Abstract
Background
Benefits of exercise on dialysis (EOD) are well established, however, uptake in our local satellite haemodialysis units is low. The implications of the status quo are risks to treatment efficiency, equity and patient centredness in managing personal health risks. The current study aimed to identify and address barriers to exercise participation while on dialysis by substantiating local EOD risks, assigning context, implementing changes and evaluating their impact. Our primary objective was to increase the uptake of EOD across our five dialysis units.
Methods
Semi-structured interview and questionnaire data from patients and nursing staff were used to inform a root-cause analysis of barriers to exercise participation while on dialysis. Intervention was subsequently designed and implemented by a senior physiotherapist. It consisted of patient and nursing staff education, equipment modification and introduction of patient motivation schemes.
Results
Staff knowledge, patient motivation and equipment problems were the main barriers to EOD. A significant increase in the uptake of EOD from 23.3% pre-intervention to 74.3% post-intervention was achieved [χ2 (1, N = 174) = 44.18, P < 0.001].
Conclusions
Barriers to EOD are challenging, but there is evidence that patients wish to participate and would benefit from doing so. The input of a physiotherapist in the dialysis units had a significant positive effect on the uptake of EOD. National guidelines should encourage dialysis units to include professional exercise provision in future service planning.
Keywords: chronic kidney disease, dialysis, exercise, quality improvement
Introduction
Background
Chronic kidney disease (CKD) prevalence is increasing because of its association with the rising burdens of diabetes and hypertension [1]. Patients with CKD who progress to stage 5 will require renal replacement therapy (RRT; dialysis or kidney transplantation) for long-term survival. The 2010 Health Survey for England estimated that 6% of men and 7% of women have stage 3–5 CKD [2] and as of 2012 there were 54 824 patients receiving RRT UK-wide, with 42.7% (∼ 23 000 patients) reliant on haemodialysis (HD) [3].
The rationale for provision of exercise on dialysis (EOD) is to offer supervised exercise within the time constraints of dialysis and potentially exercise-induced supplementation of the HD process. There is a persuasive view that service users represent a ‘captive audience’ [4] who could actively exercise in parallel with dialysis and respond to health education that is reinforced by multidisciplinary team (MDT) members [5]. EOD has been shown to be feasible and safe [6] and provides biopsychosocial benefits including cardiopulmonary fitness, muscle strength, blood pressure control, health-related quality of life (HRQoL), physical function and potential improvements in urea and creatinine clearance [7–10]. A Cochrane Review concluded that adults with CKD, including those undergoing HD, could significantly improve their cardiovascular fitness, functional capacity, blood pressure and HRQoL by participating in exercise three times a week for >30 min/session [11].
Local problem
Despite agreement that EOD is beneficial in CKD management, uptake and long-term participation is low and CKD patients are often particularly inactive [12]. Patient-perceived barriers reported in the literature include low motivation, a perceived lack of time during dialysis, CKD-related shortness of breath [13], fatigue, muscle cramps, decreased muscle strength and the presence of multiple comorbidities [13, 14]. While patients on HD are a captive audience, it seems they are not captivated by EOD.
Clinical practice guidelines state that clinical staff should provide encouragement and education to HD patients to improve levels of physical activity (PA) [15]. However, there remains uncertainty about professional responsibility for EOD provision. Barriers reported by clinical staff have included lack of time or expertise and concerns regarding adverse effects [16]. It is recognized that exercise professionals, including physiotherapists, possess the requisite skills to provide EOD education, advice and support to patients and MDT members working within HD environments [17, 18].
Study aims
Our aims were to determine specific local barriers to EOD and to develop and implement a structured, physiotherapy-led intervention to address these across our satellite HD units. The objective was to increase the uptake of EOD.
Materials and methods
Ethical approval
This quality improvement (QI) project was designed to measure change following the introduction of clinical improvement strategies and did not require ethical approval beyond registration and governance by the local clinical audit committee (audit no. 3218).
Setting
The QI project was conducted across five satellite dialysis units, diverse in location, size and organization (Table 1). A, senior physiotherapist (project lead) was recruited to lead the 9-month project.
Table 1.
Satellite dialysis units
| Dialysis capacity |
Total staffa |
||||||
|---|---|---|---|---|---|---|---|
| Site | Location | Organization | Bed/chair | Self-care | Nurse | HCAb | Shifts |
| A | IL (S) | NHS | 21 | 2 | 15 (4) | 3 (2) | AM, PM |
| B | GL (SE) | NHS | 20 | 4 | 13 (4) | 3 (1) | AM, PM |
| C | Kent (W) | NHS | 28 | 8 | 21 (7) | 4 (1) | AM, PM |
| D | GL (SE) | Privateb | 12 | 0 | 10 (3) | 3 (1) | AM, PM, Twilight |
| E | GL (S) | Private | 15 | 1 | 12 (4) | 3 (1) | AM, PM, Twilight |
GL, Greater London; HCA, health care assistant; IL, Inner London; S, south; SE, southeast; W, west.
Minimum staffing per shift in parentheses.
Divarum.
Planning the intervention
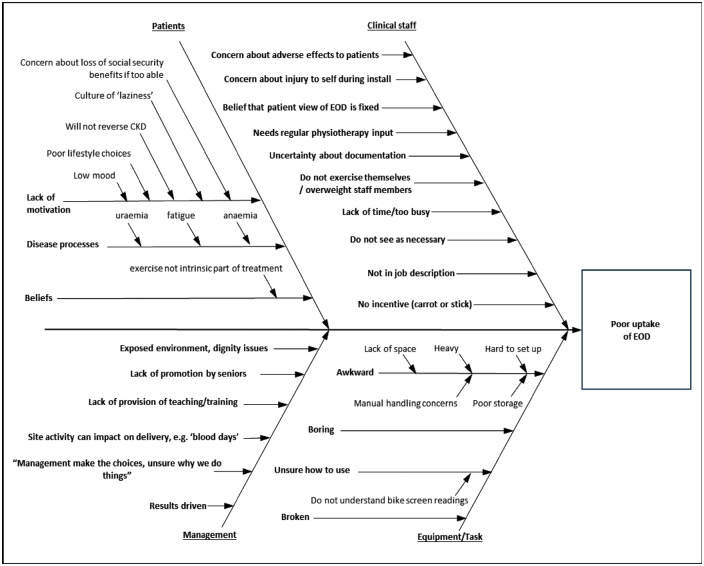
The 4Es translation model was applied [19] by utilizing a root-cause analysis to identify local barriers to EOD at site A, selected for convenience of its location (Table 1). A series of questionnaires (Supplementary appendix 2), designed by the project lead based on existing tools [20], were piloted and refined at site A and revealed local barriers to EOD. A fishbone diagram summary of modal themes was produced [21] from both questionnaire and semi-structured interview data from a sample of representative clinical staff and patients (Figure 1). Further details can be found in the online supplement (Supplementary appendices 1–9). Identified themes informed the generation of intervention elements, including education and motivation, deemed necessary to affect the primary outcome measure (rate of EOD among patients), and secondary measures (staff and patient perceptions of EOD). The intervention was deployed across all sites and evaluated across all sites except site A (Supplementary appendix 6).
Fig. 1.
Root-cause analysis: barriers to EOD.
Implementation of i ntervention. Final intervention strategies (Table 2) were implemented independently at all sites to maintain the equity of QI. The satellite sites consisted of a mixture of National Health Service (NHS) and private sector providers. Private sector provision was undertaken by Europe’s largest product-independent renal care service provider company, which is authorized by the Care Quality Commission in an outcomes-based prime contractor arrangement with the NHS Trust (prime contractor). The provision of services was, therefore, subject to consistent clinical governance across all sites. A 12-week timetable detailed the introduction of intervention processes (Supplementary appendix 7). This was conducted pragmatically [19]; interventions were adaptable, recognizing variation in management structures, working systems and available resources across sites. The project lead and local stakeholders planned changes to environment, practice and training/development collaboratively.
Table 2.
Interventions
| Intervention component | Details | |
|---|---|---|
| 1. | Clinical staff education sessions |
|
| 2. | Appointment of permanent clinical staff advocate for EOD (Exercise Link Practitioner) |
|
| 3. | Motivational schemes and improved access to literature promoting EOD |
|
| 4. | Patient education sessions |
|
| 5. | Improvement and standardization in documentation of EOD |
|
| 6. | Provision of appropriate and fully functioning equipment |
|
| 7. | Provision of training and support for clinical staff |
|
Staff and patient participants. Unit staff were excluded if they were not present during the first 3 weeks of the intervention. Patients were excluded if they declined to participate, had severe cognitive impairment, were unable to communicate in English or were receiving dialysis away from their regular unit.
Planning the study of the intervention
This was a quasi-experimental, pre-/post-intervention QI project. EOD uptake was measured by self-reported use of cycle ergometry while on dialysis from questionnaires at weeks 1 and 12 (Supplementary appendix 3). Changes in patient and staff beliefs/behaviours were determined from questionnaires at weeks 1 and 12 (Supplementary appendices 3–6).
Patient demographic factors (age, sex and ethnicity), length of time on dialysis (days), comorbidity measures (modified Charlson comorbidity index [22]) and hand grip strength (standardized hand-held dynamometry protocol [23] from calibrated dynamometers; Jamar Hydraulic Hand Dynamometer) as a surrogate measure of frailty [24] were collected to describe the population at sites A–E. To avoid bias, the results of site A’s data were not used in the pre-/post-intervention analyses. Patients and staff were encouraged to participate and were provided with a full explanation of the purpose and potential benefits of the study. Every effort was made to recover questionnaires and minimize missing data. Few exclusion criteria and multiple sites were used in an attempt to maximize generalizability.
Methods of evaluation
Semi-structured interviews for assessing root-cause analysis, questionnaires for assessing patients’ and staff’s beliefs and documented/self-reported level of participation in exercise were utilized and are detailed in the Supplementary data. Participants were assigned a unique identification number by the project lead and questionnaire order was randomized using predetermined orders derived from commercially available software (Microsoft Excel, Microsoft, Redmond, WA, USA, 2003).
To ensure robustness and feasibility of the questionnaires, they were piloted among staff and patients at site A and adapted based on feedback prior to use in the study. Any patients who required help with literacy were aided by the project lead appropriately.
Data analysis
Categorical data were summarized as frequency (%) and continuous data as mean [standard deviation (SD)] or median [interquartile range (IQR)] as appropriate. Characteristics of participants from each of the four sites were compared using a one-way analysis of variance, Kruskal–Wallis test, χ2 test or Fisher’s exact test, as appropriate. Proportions of participants reporting cycle ergometry while on dialysis pre- and post-intervention were compared using a χ2 test. For all other outcomes, all pre- and post-intervention data were summarized and compared using a two-sample t-test, χ2 test or Fisher’s exact test, as appropriate. Perception and belief data from questionnaire responses were dichotomized into agreeing or disagreeing by combining responses ‘agree’ or ‘strongly agree’ and ‘disagree’ or ‘strongly disagree’. An alpha level of 0.05 was used for all statistical tests. Uptake of EOD was expected to increase to 40% of patients. A power calculation based on an approximate current uptake of exercise of 25% and a 95% confidence interval determined a sample size of 152 patients to give statistical power of 99%. Power calculation and all other statistical analyses were undertaken using STATA 11ME (StataCorp, College Station, TX, USA) and SPSS version 17 (SPSS, Chicago, IL, USA).
Results
Outcomes
Participants and EOD s ites. Three hundred and five dialysis patients were available for inclusion in the study. After applying eligibility criteria, 177 patients and 86 members of clinical staff [nurses and health care assistants (HCAs)] were eligible for study inclusion.
Non-uniformity of patient groups across sites was confirmed (Table 3).
Table 3.
Comparison of participant characteristics across control site (A) and intervention sites (B-E)
| groups | Site A | Site B | Site C | Site D | Site E | P-value |
|---|---|---|---|---|---|---|
| Age (years), mean (SD) | 65 (14) | 56.2 (13.1) | 73 (11.9) | 70 (10.9) | 66 (13.5) | <0.001 |
| Sex, n (%) | 0.461 | |||||
| Male | 45 (54.9) | 24 (53.3) | 31 (57.4) | 24 (70.6) | 28 (60.9) | |
| Female | 37 (45.1) | 21 (46.7) | 23 (42.6) | 10 (29.4) | 18 (39.1) | |
| Ethnicitya, n (%) | <0.001 | |||||
| White | 34 (41) | 13 (29.6) | 54 (100) | 30 (90.9) | 16 (34.8) | |
| Black | 40 (48.8) | 25 (56.8) | 0 (0) | 1 (3) | 24 (52.2) | |
| Other | 8 (20) | 6 (13.6) | 0 (0) | 2 (6.1) | 6 (13) | |
| Length of time on dialysisa (days), mean (range) | 1808.7 (37–6574) | 1229 (789–2197) | 502.5 (865–2103) | 915 (670–1735) | 1503 (1133–2077) | 0.019 |
| Charlston comorbidity indexa, mean (SD) | 4.5 (1.85) | 4.53 (1.8) | 6.5 (2.2) | 6.4 (1.9) | 5.5 (18) | <0.001 |
| Hand grip strength, median (IQR) | unavailable | 20.6 (16.6–25.3) | 19.0 (10.0–28.0) | 18.6 (10.7–28) | 18.7 (14–26.6) | 0.702 |
Significant differences across.
All 177 eligible patients completed questionnaires pre-intervention and 134 (76%) were completed post-intervention. Reasons for non-completion included patient unavailable (n = 20), patient declined (n = 14), medical condition changed (e.g. transplant; n = 5), death (n = 2), acute illness (n = 1) and out of trust transfer (n = 1).
Return rates for initial questionnaires from clinical staff were 28 (33%) for exercise behaviour and 32 (37%) for exercise belief. This decreased to 21 (24%) and 20 (24%), respectively, at week 12. Low response rates were partly attributed to staff absence due to planned leave or sickness.
EOD uptake. The proportion of patients across all sites who reported a lifetime prevalence of EOD participation showed a statistically significantly increase from 43.9% pre-intervention to 77.9% post-intervention [χ2 (1, N = 127) = 35.37, P < 0.001]. Proportions of patients reporting a 3-month prevalence of EOD participation increased from 23.3% to 74.3% [χ2 (1, N = 174) = 44.18, P < 0.001]. Of those exercising, frequency and duration of EOD showed no statistically significant change. The percentage of individuals who reported using weights when on dialysis decreased from 30.2 to 13.9% [χ2 (1, N = 136) = 9.00, P = 0.003]. There was no significant change in patient-reported exercise behaviour outside dialysis sessions [χ2 (1, N = 162) = 0.87, P = 0.352].
Patient belief. There were positive changes in beliefs that exercise slows down the rate of bone disease, with the proportion of respondents agreeing increasing from 73.8 to 90.6%, (P = 0.010, Fisher’s exact test). Participants disagreeing with the statement ‘I have a lack of understanding on how to carry out exercise’ increased from 64.1 to 79% (P = 0.016, Fisher’s exact test). There was a small but statistically significant increase in those disagreeing with the belief that exercise is harmful to the health of dialysis patients: 11.3 to 14.2% [χ2 (3, N = 141) = 8.23, P = 0.042].
Post-intervention, patients more often identified the following reasons for not exercising: not yet being assessed to exercise (P < 0.001, Fisher’s exact test), exercising enough already elsewhere [χ2 (1, N = 311) = 5.7056, P = 0.017] and being busy at home [χ2 (1, N = 311) = 8.5722, P = 0.003] (Supplementary appendix 8). There were no statistically significant changes in patient-reported variables that would encourage more frequent exercise pre-intervention to post-intervention.
Clinician perception and beliefs. All clinicians reported a significant increase in confidence preparing a patient for EOD (Supplementary appendix 9), with median responses rising post-intervention (P = 0.008, Fisher’s exact test). The proportion of clinical staff reporting that HCAs were most responsible for encouraging EOD rose from 21.4 to 61.9% (P = 0.004, Fisher’s exact test). There was an increase in the proportion of respondents who agreed or strongly agreed with the statement ‘nursing staff do not always have the time to help with exercise equipment’, from 45.2 to 66.6% (P = 0.055, Fisher’s exact test). Although the proportion of patients who disagreed with the statement ‘frequent tiredness stops exercise participation’ increased from 0 to 14.3% (P = 0.028, Fisher’s exact test), there was an increase in those agreeing that exercise is not suitable for patients with multiple health problems, from 12.6 to 38.1% (P = 0.013, Fisher’s exact test).
Discussion
Summary
This study is the first to report on the planning and introduction of practice improvement across multiple dialysis sites with non-uniform patient demographics and clinical presentations. Improvement strategies were identified and effectively and efficiently introduced through a transparent, reproducible 12-week plan that aimed to address barriers to EOD.
A significant increase in the proportion of patients trying EOD and the proportion of those reporting participation in EOD in the previous quarter ensued. Our results suggest that there is potential to address barriers and increase acceptance of EOD to establish an exercise culture within satellite units. However, there remains, mixed beliefs of patients and staff suggesting a mismatch between need and operational provision.
Relation to other evidence
Most literature regarding EOD focuses on the biomedical benefits [7, 9], which provided the rationale for local EOD delivery and prompted this QI project. However, EOD becomes questionable if patient uptake limits its application. Clinical trials demand strict adherence to EOD protocols to establish causal relationships. This does not translate directly to a complex clinical context, with fluctuating motivation levels, logistic factors and equipment issues. Within this real-world, pragmatic context, patient-perceived markers are arguably more relevant to ensure sustained participation. Considering not only the physical presentation but also the psychological components allows a more targeted approach in implementing EOD, as demonstrated in this study. While we have shown that it is possible to improve EOD uptake across diverse satellite units, our data and that of others [12, 13] show that not all patients participate in EOD. The specific EOD type, intensity, frequency and support for patients within their socio-medical context is now the challenge, with realist approaches promising a method of inquiry [25].
Patient-perceived physical barriers cited in the literature, such as shortness of breath, fatigue, muscle cramps, decreased muscle strength and the presence of comorbidities [13, 14], are not in themselves contraindications to EOD. Instead, they have been argued to represent patients’ perception of the disease burden, and our results suggest that there is interest from patients to participate in EOD despite perceived physical barriers, reflecting existing published research [13]. Motivation and structure provided by physiotherapist-led intervention and streamlining of service delivery are the most likely causes of increased EOD participation.
Interpretation
Clinician-reported beliefs demonstrated a favourable understanding of exercise at baseline, with significant positive change in perceptions of confidence in setting up patients on EOD post-intervention. Patients’ baseline exercise beliefs demonstrated a generally good level of understanding too, and significant improvements were reported post-intervention with regard to the impact of exercise on bone health and ability to carry out EOD. The pre-intervention root-cause analysis conducted at site A revealed that clinical staff felt they lack time, deprioritizing EOD in the dialysis process. Despite educational components of the intervention focusing on the suspected benefits of EOD and training demonstrating the safety and ease of set-up, a high proportion of clinical staff post-intervention continued to report not having time to help with exercise equipment. It is possible that patient and clinical staff non-response to the study may reflect a level of apathy towards EOD or the QI project. Nursing staff may continue to believe that EOD provision is outside of their clinical role, reflected by the captured belief that EOD is the domain of physiotherapy (95.2% post-intervention). These findings suggest that clinicians and patients have an understanding of the benefits of exercise and welcomed strategies to engender it, but enacting changes in behaviours is not optimal. Psycho-educative methodologies [26, 27] may be the next step in grounding change at the clinician and patient level.
Delgado and Johansen [13] have argued for the development of motivational strategies that acknowledge patient-perceived time and disease burdens, and the importance of integrating self-management education into CKD exercise rehabilitation has been recognized with exercise counselling in conjunction with exercise being promoted [28, 29]. While the results of this QI project may be subject to response bias, the fact that patient-reported rates for trialling EOD/participating in the last 3 months increased so significantly suggests that, at least, the benefits of EOD were understood.
Both the reduction in patients undertaking resistance training and the increase in patients believing that exercise is unsuitable for people with multiple health problems were intriguing results. Since our purpose was to increase the uptake of EOD, these findings should be explored in future studies. Nonetheless, our interpretation is that being exposed to systematic EOD might have caused patients’ fear of movement, or kinesiophobia, to manifest and resistive training (i.e. using weights in open kinematic-chain reciprocal movement) represented a greater threat than cycle ergometry. Kinesiophobia is an attribute typically associated with chronic pain syndromes, but might also relate with other chronic conditions [30], with high levels of kinesiophobia associated with low self-efficacy, low self-regulated PA, length of dialysis and reduced creatinine clearance in renal transplant patients [31]. While we are unable to provide any data to support it, these surprising findings might be attributed to an interaction between exercise type and illness vulnerability cognitions and perceived threat of injury or re-injury resulting in fear of movement.
In addition, it was interesting that patients’ reports of not yet being assessed for exercise increased during the QI intervention despite an exercise assessment being included in the intervention. Two factors might explain this finding. First, confidence to participate in PA might be influenced by co-morbidity where exercise-induced physical symptoms are misinterpreted as health threats, causing fear and distress that can decrease self-efficacy [32]. Second, our pre-assessment informed the EOD dose that deliberately worked patients at a moderate intensity to minimize high-intensity unpleasantness [33] and was also designed to convey a positive knowledge about exercising. While knowledge acquisition is a necessary factor in changing behaviour, it is not usually sufficient by itself and influences to change health behaviour must usually come from sources in addition to, or instead of, factual knowledge, including behavioural modification interventions [34]. Inadvertently, it is, therefore, possible that our exercise pre-assessment adversely affected patients’ perceptions of health risk and exercise knowledge.
Supervision of PA interventions is contentious; meta-analyses suggest it is costly and no more effective than independent motivational or behavioural educational approaches in increasing PA in patients with chronic health conditions [35]. However, we agree with the position that rehabilitation models promoting EOD self-management, including behavioural modification interventions [11], requires sustained involvement of renal physiotherapists [18] and initial capital costs with which to acquire PA equipment (e.g. cycle ergometers). This would reduce the time pressures that continue to be reported by other clinical staff and concurs with Ridley et al.’s [36] proposition that an exercise professional should be present at dialysis units for at least 2 days per week. The supervision and support this would confer could act to offset reports of EOD rates reducing over time, and is in keeping with evidence of longer-term compliance with supervised exercise programmes [11]. While it is well accepted that PA as a health promotion activity has significant cost benefits to society [37], to our knowledge there is no robust study examining health care utilization cost benefits in increasing PA for patients with established chronic conditions, and future work is needed to establish this.
Limitations
This study was designed to be quasi-experimental, because it would have been unethical to abbreviate existing exercise provision at the units. Furthermore, we were not confident that we could control contamination between patient groups within the diverse units, and selection of a control site would be a challenge. We acknowledge, therefore, that our results cannot be causally related nor generalized. Nonetheless, a strength of the study is that we have provided evidence that EOD uptake can be positively influenced across multiple sites, and future work can investigate causal relationships. Nominal development of the questionnaires used was due to available resources and we acknowledge susceptibility to response bias. A limitation of the adapted questionnaire [20] was the revision of response categories to ‘strongly agree’, ‘agree’, ‘disagree’ and ‘strongly disagree’, with no intermediate option. Respondents were required to make a polarized decision that might not capture their view.
A single project lead across multiple geographical sites meant it was a challenge with staff shifts and patient appointment times to collect completed questionnaires, reflected in our disappointing return rates, which we acknowledge. Future multisite studies should allocate appropriate research staff costs to maximize return rates.
Consultant nephrologists, while involved in the consultation and broad implementation of intervention strategies for this QI project, were not involved with the specific interventions to promote organizational change. Arguably, change in clinical environments occurs from the top down; in the future, senior clinicians should be fully integrated in the QI activity.
The use of self-reports of EOD frequency and duration rather than objective measurement limits reliability of the results and may be subject to social desirability bias. However, such measurement was beyond the scope of clinical governance we were subject to and the pragmatics of real QI.
Conclusion
We successfully implemented a positive change in the uptake of EOD in five disparate satellite dialysis units and have provided evidence of the specific challenges that remain. The sustainability of the intervention is unknown and EOD maintenance requires further investigation. Nonetheless, these results provide evidence that an exercise culture within multiple dialysis units can be established. National guidelines should encourage that exercise provision be incorporated into future service planning to facilitate this captive audience towards self-management of this global health problem.
Supplementary data
Supplementary data are available online at http://ckj.oxfordjournals.org.
Supplementary Material
Acknowledgements
The authors would like to thank Anne Bisset-Smith, Clinical Specialist Physiotherapist, for her support in planning and undertaking the project; Siobhan Crichton, King’s College London, for her assistance with the statistical analyses and Dr Jacky Jones, Head of Physiotherapy Guy’s & St. Thomas’ NHS Foundation Trust, for reviewing the manuscript. Due acknowledgement is given to Ros Tibbles, Service Improvement Nurse, and Debra Mundle, Clinical Nurse Manager Satellite Haemodialysis, for their assistance in planning the project. In addition, we thank the staff and patients at all the dialysis units for their participation. This study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement
None declared.
References
- 1. Zhang QL, Rothenbacher D.. Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health 2008; 8: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Office for National Statistics and NHS Information Centre for Health and Social Care. Health Survey for England 2010: Health and Lifestyles, Office for National Statistics, Leeds, 2011 [Google Scholar]
- 3. Shaw C, Pitcher D, Pruthi R. et al. UK Renal Registry 16th Annual Report: Chapter 2 UK RRT Prevalence in 2012: National and Centre-specific Analyses. Health and Social Care Trust, Bristol, UK, 2013 [DOI] [PubMed] [Google Scholar]
- 4. Desai AA, Bolus R, Nissenson A. et al. Identifying best practices in dialysis care: results of cognitive interviews and a national survey of dialysis providers. Clin J Am Soc Nephrol 2008; 3: 1066–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McMurray SD, Johnson G, Davis S. et al. Diabetes education and care management significantly improve patient outcomes in the dialysis unit. Am J Kidney Dis 2002; 40: 566–575 [DOI] [PubMed] [Google Scholar]
- 6. Mohseni R, Emami Zeydi A, Ilali E. et al. The effect of intradialytic aerobic exercise on dialysis efficacy in hemodialysis patients: a randomized controlled trial. Oman Med J 2013; 28: 345–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Anderson JE, Boivin MR Jr, Hatchett L.. Effect of exercise training on interdialytic ambulatory and treatment-related blood pressure in hemodialysis patients. Ren Fail 2004; 26: 539–544 [DOI] [PubMed] [Google Scholar]
- 8. Kopple JD, Storer T, Casburi R.. Impaired exercise capacity and exercise training in maintenance hemodialysis patients. J Ren Nutr 2005; 15: 44–48 [DOI] [PubMed] [Google Scholar]
- 9. Parsons TL, Toffelmire EB, King-VanVlack CE.. Exercise training during hemodialysis improves dialysis efficacy and physical performance. Arch Phys Med Rehabil 2006; 87: 680–687 [DOI] [PubMed] [Google Scholar]
- 10. Reboredo M, Henrique DM, de Souza Faria R. et al. Exercise training during hemodialysis reduces blood pressure and increases physical functioning and quality of life. Artif Organs 2010; 34: 586–593 [DOI] [PubMed] [Google Scholar]
- 11. Heiwe S, Jacobson SH.. Exercise training for adults with chronic kidney disease. Cochrane Database Syst Rev 2011; 10: CD003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johansen KL, Chertow GM, Ng AV. et al. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int 2000; 57: 2564–2570 [DOI] [PubMed] [Google Scholar]
- 13. Delgado C, Johansen KL.. Barriers to exercise participation among dialysis patients. Nephrol Dial Transplant 2012; 27: 1152–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heiwe S, Clyne N, Tollback A. et al. Effects of regular resistance training on muscle histopathology and morphometry in elderly patients with chronic kidney disease. Am J Phys Med Rehabil 2005; 84: 865–874 [DOI] [PubMed] [Google Scholar]
- 15. National Kidney Foundation. K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 2005; 45(4 Suppl 3): S1–S153 [PubMed] [Google Scholar]
- 16. Johansen KL, Sakkas GK, Doyle J. et al. Exercise counseling practices among nephrologists caring for patients on dialysis. Am J Kidney Dis 2003; 41: 171–178 [DOI] [PubMed] [Google Scholar]
- 17. Bennett PN, Breugelmans L, Barnard R. et al. Sustaining a hemodialysis exercise program: a review. Semin Dial 2010; 23: 62–73 [DOI] [PubMed] [Google Scholar]
- 18. Greenwood S. The role of the physiotherapist in the renal unit. J Ren Nurs 2010; 2: 292–295 [Google Scholar]
- 19. Pronovost PJ, Berenholtz SM, Needham DM.. Translating evidence into practice: a model for large scale knowledge translation. BMJ 2008; 337: a1714. [DOI] [PubMed] [Google Scholar]
- 20. Zheng J, You LM, Lou TQ. et al. Development and psychometric evaluation of the dialysis patient-perceived exercise benefits and barriers scale. Int J Nurs Stud 2010; 47: 166–180 [DOI] [PubMed] [Google Scholar]
- 21. Fernandes CM, Walker R, Price A. et al. Root cause analysis of laboratory delays to an emergency department. J Emerg Med 1997; 15: 735–739 [DOI] [PubMed] [Google Scholar]
- 22. Beddhu S, Bruns FJ, Saul M. et al. A simple comorbidity scale predicts clinical outcomes and costs in dialysis patients. Am J Med 2000; 108: 609–613 [DOI] [PubMed] [Google Scholar]
- 23. Fess EE. Grip strength In: Casanova JS. (ed). Clinical Assessment Recommendations. Chicago, IL: American Society of Hand Therapists, 1992, 41–45 [Google Scholar]
- 24. de Vries NM, Staal JB, van Ravensberg CD. et al. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev 2011; 10: 104–114 [DOI] [PubMed] [Google Scholar]
- 25. Thompson S, Clark A, Molzahn A. et al. Increasing the uptake of exercise programs in the dialysis unit: a protocol for a realist synthesis. Syst Rev 2016; 5: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aldcroft SA, Taylor NF, Blackstock FC. et al. Psychoeducational rehabilitation for health behavior change in coronary artery disease: a systematic review of controlled trials. J Cardiopulm Rehabil Prev 2011; 31: 273–281 [DOI] [PubMed] [Google Scholar]
- 27. Tones BK. Health education, behaviour change and public health In: Dettels R, McEwen J (eds). Oxford Textbook of Public Health. Oxford: Oxford University Press, 1997, 786 [Google Scholar]
- 28. Tawney KW, Tawney PJ, Kovach J.. Disablement and rehabilitation in end-stage renal disease. Semin Dial 2003; 16: 447–452 [DOI] [PubMed] [Google Scholar]
- 29. van Vilsteren MC, de Greef MH, Huisman RM.. The effects of a low-to-moderate intensity pre-conditioning exercise programme linked with exercise counselling for sedentary haemodialysis patients in the Netherlands: results of a randomized clinical trial. Nephrol Dial Transplant 2005; 20: 141–146 [DOI] [PubMed] [Google Scholar]
- 30. Hart DL, Werneke MW, George SZ. et al. Screening for elevated levels of fear-avoidance beliefs regarding work or physical activities in people receiving outpatient therapy. Phys Ther 2009; 89: 770–785 [DOI] [PubMed] [Google Scholar]
- 31. Zelle DM, Corpeleijn E, Klaassen G. et al. Fear of movement and low self-efficacy are important barriers in physical activity after renal transplantation. PLoS One 2016; 11: e0147609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev 1977; 84: 191–215 [DOI] [PubMed] [Google Scholar]
- 33. Vandoni M, Codrons E, Marin L. et al. Psychophysiological responses to group exercise training sessions: does exercise intensity matter? PLoS One 2016; 11: e0149997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. French DP, Olander EK, Chisholm A. et al. Which behaviour change techniques are most effective at increasing older adults' self-efficacy and physical activity behaviour? A systematic review. Ann Behav Med 2014; 48: 225–234 [DOI] [PubMed] [Google Scholar]
- 35. Conn VS, Hafdahl AR, Brown SA. et al. Meta-analysis of patient education interventions to increase physical activity among chronically ill adults. Patient Educ Couns 2008; 70: 157–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ridley J, Hoey K, Ballagh-Howes N.. The exercise-during-hemodialysis program: report on a pilot study. CANNT J 1999; 9: 20–26 [PubMed] [Google Scholar]
- 37. Allender S, Foster C, Scarborough P. et al. The burden of physical activity-related ill health in the UK. J Epidemiol Commun Health 2007; 61: 344–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.