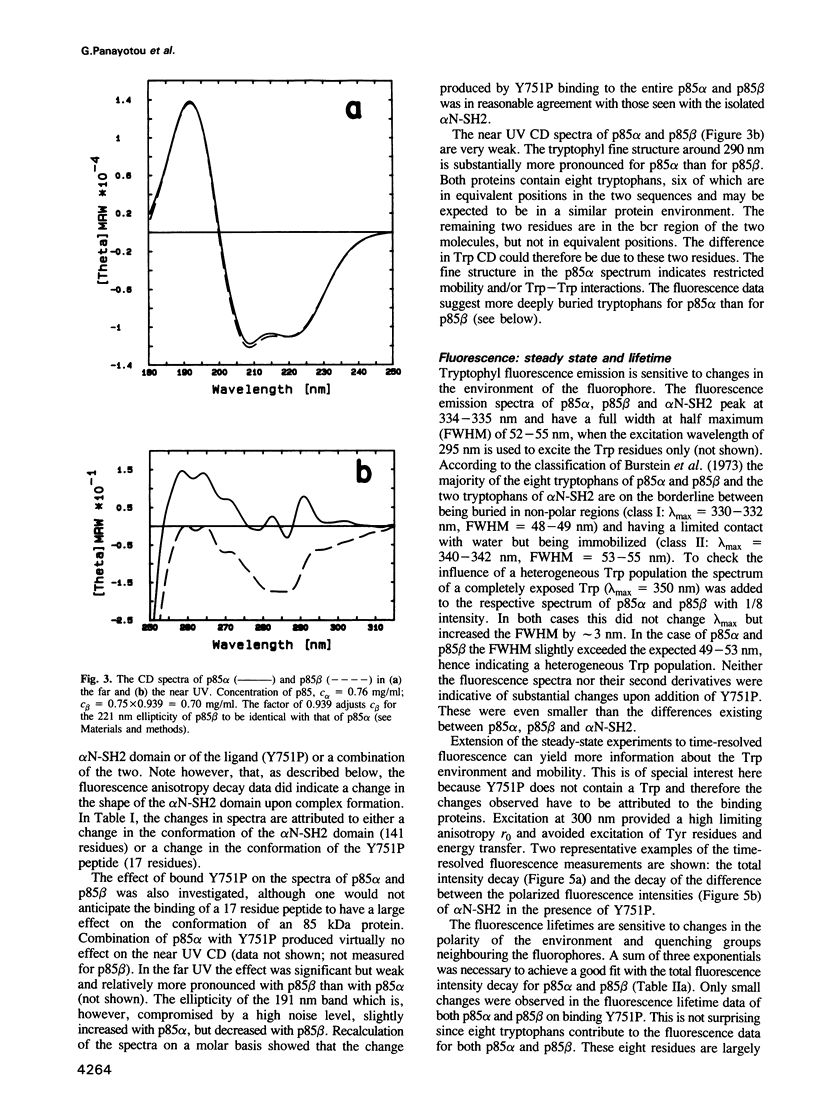
PDF
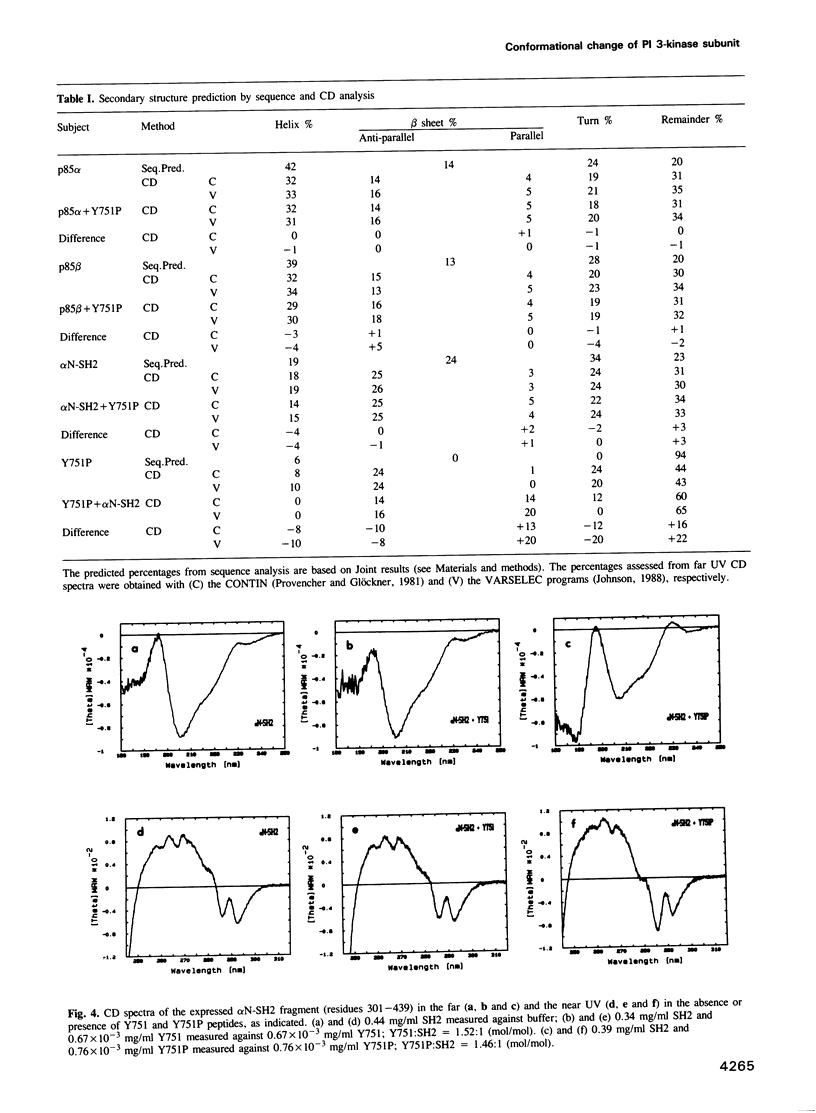
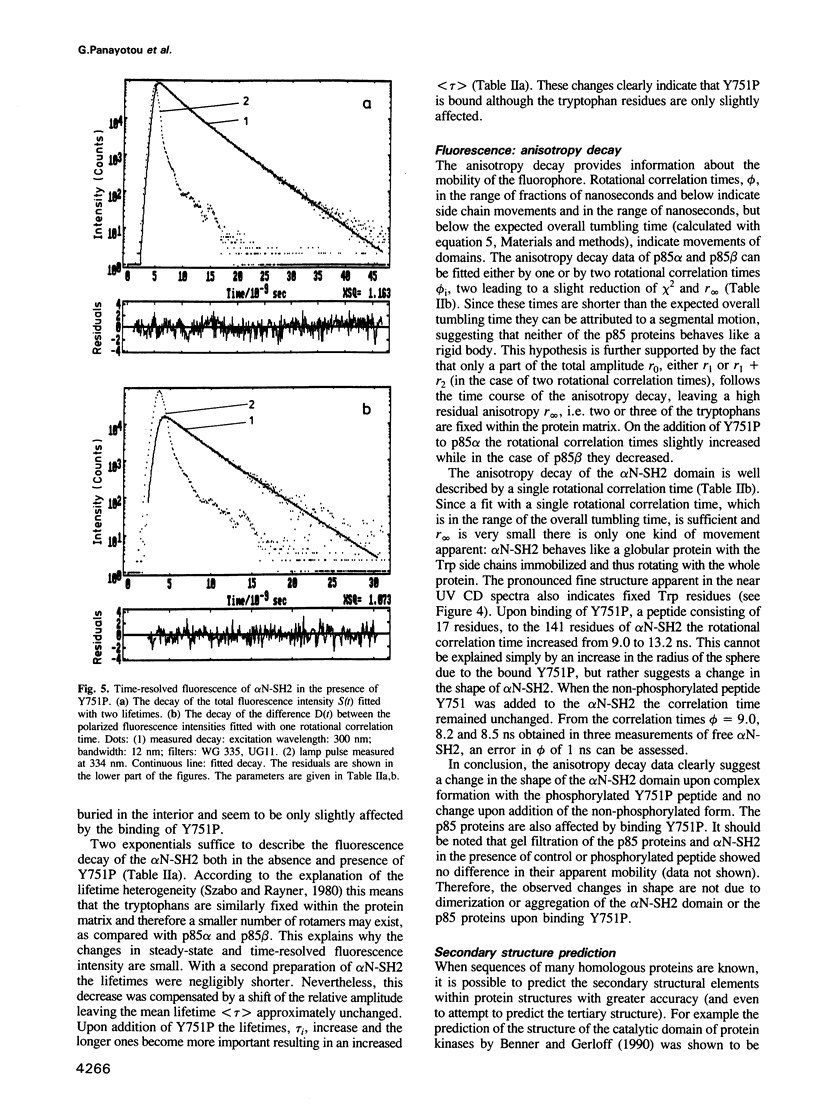
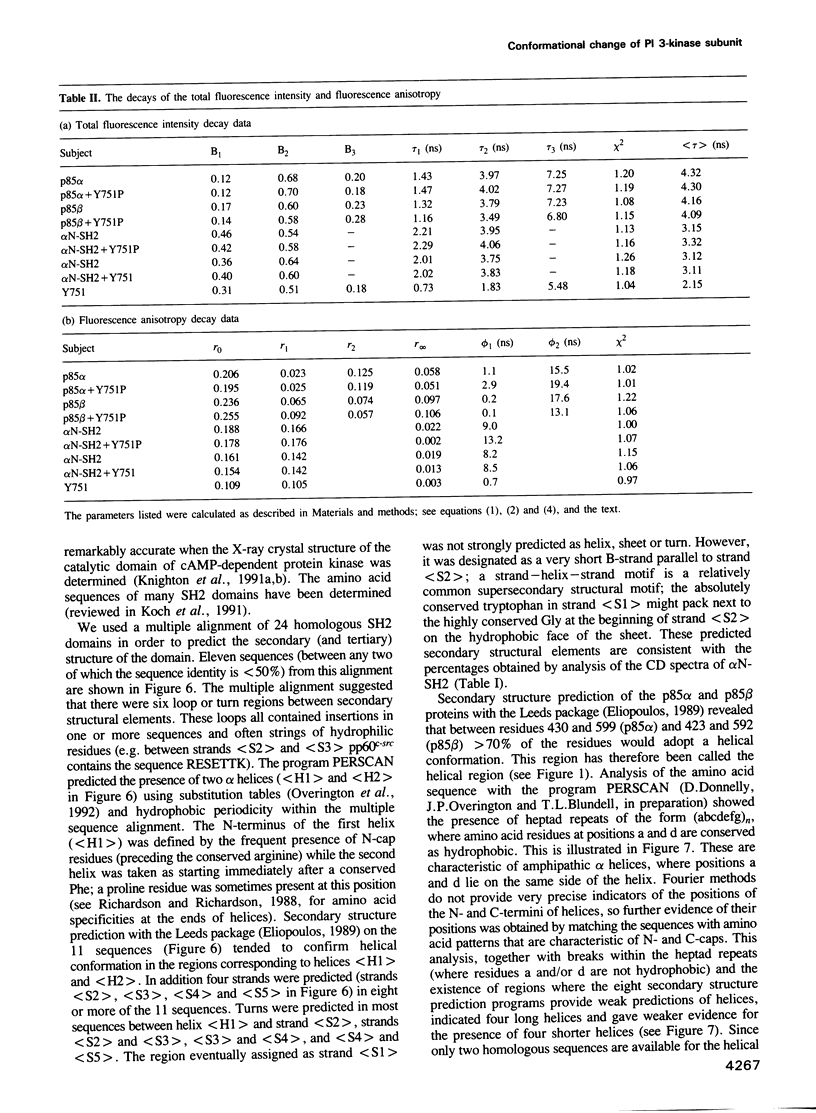





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D., Koch C. A., Grey L., Ellis C., Moran M. F., Pawson T. Binding of SH2 domains of phospholipase C gamma 1, GAP, and Src to activated growth factor receptors. Science. 1990 Nov 16;250(4983):979–982. doi: 10.1126/science.2173144. [DOI] [PubMed] [Google Scholar]
- Argos P., Palau J. Amino acid distribution in protein secondary structures. Int J Pept Protein Res. 1982 Apr;19(4):380–393. doi: 10.1111/j.1399-3011.1982.tb02619.x. [DOI] [PubMed] [Google Scholar]
- Balestrieri C., Colonna G., Giovane A., Irace G., Servillo L. Second-derivative spectroscopy of proteins. A method for the quantitative determination of aromatic amino acids in proteins. Eur J Biochem. 1978 Oct 16;90(3):433–440. doi: 10.1111/j.1432-1033.1978.tb12622.x. [DOI] [PubMed] [Google Scholar]
- Benner S. A., Gerloff D. Patterns of divergence in homologous proteins as indicators of secondary and tertiary structure: a prediction of the structure of the catalytic domain of protein kinases. Adv Enzyme Regul. 1991;31:121–181. doi: 10.1016/0065-2571(91)90012-b. [DOI] [PubMed] [Google Scholar]
- Bucci E., Steiner R. F. Anisotropy decay of fluorescence as an experimental approach to protein dynamics. Biophys Chem. 1988 Jul 15;30(3):199–224. doi: 10.1016/0301-4622(88)85017-8. [DOI] [PubMed] [Google Scholar]
- Burstein E. A., Vedenkina N. S., Ivkova M. N. Fluorescence and the location of tryptophan residues in protein molecules. Photochem Photobiol. 1973 Oct;18(4):263–279. doi: 10.1111/j.1751-1097.1973.tb06422.x. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Carpenter C. L., Duckworth B. C., Auger K. R., Cohen B., Schaffhausen B. S., Cantley L. C. Purification and characterization of phosphoinositide 3-kinase from rat liver. J Biol Chem. 1990 Nov 15;265(32):19704–19711. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Coughlin S. R., Escobedo J. A., Williams L. T. Role of phosphatidylinositol kinase in PDGF receptor signal transduction. Science. 1989 Mar 3;243(4895):1191–1194. doi: 10.1126/science.2466336. [DOI] [PubMed] [Google Scholar]
- Donnelly D., Johnson M. S., Blundell T. L., Saunders J. An analysis of the periodicity of conserved residues in sequence alignments of G-protein coupled receptors. Implications for the three-dimensional structure. FEBS Lett. 1989 Jul 17;251(1-2):109–116. doi: 10.1016/0014-5793(89)81438-3. [DOI] [PubMed] [Google Scholar]
- Downward J., Parker P., Waterfield M. D. Autophosphorylation sites on the epidermal growth factor receptor. Nature. 1984 Oct 4;311(5985):483–485. doi: 10.1038/311483a0. [DOI] [PubMed] [Google Scholar]
- Dufton M. J., Hider R. C. Snake toxin secondary structure predictions. Structure activity relationships. J Mol Biol. 1977 Sep 15;115(2):177–193. doi: 10.1016/0022-2836(77)90095-x. [DOI] [PubMed] [Google Scholar]
- Emori Y., Homma Y., Sorimachi H., Kawasaki H., Nakanishi O., Suzuki K., Takenawa T. A second type of rat phosphoinositide-specific phospholipase C containing a src-related sequence not essential for phosphoinositide-hydrolyzing activity. J Biol Chem. 1989 Dec 25;264(36):21885–21890. [PubMed] [Google Scholar]
- Escobedo J. A., Kaplan D. R., Kavanaugh W. M., Turck C. W., Williams L. T. A phosphatidylinositol-3 kinase binds to platelet-derived growth factor receptors through a specific receptor sequence containing phosphotyrosine. Mol Cell Biol. 1991 Feb;11(2):1125–1132. doi: 10.1128/mcb.11.2.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobedo J. A., Navankasattusas S., Kavanaugh W. M., Milfay D., Fried V. A., Williams L. T. cDNA cloning of a novel 85 kd protein that has SH2 domains and regulates binding of PI3-kinase to the PDGF beta-receptor. Cell. 1991 Apr 5;65(1):75–82. doi: 10.1016/0092-8674(91)90409-r. [DOI] [PubMed] [Google Scholar]
- Fantl W. J., Escobedo J. A., Martin G. A., Turck C. W., del Rosario M., McCormick F., Williams L. T. Distinct phosphotyrosines on a growth factor receptor bind to specific molecules that mediate different signaling pathways. Cell. 1992 May 1;69(3):413–423. doi: 10.1016/0092-8674(92)90444-h. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Greenfield C., Hiles I., Waterfield M. D., Federwisch M., Wollmer A., Blundell T. L., McDonald N. Epidermal growth factor binding induces a conformational change in the external domain of its receptor. EMBO J. 1989 Dec 20;8(13):4115–4123. doi: 10.1002/j.1460-2075.1989.tb08596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidaran M. A., Pierce J. H., Lombardi D., Ruggiero M., Gutkind J. S., Matsui T., Aaronson S. A. Deletion or substitution within the alpha platelet-derived growth factor receptor kinase insert domain: effects on functional coupling with intracellular signaling pathways. Mol Cell Biol. 1991 Jan;11(1):134–142. doi: 10.1128/mcb.11.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisterkamp N., Stam K., Groffen J., de Klein A., Grosveld G. Structural organization of the bcr gene and its role in the Ph' translocation. 1985 Jun 27-Jul 3Nature. 315(6022):758–761. doi: 10.1038/315758a0. [DOI] [PubMed] [Google Scholar]
- Heldin C. H. SH2 domains: elements that control protein interactions during signal transduction. Trends Biochem Sci. 1991 Dec;16(12):450–452. doi: 10.1016/0968-0004(91)90175-u. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Jr Secondary structure of proteins through circular dichroism spectroscopy. Annu Rev Biophys Biophys Chem. 1988;17:145–166. doi: 10.1146/annurev.bb.17.060188.001045. [DOI] [PubMed] [Google Scholar]
- Kabat E. A., Wu T. T. The influence of nearest-neighbor amino acids on the conformation of the middle amino acid in proteins: comparison of predicted and experimental determination of -sheets in concanavalin A. Proc Natl Acad Sci U S A. 1973 May;70(5):1473–1477. doi: 10.1073/pnas.70.5.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashishian A., Kazlauskas A., Cooper J. A. Phosphorylation sites in the PDGF receptor with different specificities for binding GAP and PI3 kinase in vivo. EMBO J. 1992 Apr;11(4):1373–1382. doi: 10.1002/j.1460-2075.1992.tb05182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H., Suzuki K. Separation of peptides dissolved in a sodium dodecyl sulfate solution by reversed-phase liquid chromatography: removal of sodium dodecyl sulfate from peptides using an ion-exchange precolumn. Anal Biochem. 1990 May 1;186(2):264–268. doi: 10.1016/0003-2697(90)90077-m. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A., Cooper J. A. Autophosphorylation of the PDGF receptor in the kinase insert region regulates interactions with cell proteins. Cell. 1989 Sep 22;58(6):1121–1133. doi: 10.1016/0092-8674(89)90510-2. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A., Cooper J. A. Phosphorylation of the PDGF receptor beta subunit creates a tight binding site for phosphatidylinositol 3 kinase. EMBO J. 1990 Oct;9(10):3279–3286. doi: 10.1002/j.1460-2075.1990.tb07527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazlauskas A., Ellis C., Pawson T., Cooper J. A. Binding of GAP to activated PDGF receptors. Science. 1990 Mar 30;247(4950):1578–1581. doi: 10.1126/science.2157284. [DOI] [PubMed] [Google Scholar]
- Kazlauskas A., Kashishian A., Cooper J. A., Valius M. GTPase-activating protein and phosphatidylinositol 3-kinase bind to distinct regions of the platelet-derived growth factor receptor beta subunit. Mol Cell Biol. 1992 Jun;12(6):2534–2544. doi: 10.1128/mcb.12.6.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knighton D. R., Zheng J. H., Ten Eyck L. F., Ashford V. A., Xuong N. H., Taylor S. S., Sowadski J. M. Crystal structure of the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):407–414. doi: 10.1126/science.1862342. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Zheng J. H., Ten Eyck L. F., Xuong N. H., Taylor S. S., Sowadski J. M. Structure of a peptide inhibitor bound to the catalytic subunit of cyclic adenosine monophosphate-dependent protein kinase. Science. 1991 Jul 26;253(5018):414–420. doi: 10.1126/science.1862343. [DOI] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Koga Y., Caccia N., Toyonaga B., Spolski R., Yanagi Y., Yoshikai Y., Mak T. W. A human T cell-specific cDNA clone (YT16) encodes a protein with extensive homology to a family of protein-tyrosine kinases. Eur J Immunol. 1986 Dec;16(12):1643–1646. doi: 10.1002/eji.1830161229. [DOI] [PubMed] [Google Scholar]
- Kumjian D. A., Wahl M. I., Rhee S. G., Daniel T. O. Platelet-derived growth factor (PDGF) binding promotes physical association of PDGF receptor with phospholipase C. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8232–8236. doi: 10.1073/pnas.86.21.8232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kypta R. M., Goldberg Y., Ulug E. T., Courtneidge S. A. Association between the PDGF receptor and members of the src family of tyrosine kinases. Cell. 1990 Aug 10;62(3):481–492. doi: 10.1016/0092-8674(90)90013-5. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R. Fluorescence studies of structural fluctuations in macromolecules as observed by fluorescence spectroscopy in the time, lifetime, and frequency domains. Methods Enzymol. 1986;131:518–567. doi: 10.1016/0076-6879(86)31054-1. [DOI] [PubMed] [Google Scholar]
- Lehmann J. M., Riethmüller G., Johnson J. P. Nck, a melanoma cDNA encoding a cytoplasmic protein consisting of the src homology units SH2 and SH3. Nucleic Acids Res. 1990 Feb 25;18(4):1048–1048. doi: 10.1093/nar/18.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S., Givol D., Yarden Y. A specific combination of substrates is involved in signal transduction by the kit-encoded receptor. EMBO J. 1991 Mar;10(3):647–654. doi: 10.1002/j.1460-2075.1991.tb07993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim V. I. Algorithms for prediction of alpha-helical and beta-structural regions in globular proteins. J Mol Biol. 1974 Oct 5;88(4):873–894. doi: 10.1016/0022-2836(74)90405-7. [DOI] [PubMed] [Google Scholar]
- Lim V. I. Structural principles of the globular organization of protein chains. A stereochemical theory of globular protein secondary structure. J Mol Biol. 1974 Oct 5;88(4):857–872. doi: 10.1016/0022-2836(74)90404-5. [DOI] [PubMed] [Google Scholar]
- Margolis B., Li N., Koch A., Mohammadi M., Hurwitz D. R., Zilberstein A., Ullrich A., Pawson T., Schlessinger J. The tyrosine phosphorylated carboxyterminus of the EGF receptor is a binding site for GAP and PLC-gamma. EMBO J. 1990 Dec;9(13):4375–4380. doi: 10.1002/j.1460-2075.1990.tb07887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M., Mayer B. J., Fukui Y., Hanafusa H. Binding of transforming protein, P47gag-crk, to a broad range of phosphotyrosine-containing proteins. Science. 1990 Jun 22;248(4962):1537–1539. doi: 10.1126/science.1694307. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Hamaguchi M., Hanafusa H. A novel viral oncogene with structural similarity to phospholipase C. Nature. 1988 Mar 17;332(6161):272–275. doi: 10.1038/332272a0. [DOI] [PubMed] [Google Scholar]
- Mayer B. J., Hanafusa H. Association of the v-crk oncogene product with phosphotyrosine-containing proteins and protein kinase activity. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2638–2642. doi: 10.1073/pnas.87.7.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B. J., Jackson P. K., Baltimore D. The noncatalytic src homology region 2 segment of abl tyrosine kinase binds to tyrosine-phosphorylated cellular proteins with high affinity. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):627–631. doi: 10.1073/pnas.88.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B. J., Jackson P. K., Van Etten R. A., Baltimore D. Point mutations in the abl SH2 domain coordinately impair phosphotyrosine binding in vitro and transforming activity in vivo. Mol Cell Biol. 1992 Feb;12(2):609–618. doi: 10.1128/mcb.12.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlade C. J., Ellis C., Reedijk M., Anderson D., Mbamalu G., Reith A. D., Panayotou G., End P., Bernstein A., Kazlauskas A. SH2 domains of the p85 alpha subunit of phosphatidylinositol 3-kinase regulate binding to growth factor receptors. Mol Cell Biol. 1992 Mar;12(3):991–997. doi: 10.1128/mcb.12.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran M. F., Koch C. A., Anderson D., Ellis C., England L., Martin G. S., Pawson T. Src homology region 2 domains direct protein-protein interactions in signal transduction. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8622–8626. doi: 10.1073/pnas.87.21.8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano K. Logical analysis of the mechanism of protein folding. I. Predictions of helices, loops and beta-structures from primary structure. J Mol Biol. 1973 Apr 5;75(2):401–420. doi: 10.1016/0022-2836(73)90030-2. [DOI] [PubMed] [Google Scholar]
- Otsu M., Hiles I., Gout I., Fry M. J., Ruiz-Larrea F., Panayotou G., Thompson A., Dhand R., Hsuan J., Totty N. Characterization of two 85 kd proteins that associate with receptor tyrosine kinases, middle-T/pp60c-src complexes, and PI3-kinase. Cell. 1991 Apr 5;65(1):91–104. doi: 10.1016/0092-8674(91)90411-q. [DOI] [PubMed] [Google Scholar]
- Overington J., Donnelly D., Johnson M. S., Sali A., Blundell T. L. Environment-specific amino acid substitution tables: tertiary templates and prediction of protein folds. Protein Sci. 1992 Feb;1(2):216–226. doi: 10.1002/pro.5560010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overington J., Johnson M. S., Sali A., Blundell T. L. Tertiary structural constraints on protein evolutionary diversity: templates, key residues and structure prediction. Proc Biol Sci. 1990 Aug 22;241(1301):132–145. doi: 10.1098/rspb.1990.0077. [DOI] [PubMed] [Google Scholar]
- Pai E. F., Kabsch W., Krengel U., Holmes K. C., John J., Wittinghofer A. Structure of the guanine-nucleotide-binding domain of the Ha-ras oncogene product p21 in the triphosphate conformation. Nature. 1989 Sep 21;341(6239):209–214. doi: 10.1038/341209a0. [DOI] [PubMed] [Google Scholar]
- Pawson T. Non-catalytic domains of cytoplasmic protein-tyrosine kinases: regulatory elements in signal transduction. Oncogene. 1988 Nov;3(5):491–495. [PubMed] [Google Scholar]
- Provencher S. W., Glöckner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981 Jan 6;20(1):33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- Reedijk M., Liu X. Q., Pawson T. Interactions of phosphatidylinositol kinase, GTPase-activating protein (GAP), and GAP-associated proteins with the colony-stimulating factor 1 receptor. Mol Cell Biol. 1990 Nov;10(11):5601–5608. doi: 10.1128/mcb.10.11.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedijk M., Liu X., van der Geer P., Letwin K., Waterfield M. D., Hunter T., Pawson T. Tyr721 regulates specific binding of the CSF-1 receptor kinase insert to PI 3'-kinase SH2 domains: a model for SH2-mediated receptor-target interactions. EMBO J. 1992 Apr;11(4):1365–1372. doi: 10.1002/j.1460-2075.1992.tb05181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renscheidt H., Strassburger W., Glatter U., Wollmer A., Dodson G. G., Mercola D. A. A solution equivalent of the 2Zn----4Zn transformation of insulin in the crystal. Eur J Biochem. 1984 Jul 2;142(1):7–14. doi: 10.1111/j.1432-1033.1984.tb08243.x. [DOI] [PubMed] [Google Scholar]
- Restall C. J., Coke M., Phillips E., Chapman D. Derivative spectroscopy of tryptophan fluorescence used to study conformational transitions in the (Ca2+ + Mg2+)-adenosine triphosphatase of sarcoplasmic reticulum. Biochim Biophys Acta. 1986 Dec 12;874(3):305–311. doi: 10.1016/0167-4838(86)90029-4. [DOI] [PubMed] [Google Scholar]
- Richardson J. S., Richardson D. C. Amino acid preferences for specific locations at the ends of alpha helices. Science. 1988 Jun 17;240(4859):1648–1652. doi: 10.1126/science.3381086. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Moras D., Olsen K. W. Chemical and biological evolution of nucleotide-binding protein. Nature. 1974 Jul 19;250(463):194–199. doi: 10.1038/250194a0. [DOI] [PubMed] [Google Scholar]
- Rottapel R., Reedijk M., Williams D. E., Lyman S. D., Anderson D. M., Pawson T., Bernstein A. The Steel/W transduction pathway: kit autophosphorylation and its association with a unique subset of cytoplasmic signaling proteins is induced by the Steel factor. Mol Cell Biol. 1991 Jun;11(6):3043–3051. doi: 10.1128/mcb.11.6.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki F., Homma Y., Takenawa T. Two types of phosphatidylinositol 3-kinase from bovine thymus. Monomer and heterodimer form. J Biol Chem. 1991 May 5;266(13):8108–8114. [PubMed] [Google Scholar]
- Shtivelman E., Lifshitz B., Gale R. P., Roe B. A., Canaani E. Alternative splicing of RNAs transcribed from the human abl gene and from the bcr-abl fused gene. Cell. 1986 Oct 24;47(2):277–284. doi: 10.1016/0092-8674(86)90450-2. [DOI] [PubMed] [Google Scholar]
- Shurtleff S. A., Downing J. R., Rock C. O., Hawkins S. A., Roussel M. F., Sherr C. J. Structural features of the colony-stimulating factor 1 receptor that affect its association with phosphatidylinositol 3-kinase. EMBO J. 1990 Aug;9(8):2415–2421. doi: 10.1002/j.1460-2075.1990.tb07417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurtleff S. A., Downing J. R., Rock C. O., Hawkins S. A., Roussel M. F., Sherr C. J. Structural features of the colony-stimulating factor 1 receptor that affect its association with phosphatidylinositol 3-kinase. EMBO J. 1990 Aug;9(8):2415–2421. doi: 10.1002/j.1460-2075.1990.tb07417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnik E. Y., Margolis B., Mohammadi M., Lowenstein E., Fischer R., Drepps A., Ullrich A., Schlessinger J. Cloning of PI3 kinase-associated p85 utilizing a novel method for expression/cloning of target proteins for receptor tyrosine kinases. Cell. 1991 Apr 5;65(1):83–90. doi: 10.1016/0092-8674(91)90410-z. [DOI] [PubMed] [Google Scholar]
- Small E. W., Isenberg I. Hydrodynamic properties of a rigid molecule: rotational and linear diffusion and fluorescence anisotropy. Biopolymers. 1977 Sep;16(9):1907–1928. doi: 10.1002/bip.1977.360160907. [DOI] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Varticovski L., Druker B., Morrison D., Cantley L., Roberts T. The colony stimulating factor-1 receptor associates with and activates phosphatidylinositol-3 kinase. Nature. 1989 Dec 7;342(6250):699–702. doi: 10.1038/342699a0. [DOI] [PubMed] [Google Scholar]
- Vogel U. S., Dixon R. A., Schaber M. D., Diehl R. E., Marshall M. S., Scolnick E. M., Sigal I. S., Gibbs J. B. Cloning of bovine GAP and its interaction with oncogenic ras p21. Nature. 1988 Sep 1;335(6185):90–93. doi: 10.1038/335090a0. [DOI] [PubMed] [Google Scholar]
- Wilmot C. M., Thornton J. M. Beta-turns and their distortions: a proposed new nomenclature. Protein Eng. 1990 May;3(6):479–493. doi: 10.1093/protein/3.6.479. [DOI] [PubMed] [Google Scholar]
- Yu J. C., Heidaran M. A., Pierce J. H., Gutkind J. S., Lombardi D., Ruggiero M., Aaronson S. A. Tyrosine mutations within the alpha platelet-derived growth factor receptor kinase insert domain abrogate receptor-associated phosphatidylinositol-3 kinase activity without affecting mitogenic or chemotactic signal transduction. Mol Cell Biol. 1991 Jul;11(7):3780–3785. doi: 10.1128/mcb.11.7.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Geer P., Hunter T. Identification of tyrosine 706 in the kinase insert as the major colony-stimulating factor 1 (CSF-1)-stimulated autophosphorylation site in the CSF-1 receptor in a murine macrophage cell line. Mol Cell Biol. 1990 Jun;10(6):2991–3002. doi: 10.1128/mcb.10.6.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]



