Abstract
Carbon-ion radiotherapy (CIRT) is an advanced radiotherapy and has achieved good local control, even in tumors that are resistant to conventional photon beam radiotherapy (PBRT). However, distant metastasis control is an important issue. Recently, the combination of radiotherapy and immunotherapy has attracted the attention. In immunotherapy, dendritic cells (DCs) play a pivotal role in the anti-tumor immune system. However, the mechanisms underlying the combination therapy of DCs and radiotherapy have been unclear. In the present study, we evaluated anti-metastatic effects of this combination therapy, focused on the irradiation type and the route of DC administration, using a mouse model. C3H/He mice bearing NR-S1 cells were treated with CIRT or PBRT, using biologically equivalent doses. Subsequently, DCs were administered intratumorally (IT) or intravenously (IV). IV and IT DC administrations combined with CIRT to the local tumor, but not alone, significantly suppressed pulmonary metastasis, whereas the combination of DCs with PBRT suppressed metastasis at a relatively higher dose. Additionally, the anti-metastatic effect was greater in IV DC administration compared with in IT DC administration in both CIRT and PBRT. The expression levels of CD40 and IL-12 in DCs were significantly increased after co-culturing with CIRT-treated NR-S1 cells. In addition, IV administration of those co-cultured DCs significantly suppressed pulmonary metastasis. Furthermore, ecto-calreticulin levels from CIRT-treated NR-S1 cells significantly increased compared with those of a PBRT-treated tumor. Taken together, these results suggest that local CIRT combined with IV DCs augments an immunogenicity of the tumor cells by ecto-calreticulin expression and the maturation of DCs to stimulate anti-tumor immunity to decrease lung metastases.
Keywords: calreticulin, carbon-ion radiotherapy, CD40, dendritic cell, metastasis
INTRODUCTION
Particle radiotherapy such as proton and carbon-ion (C-ion) radiotherapies has become widespread in recent years. In particular, C-ion radiotherapy (CIRT) possesses excellent dose distribution and strong cell-killing effect, and is considered to be one of the most outstanding breakthroughs in the field of cancer therapy [1]. Since 1994, more than 8000 patients with a variety of malignant tumors have been treated with CIRT at the National Institute of Radiological Sciences (NIRS) in Chiba, Japan, which have resulted in favorable local controls in most cases [2]. However, distant metastasis after local therapy still remains a major concern for long-term survival. In recent years, immunotherapy has been heralded as one of the most important advances in the field of cancer treatment. Particularly, immunotherapies targeting immune checkpoint receptors, such as cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4), programmed cell-death 1 (PD-1), and programmed cell-death ligand 1 (PD-L1) have demonstrated good clinical results in a wide variety of tumors [3–5]. In anti-tumor immunity, dendritic cells (DCs), which are the most potent professional antigen-presenting cells, play central roles in initiating the immune response after receiving signals from pathogens [6, 7]. In addition, there has been accumulating evidence supporting the concept that radiotherapy (RT) to the local tumor induces the host's immune responses [8–10]. To date, however, there have only been a few studies that have investigated the effectiveness of combination therapy with CIRT plus immunotherapy.
We have previously demonstrated that a combination of CIRT and DC-based immunotherapy significantly suppressed pulmonary metastases in a mouse model [11]. In that study, local administration of DCs to tumor allografts after CIRT significantly decreased the number of lung metastases. These results indicate that DC-based immunotherapy could become an effective anti-metastatic strategy for patients treated with CIRT. However, an optimal way of DC-administration has not been evaluated in previous studies. In addition, despite the significant anti-metastatic effect of the combined treatment, the underlying mechanisms of how irradiated tumor cells and the administered DCs effect anti-metastasis remain largely unknown. Moreover, it is important that certain treatments induce immunogenic cell death (ICD) and consequent DC maturation to elicit effective anti-tumor immunity. ICD, a type of cell death, which involves the expression of the mediator of the cell surface (such as calreticulin: CALR) as well as the release of soluble mediators (such as high-mobility group box 1: HMGB1). These mediators induce DC maturation to stimulate the presentation of tumor antigens to T cells [12–14]. DCs evolve from immature cells, which capture antigens, to mature cells, which perform antigen-presenting and T cell priming. Matured DCs convert antigens to immunogenic antigens and express molecules such as cytokines, chemokines, costimulatory molecules (such as CD40, CD80 and CD86) and proteases to initiate an immune response [15].
In the present study, we evaluated an optimal method of DC administration, irradiation, and the underlying mechanism of how the administered DCs exerted the anti-metastatic effect, focusing on ICD and DC maturation by local tumor irradiation in combination therapy with DCs.
MATERIALS AND METHODS
Mice and tumors
C3H/He female mice (7–8 weeks old) were purchased from Japan Shizuoka Laboratory Animal Center (Shizuoka, Japan). Mice were bred and maintained under specific-pathogen-free conditions. A murine squamous cell carcinoma cell line arose from buccal mucosa, NR-S1 (kindly provided by Dr Koichi Ando, National Institute of Radiological Science, Chiba, Japan) were maintained in DMEM medium (Nissui Pharmaceutical Co., Tokyo, Japan) with 10% fetal bovine serum and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA) at 37°C in a humidified atmosphere of 5% CO2 [16, 17]. The animal experiments were carried out with the permission and under regulation of the NIRS Institutional Animal Care and Use Committee (Permit numbers 08–2008 and 11–2021).
Preparation of bone marrow–derived immature DCs
The preparation of DCs has been described previously [18]. Briefly, cells obtained from flushed marrow cavities of femurs and tibias of C3H/He mice were cultured in DC complete media supplemented with 20 ng/ml recombinant murine granulocyte–macrophage colony-stimulating factor (GM-CSF) (Wako Pure Chemical Industries, Osaka, Japan) for 8 days. The immune response of the collected DCs was confirmed by lipopolysaccharide (LPS) treatment [19]. The DCs were washed twice with phosphate buffered saline (PBS), and a suspension of 1 × 106 cells in 0.02 ml PBS, and a similar suspension in 0.2 ml PBS, were immediately used for intratumoral (IT) and intravenous (IV) injection to mice, respectively.
Irradiation characteristics
CIRT was carried out using the Heavy Ion Medical Accelerator in Chiba (HIMAC) facility at the NIRS [20]. Tumor allografts and cultured cells were irradiated with 290 MeV/nucleon, 70~80 keV/µm C-ion beams at the center of the spread-out Bragg peak (6 cm SOBP). In photon beam radiation therapy (PBRT), 137Cs γ rays at a dose rate of 1.3 Gy/min at 21 cm from source to surface and X-rays (200 kV, 20 mA, 80 cm from X-ray focus to incident surface; 0.47–0.50 Gy/min) using the PANTAK HF-320S X-Ray Unit (Shimadzu, Kyoto, Japan) were utilized for tumor allografts and cultured cells, respectively.
Clonogenic assay
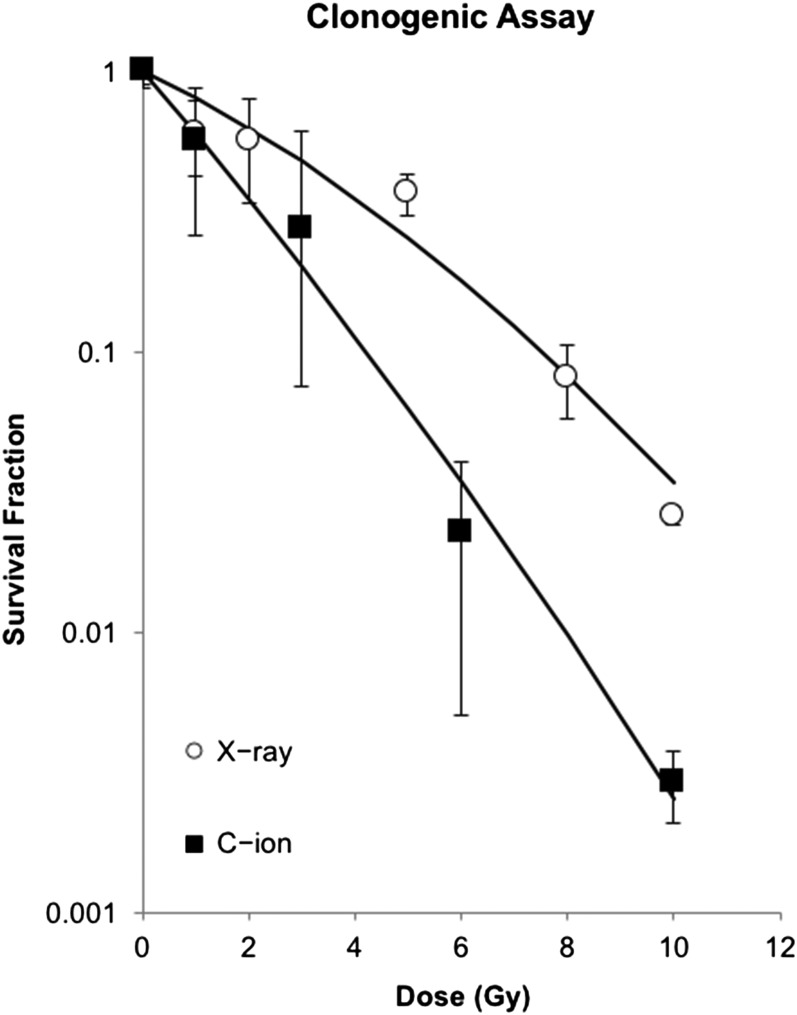
NR-S1 cells were seeded on dishes for colony formation assay. One day after seeding, cells were irradiated by C-ions or X-rays. Two weeks after irradiation, cells were fixed with 100% ethanol and stained with 2% crystal violet. Colonies consisting of more than 50 cells were counted. Surviving fractions against physical doses were plotted and fitted to survival curves using the following linear–quadratic model: SF = exp(−αD − βD2), where SF is the surviving fraction and D is the physical dose. The relative biological effectiveness (RBE) of C-ion beams compared with that of X-rays was calculated at the D0 (dose of radiation reducing the surviving fraction to 37%).
Inoculation of tumor cells and irradiation for assessment of pulmonary metastasis
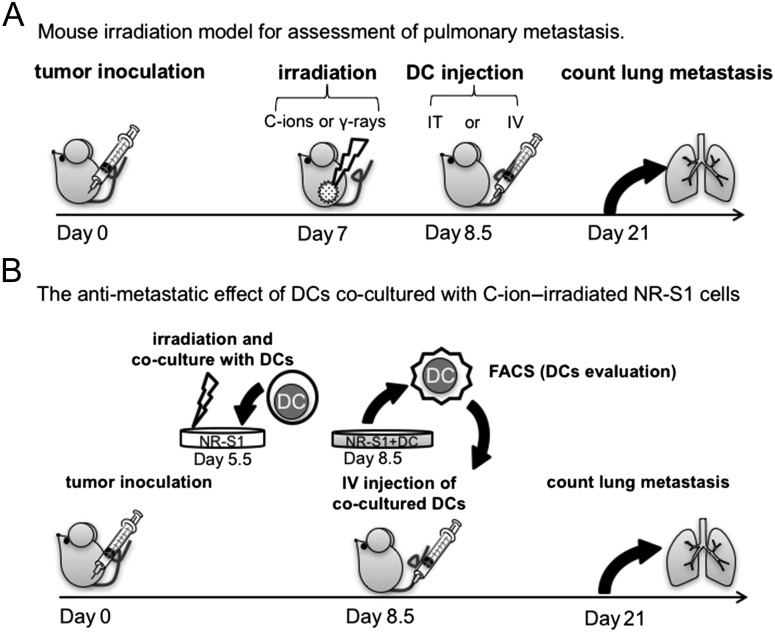
The mouse irradiation model for assessment of pulmonary metastases is shown in Fig. 1A. On Day 0, mice were inoculated with 1 × 106 cancer cells subcutaneously into the right thigh. After one week, by which the tumor was ~7 mm in diameter, they were irradiated with 2 Gy for CIRT or 4 Gy for PBRT. The irradiation system and the biophysical characteristics of the beam have been described elsewhere [20]. At 1.5 days after irradiation, DCs were injected (1 × 106 cells/mouse) IT or IV. The control mice received anesthesia only (i.e. no irradiation).
Fig. 1.
Experimental schema. (A) Mouse model for assessment of pulmonary metastasis treated with DC administration combined with irradiation to local tumor. Inoculated tumors were irradiated with C-ions or γ-rays on Day 7, and DCs were injected intratumorally or intravenously at 1.5 days after irradiation. Two weeks after irradiation, the mice were subjected to pneumonectomy (n = 7 per group). (B) Mouse model for assessment of pulmonary metastasis treated with ex vivo pulsed DCs. DCs were co-incubated with C-ion–irradiated (6 Gy) or non-irradiated NR-S1 cells for 3 days. The co-cultured DCs were injected intravenously into the NR-S1–transplanted mice at 8.5 days after tumor inoculation. The number of lung metastases was measured at 21 days after tumor inoculation (n = 7 per group).
Lung metastasis assay
At 2 weeks after irradiation, the bilateral lungs were initially placed in Bouin solution overnight. The metastatic nodules on the surfaces of all the pulmonary lobes were macroscopically counted (n = 7 per group). The average incidence of lung metastases was expressed as relative values, compared with that of the control group.
Tumor growth delay assay
After CIRT or PBRT, the tumor size was measured with calipers at the time of irradiation and also at the endpoint (n = 7 per group). Tumor volume was calculated according to the following formula: (a × b × c × π)/6, where a, b and c represent the three orthogonal diameters of the tumor, as previously described [21].
Detection of HMGB1 released from tumor cells relating to immunogenic cell death
NR-S1 cells were cultured in a 6-cm dish with fresh medium. Three days after irradiation, cultured media were collected for assay. HMGB1 in the culture medium was detected using a two-step sandwich ELISA kit (HMGB1 ELISA Kit II, SHINO-TEST, Kanagawa, Japan), according to the manufacturer's instructions (n = 3 per group) [22]. Fresh medium was used as internal standards.
Detection of ecto-CALR expressed on tumor cells relating to immunogenic cell death
Irradiated NR-S1 cells were washed with stain buffer (1 × PBS, 2% FBS and 0.09% sodium azide) and then incubated with anti-mouse CALR antibody (1:150, Abcam, Cambridge, UK) in stain buffer at 4°C for 30 min. After washing with stain buffer, the cells were incubated with anti-mouse IgG DyLight488 conjugates (1:200, Jackson ImmunoReseaerch, West Grove, PA, USA) in stain buffer at 4°C for 30 min. After washing three times with the stain buffer, ecto-CALR on the cell surface on propidium iodide (PI)-negative cells was detected by Gallios flow cytometer (Beckman Coulter, Miami, FL, USA) (n = 3 per group).
Maturation of DCs co-cultured with irradiated tumor cells in vitro
Bone marrow–derived DCs (1 × 106 cells) were co-cultured for 3days with irradiated or non-irradiated NR-S1 cells (5 × 105 cells) in DC complete media supplemented with 20 ng/ml recombinant murine GM-CSF. Collected DCs were analyzed for phenotypic maturation by flow cytometry and real-time qPCR (RT-qPCR). Co-cultured DCs were stained using a combination of PE-conjugated anti-CD11c antibody for detection of DCs and FITC-conjugated antibodies, anti-CD40, anti-CD80 or anti-CD86 (BioLegend, San Diego, CA, USA), following the manufacturer's instructions. Analyses of fluorescence staining were performed with a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) and CELLQuest Software (BD Biosciences). For RT-qPCR, total RNA was purified using NucleoSpin RNA II (Takara Bio, Shiga, Japan) according to the manufacturer's instructions. Isolated total RNA was reverse-transcribed using the PrimeScript RT-PCR Kit with random primers N6 (Takara Bio). Real-time PCR was performed with probes of IL12 p35, IL12 p40, CD40 and CD80 genes belonging to LightCycler 480 probes Master (Roche Diagnostics, Mannheim, Germany) as previously described [23]. Primer and probe sets used in this study are shown in Table 1. As a negative control, DCs were incubated without tumor cells. 18S ribosomal RNA (18S rRNA) was used as an internal control. Three independent experiments were performed with flow cytometry and RT-qPCR.
Table 1.
PCR primer and probe
| Gene | Directiona | Primer sequence (5′ to >3′) | Product size (bp) | Probeb |
|---|---|---|---|---|
| Il12 p35 | F | ccaggtgtcttagccagtcc | 94 | #62 |
| R | gcagtgcaggaataatgtttca | |||
| Il12 p40 | F | gaactggcgttggaagca | 98 | #84 |
| R | aagttcttgggcgggtct | |||
| CD40 | F | ccatgtgactcaggcgaat | 94 | #26 |
| R | taacccgaagcccttgatt | |||
| CD80 | F | tcgtctttcacaagtgtcttcag | 127 | #91 |
| R | ttgccagtagattcggtcttc | |||
| 18S RNA | F | gcaattattccccatgaacg | 68 | #48 |
| R | gggacttaatcaacgcaagc |
aDirection of primer sequences: F = forward, R = reverse.
bProbe number of Universal ProbeLibrary probes (Roche).
The anti-metastatic effect of DCs co-cultured with C-ion–irradiated NR-S1 cells
The anti-metastatic effect of the co-cultured DCs irradiated with C-ions was investigated by using the mouse model for lung metastases as shown in Fig. 1B. DCs were co-cultured with 6 Gy C-ion–irradiated NR-S1 cells in vitro for 3 days. The DCs were collected from co-cultured supernatants. Co-cultured DCs were then intravenously administered to C3H/He mice bearing NR-S1 allografts. The number of lung metastatic nodules was counted macroscopically at 21 days after tumor inoculation (n = 7 per group).
Statistical analysis
Statistical significance was tested by means of the Student's t test or the Steel–Dwass test where appropriate. Statistical analyses were performed with SPSS statistics 19.0 (SPSS Inc, Chicago, IL) and statistical package R (ver. 3.2.0; available as a free download from http://www.R-project.org). We considered P < 0.05 to be statistically significant.
RESULTS
Equivalent biological dose between photon and C-ion irradiations of NR-S1 cells by clonogenic assays
In order to determine the equivalent dose of PBRT to CIRT for clonogenicity of NR-S1 cells, cell survival curves of NR-S1 cells by PBRT and CIRT were calculated as shown in Fig. 2. Average D0 values were 1.89 Gy and 3.83 Gy for X-rays and C-ions, respectively. The RBE of NR-S1 cells was almost 2. Therefore, 4 Gy of PBRT and 2 Gy of CIRT were used as equivalent doses for comparison of their biological effectiveness.
Fig. 2.
Clonogenic survival curves after X-ray or C-ion irradiation for NR-S1 cells. Surviving fractions against physical doses were plotted and fitted to survival curves using the following linear–quadratic model: SF = exp(−αD − βD2), where SF is the surviving fraction and D is the physical dose.
Anti-metastasis effect of intravenous DC administration combined with local tumor irradiation by C-ions in vivo
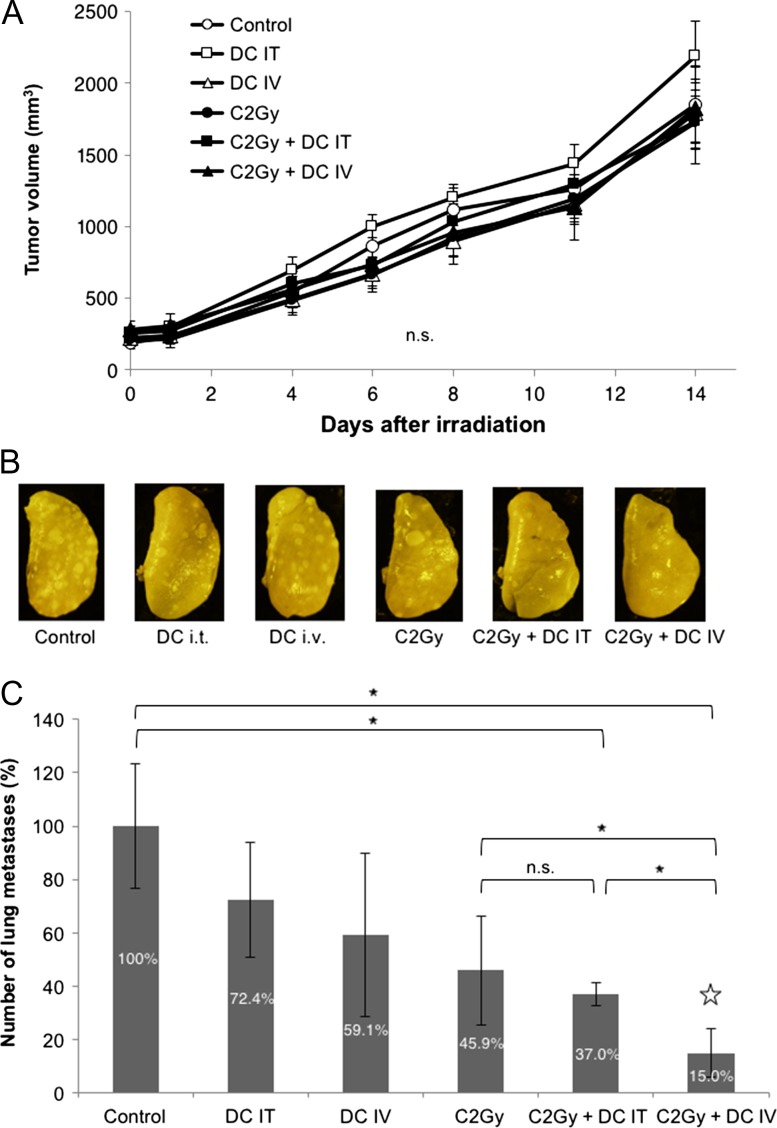
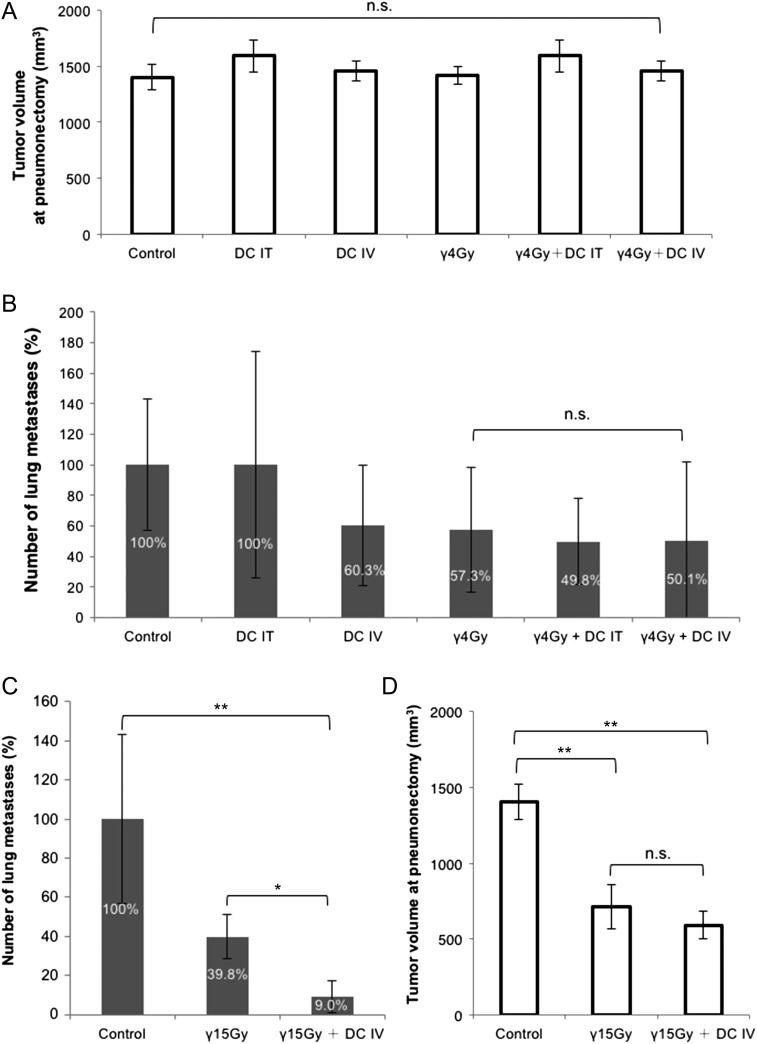
Two administration methods, IT and IV, of DCs were evaluated for their suppression effect of lung metastasis after two types of irradiation, CIRT and PBRT (Figs 3 and 4). First, in CIRT there were no significant differences in the tumor growth of allografts between each of the treatment modalities (Fig. 3A). The macroscopic features of lungs with metastases after various conditions of experiments are shown in Fig. 3B, and the metastatic nodules varied both in size and in numbers. The number of lung metastases tended to decrease in the mice treated with either IT or IV DC injections or with CIRT alone. Meanwhile, lung metastases were significantly suppressed when CIRT was combined with IV DC injection (indicated by star in Fig. 3C) compared with CIRT alone and CIRT combined with IT-DC injection (Fig. 3C, P < 0.05 for the C-2Gy group, and for the C-2Gy + DC IT group). Second, in PBRT there were no significant differences in the tumor volume of allografts at pneumonectomy (which corresponded to the tumor volumes at 14 days after CIRT in Fig. 3A) between each of the treatment modalities, as in CIRT (Fig. 4A). However, IT DCs and IV DCs combined with 4 Gy of local PBRT did not significantly decrease the number of lung metastases compared with PBRT alone, whereas the number tended to decrease in the mice treated with IV DC injection or PBRT alone (Fig. 4B). On the other hand, significant suppression of lung metastases was observed by the combination of IV DC administration with a high dose (15 Gy) of PBRT compared with PBRT alone (P < 0.05), as shown in Fig. 4C. However, significant tumor volume reductions were also observed in high-dose-PBRT–treated mice compared with in the control group (Fig. 4D, P < 0.01).
Fig. 3.
Anti-metastatic effect of DCs combined with C-ion irradiation. (A) Comparison of tumor growth after local tumor irradiation with 2 Gy of C-ions. (B) Macroscopic images of lung metastases. Representative lung images of each treatment group are shown. (C) Comparison of lung metastasis after CIRT. Suppression rates are indicated inside each bar. The data are presented as mean ± SD and shown as relative values, with the control group being 100%. One asterisk indicates P < 0.05 by the Steel–Dwass test. C = C-ion irradiation, IT = intratumoral administration, IV = intravenous administration.
Fig. 4.
Anti-metastatic effect of DCs combined with γ-ray irradiation. (A) Comparison of tumor volume at pneumonectomy after PBRT. The data are presented as mean ± SD. (B) Comparison of lung metastasis after local tumor irradiation with 4 Gy of γ-rays. Suppression rates are indicated inside each bar. The data are presented as mean ± SD and shown as relative values, with the control group being 100%. (C) Repression of lung metastasis by combination with high dose (15 Gy) γ-ray irradiation and DCs. Suppression rates are indicated inside each bar. The data are presented as mean ± SD and shown as relative values, with the control group being 100%. Two asterisks indicate P < 0.01; one asterisk indicates P < 0.05 by the Steel–Dwass test. (D) Comparison of tumor volume after a high dose of PBRT. The data are presented as mean ± SD. Two asterisks indicate P < 0.01 by the Steel–Dwass test. The error bars in figures indicate standard deviations. γ = γ-ray irradiation, IT = intratumoral administration, IV = intravenous administration.
Phenotype of DCs co-cultured with NR-S1 cancer cells irradiated with C-ions or photons
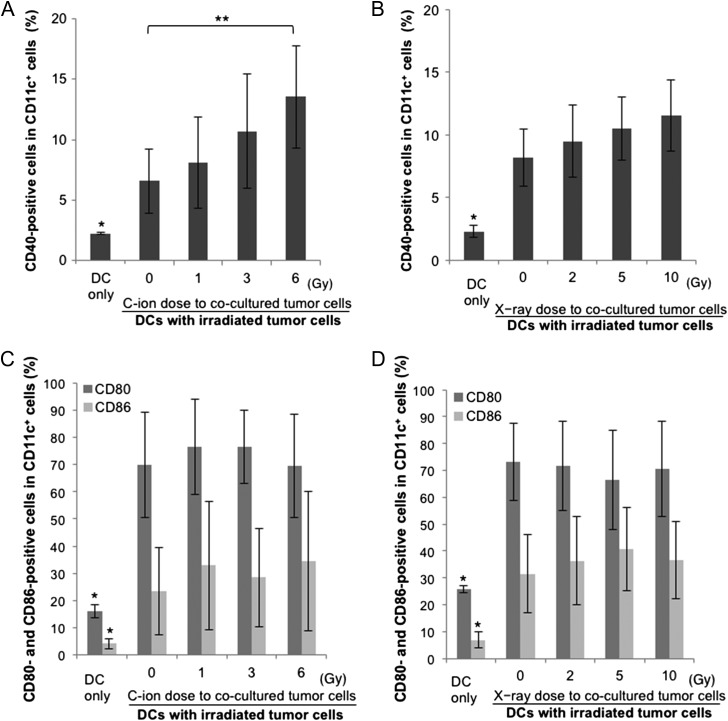
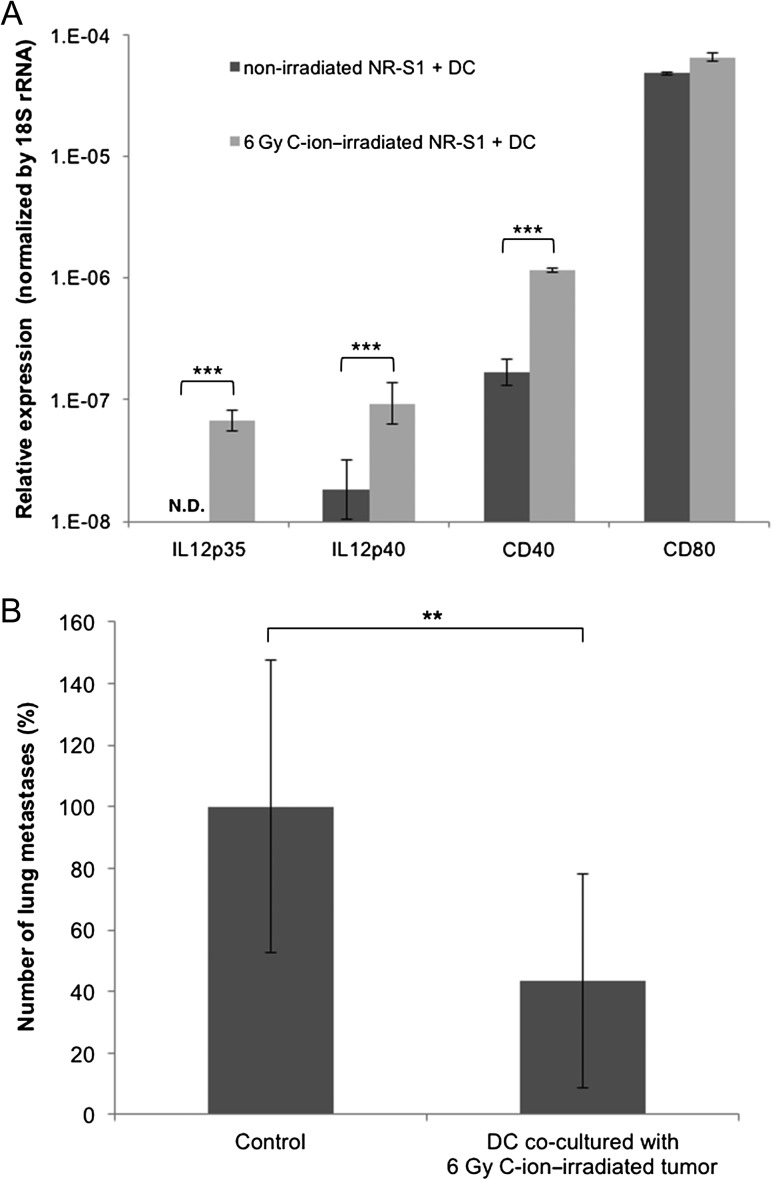
To clarify the mechanisms underlying combination therapy, phenotypes of DCs co-cultured with irradiated NR-S1 cancer cells were investigated. Expression levels of CD40, CD80 and CD86 on CD11c+ DCs after co-culture with NR-S1 cancer cells irradiated with C-ions or X-rays were evaluated to assess the maturation status of the DCs. Expression of CD40, CD80 and CD86 on DCs was significantly increased by co-culturing with NR-S1 alone (Fig. 5A–D). There were significant dose-dependent increments in the expression levels of CD40 in DCs co-cultured with C-ion–irradiated NR-S1 cells (Fig. 5A, P < 0.05, 0 Gy vs. 6 Gy). Co-culturing with X-ray–irradiated NR-S1 cells also induced CD40 expression on DCs with some trends of dose dependency (Fig. 5B). In contrast, there was no significant enhancement of CD80 or CD86 expression on DCs by co-culture with NR-S1 cells irradiated with both C-ions and X-rays compared with on DCs co-cultured with non-irradiated cells (Fig. 5C and D). To increase the reliability of the CD40 enhancement, mRNA expression levels of IL-12, CD40 and CD80 on DCs after co-culturing with NR-S1 cells irradiated with or without C-ions (6 Gy) were assessed by RT-qPCR (Fig. 6A). As observed in the flow cytometry assay, there was a significant increase in the expression levels of IL-12 or CD40, but no significant increase in CD80 (all P < 0.001 for IL-12p35, IL-12p40 and CD40).
Fig. 5.
Comparison between C-ion and photon beam in maturation of co-cultured DCs. (A, B) Dose-dependent maturation ability of C-ion–irradiated cells (A) or photon–irradiated cells (B). The percentage of CD11c+ DCs expressing CD40 is shown. The data are presented as mean ± SD. One asterisk indicates P < 0.05 by the Student's t test, compared with all the other groups. Two asterisks indicate P < 0.05 by the Student's t test, compared with 0 Gy. (C, D) Dose-dependent expression of co-stimulatory molecules on DCs co-cultured with C-ion–irradiated cells (C) or photon-irradiated cells (D). The percentage of CD11c+ DCs expressing CD80 and CD86 are shown. One asterisk indicates P < 0.05 by the Student's t test, compared with all the other groups. The data are presented as mean ± SD.
Fig. 6.
Gene expressions of DCs co-cultured with C-ion–irradiated cells and their anti-metastatic effect. (A) Gene expressions were analyzed by RT-qPCR. The data were normalized by 18S rRNA levels and presented as a logarithmic plot of relative expression levels, with the 18S rRNA expression being 1. The data are presented as mean ± SD. ND = non-detected; the signal is below the sensitivity limit of the assay. Three asterisks indicate P < 0.001 by the Student's t test. (B) Repression of lung metastasis by co-cultured DCs. The data are presented as mean ± SD and shown as relative values, with the control group being 100%. Two asterisks indicates P < 0.01 by the Student's t test.
Anti-metastatic effect of injection of DCs co-cultured with irradiated cancer cells
To assess whether the in vitro co-culturing assay reflected an in vivo immune response of the combination therapy, we investigated the anti-metastatic effect of IV injection of DCs co-cultured with 6 Gy C-ion–irradiated NR-S1 cells into non-irradiated NR-S1–bearing mice (Fig. 1B). The number of pulmonary metastatic nodules after administration of DCs co-cultured with C-ion–irradiated NR-S1 cells was significantly decreased compared with the control group (P < 0.01, Fig. 6B).
Immunogenicity of NR-S1 cancer cells irradiated with C-ions or photons
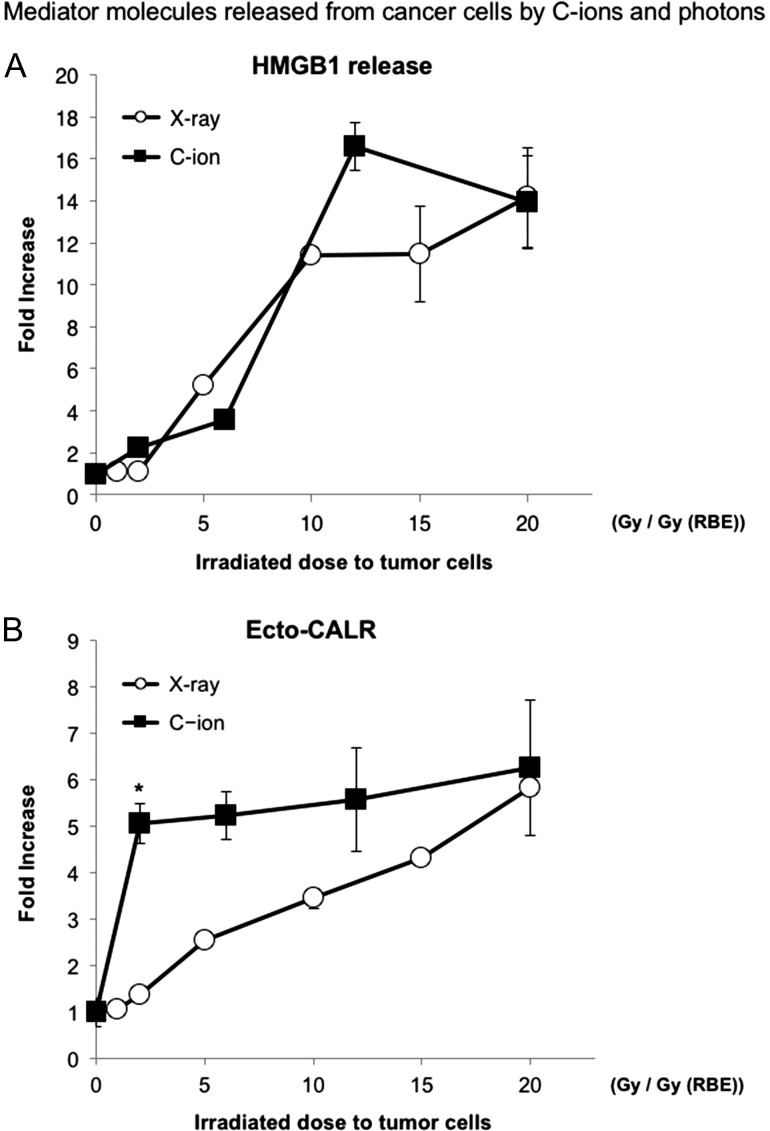
To compare the efficiency of inducing immunogenic cell death between CIRT and PBRT, the levels of HMGB1 released from irradiated tumor cells and the CALR level on the irradiated tumor cell surface after irradiation were analyzed. There was no significant difference in HMGB1 levels in irradiated tumors between C-ions and X-rays (Fig. 7A). On the other hand, at a lower dose of from 1 Gy (RBE) to 10 Gy (RBE), CALR levels in a C-ion–irradiated tumor significantly increased compared with those in X-ray–irradiated tumors (P < 0.05) (Fig. 7B). At >10 Gy (RBE), no significant increment in CALR levels was observed between C-ions and X-rays.
Fig. 7.
Comparison between C-ion and photon beam in terms of response of tumor cell. HMGB1 release levels (A) and ecto-CALR levels (B) after photon or C-ion beam irradiation for NR-S1 cells. The ecto-CALR level is plotted against Gy (RBE), which is the biologically equivalent dose of X-rays according to clonogenic cell death. C-ion dose (Gy) (RBE) is equal to physical dose (Gy) × 2 :(RBE = 2). The values were expressed as relative values, with 0 Gy being 1. One asterisk indicates P < 0.05 by the Student's t test.
DISCUSSION
Recently, it has been highlighted that RT has significant potential for activating a host's anti-tumor immunity in certain conditions [8–10]. Thus, it is strongly expected that the combination of immunotherapy and RT is able to improve outcomes compared with RT alone [24, 25].
The present study demonstrated that the number of lung metastases notably decreased in mice treated with DCs and CIRT (Fig. 3B and C) and also in mice treated with DCs and PBRT at a high dose (Fig. 4C). These results and our previous reports clearly indicated that DC-based immunotherapy combined with RT might be an effective method of suppressing metastasis [11, 26]. However, there is no consensus on the optimal method for DC administration for augmenting anti-tumor immunity. In our previous report, DCs injected into the irradiated tumor augment anti-tumor effect significantly [11], we expected the increase in a chance to expose DCs with tumor antigens by IT DC injection might be important. On the other hand, in the present study, IV DCs showed equivalent or better anti-metastatic effect compared with IT DCs in both CIRT (Fig. 3B and C) and in PBRT (Fig. 4C). Therefore, IV DCs could be an optimal method for DC administration in combination with RT for the augmentation of anti-metastatic effect to the lung.
Our result introduced a new question concerning why IV DCs were able to induce a similar anti-metastatic effect like that of IT DCs in combination with RT. Several reports have shown that more DCs are accumulated in the lung after IV DC administration than after IT DC administration [27–29]. In our study by using fluorescent dye–labeled DCs, more lung-accumulated DCs were detected at 18 h after IV DC administration compared with after IT DC administration (data not shown). Furthermore, some reports indicated that IV DCs was able to induce rejection against tumor re-inoculation [30, 31]. Thus, DC accumulation in the lung seems to be associated with the suppression of lung metastases.
The in vitro co-culturing experiments with the DCs and the irradiated tumors were conducted to assess the maturation of the DCs according to the immunologic modulation of molecular expression of CD40, CD80 and CD86. The CD40, CD80 and CD86, costimulatory molecules on DCs, play important roles in interaction between DCs and T-helper cells and induce cytotoxic T-lymphocyte (CTL) priming [32]. In particular, CD40 is used as a key marker for distinguishing mature DCs from immature DCs [33]. The present study indicated that the maturation of DCs identified by CD40 expression was induced by co-culture with both X-ray– and C-ion–irradiated cancer cells. In addition, when DCs were co-cultured with C-ion–irradiated NR-S1 cells, the expression level of CD40 was significantly increased in a dose-dependent manner (Fig. 5A). Moreover, expression levels of CD40 and IL-12 mRNA were significantly increased after co-culturing with the C-ion–irradiated NR-S1 cells (Fig. 6A). Interaction between CD40 and its ligand causes maturation of the DCs and licenses the DCs to induce effective activation of CTLs [34, 35]. In addition, IL-12 has been considered to promote cell-mediated immune activity via the interaction of CD40 and its ligand [36]. A previous study has demonstrated that tumor cell death induced by RT matured DCs, leading to the enhancement of T-cell–mediated immunity [37]. Additionally, the present study demonstrated that IV administration of DCs co-cultured with C-ion–irradiated NR-S1 cells significantly decreased the number of pulmonary metastatic nodules (Fig. 6B). This result indicated that the DCs matured by co-culture with C-ion–irradiated cancer cells have enough capacity to suppress lung metastasis, and it also suggested that our in vitro experiments might mimic the immune responses induced in mouse treated by the combination treatment. The DCs seems to play important roles in the prevention of metastasis after CIRT through their maturation via the interaction of CD40 and its ligand stimulated by irradiated cancer cells. Hence, CIRT to a local tumor in vivo probably elicits licensed DCs effectively, which seems to play an important anti-metastatic role in this combination therapy. On the other hand, the difference in the CD40 induction on co-cultured DCs between CIRT and PBRT was weaker than suppression of lung metastasis by the combination treatments at the relatively lower dose. This might indicate that DC maturation by irradiated tumor cells is a key process for the combination treatment, but that other factors are possibly involved in the mechanisms for the difference observed in the mouse experiments between CIRT and PBRT.
CD80 and CD86 expression on DCs was significantly increased by co-culturing with NR-S1 alone. Additionally, neither C-ion– nor X-ray–irradiated NR-S1 cells did not increase CD80 or CD86 expression on DCs (Fig. 5C and D). Several studies showed that CD28 and CD152, receptors on T-cells that bind to both CD80 and CD86, have two opposing functions in T-cell activation, where CD28 acts as a co-stimulator and CD152 acts as an inhibitor [38]. Hence, CD80 and CD86 were not specific markers for explaining the effectiveness of co-cultured DC maturation induced by irradiated tumor cells.
We also investigated whether there is a difference in the immune response of tumor cells between CIRT and PBRT. It is well known that PBRT induces ICD, and X-rays have frequently been used for DC experiments as inducer of the DCs [39]. In the current study, combination IV DCs with CIRT, even at dose as low as 2 Gy, showed significant anti-metastatic effect (Fig. 3C). In contrast, those with 4 Gy of γ-rays, which is the biological equivalent dose to 2 Gy of C-ions in the colony-forming assay, did not suppress pulmonary metastasis significantly (Fig. 4B). However, significant suppression of lung metastases was observed by the combination of IV DC administration with high-dose (15 Gy) PBRT (Fig. 4C). Although the difference between CIRT and PBRT in anti-metastatic effect was observed in vivo at a low dose, it is difficult to explain this difference in the status of DCs co-cultured with an irradiated tumor in vitro. A possible explanation for this difference is the immune response of tumor cells. Both CALR and HMGB1 are key molecules expressed on cancer cells to enhance tumor immunogenicity and mediators of signals induced by the immunogenic cell death [13, 14]. In the present study, the levels of HMGB1 and CALR, which elicit DC-based anti-tumor immunity, were measured to evaluate differences in expression of the molecular markers of irradiated tumor cells between C-ion and photon beams. In the irradiation doses, which show equivalent biological effect in the colony-forming assay, no significant differences were observed in HMGB1 levels after irradiation between C-ion and photon irradiation (Fig. 7A), which was consistent with the result of the colony-formation assay and also agreed with a previous study that showed C-ion beams induced HMGB1 comparable with that of X-ray irradiation at the same dose levels of cell killing [40]. Since the release of extracellular HMGB1 could be induced not only in ICD but also by the collapse of the cell membrane due to various conditions, such as secondary necrosis after apoptosis, HMGB1 might not be a suitable marker for assessing the immunogenicity of irradiated cells, and it was difficult to explain the difference in the immunologic effect between the C-ion beam and the photon beam. In contrast, significant increases in CALR levels were observed in C-ion–irradiated tumor cells compared with X-ray–irradiated cells at a low dose (Fig. 7B). Strikingly, even in the same biological dose in clonogenicity, ecto-CALR levels were significantly higher in the CIRT than in the PBRT group. These differences might be related to the previous results that only a high dose of PBRT suppressed lung metastasis (Fig. 4C). CALR is regarded to be induced on the cell surface of cells dying by ICD and is critical for the recognition and engulfment of dying tumor cells by DCs [41, 42]. These results may indicate that a higher ratio of CALR exposed on surface membrane cancer cells by CIRT may contribute to increasing DC maturation more compared with PBRT at a low dose. This result might highlight that not only conventional radiation induced cell death, but also that ICD is a key factor causing the different anti-metastatic response between PBRT and CIRT in the combination of RT and immunotherapy.
Our findings may significantly contribute to the development of combination of RT with immunotherapy. Recently, many clinical trials that combined RT and immune checkpoint blockades have been conducted [43]. However, the optimal modality of immunotherapy combined with RT is unclear. DCs play a central role in the anti-tumor immune system in many types of immunotherapy. Acquiring the tumor-specific antigen is essential for establishing DC-related anti-tumor immunity. It is believed that tumor antigens are case-specific and have huge diversity. In this regard, usage of the tumor tissue taken from the corresponding patient is considered to be more effective for activating DCs than usage of synthetized cancer peptides [44]. CIRT can deliver concentrated doses to the deep-seated tumors and gain tumor antigens with less invasiveness compared with biopsy, surgery and PBRT. CIRT can be a useful tool for the maturation of DCs and augment immunogenicity of the irradiated cancer cells, so that the combination of CIRT with IV DCs might have beneficial effects on controlling distant metastasis as well as in local control of tumors in clinical application.
Our study demonstrated that CIRT combined with IV DCs more efficiently decreased lung metastases of NR-S1 murine squamous cell carcinoma cells inoculated into mice feet than PBRT with IV DCs in the biological equivalent dose. In addition, local CIRT to the NR-S1 cells combined with IV DCs efficiently augments an immunogenicity of the cells like CALR and the maturation of DCs to stimulate anti-tumor immunity for decreasing lung metastases. These results suggested that RT, especially CIRT, is a suitable candidate as a partner for combination with immunotherapy, including DC immunotherapy.
FUNDING
This work was supported by the Research Project Heavy Ions at NIRS–HIMAC [project number 11J175 and 14J175] and the Japan Society for the Promotion of Science [KAKENHI; grant number 22791235 and 24591857].
CONFLICT OF INTEREST
None.
ACKNOWLEDGEMENTS
The authors acknowledge Mayumi Iwakawa, Miyako Nakawatari, Etsuko Nakamura, Hiroyuki Moritake, Takeshi Maeda, the FACS support team for their kind discussion and technical assistance, and HIMAC (research project with heavy ions at NIRS-HIMAC).
REFERENCES
- 1. Ohno T. Particle radiotherapy with carbon ion beams. EPMA J 2013;4:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tsujii H, Kamada T. A review of update clinical results of carbon ion radiotherapy. Jpn J Clin Oncol 2012;42:670–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. New Engl J Med 2010;363:711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015;373:123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dhodapkar MV, Dhodapkar KM, Palucka AK. Interactions of tumor cells with dendritic cells: balancing immunity and tolerance. Cell Death Differ 2008;15:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012;12:265–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Suzuki Y, Mimura K, Yoshimoto Y, et al. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res 2012;72:3967–76. [DOI] [PubMed] [Google Scholar]
- 9. Takeshima T, Chamoto K, Wakita D, et al. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Res 2010;70:2697–706. [DOI] [PubMed] [Google Scholar]
- 10. Yoshimoto Y, Suzuki Y, Mimura K, et al. Radiotherapy-induced anti-tumor immunity contributes to the therapeutic efficacy of irradiation and can be augmented by CTLA-4 blockade in a mouse model. PLoS One 2014;9:e92572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ohkubo Y, Iwakawa M, Seino K, et al. Combining carbon ion radiotherapy and local injection of alpha-galactosylceramide-pulsed dendritic cells inhibits lung metastases in an in vivo murine model. Int J Radiat Oncol Biol Phys 2010;78:1524–31. [DOI] [PubMed] [Google Scholar]
- 12. Tesniere A, Panaretakis T, Kepp O, et al. Molecular characteristics of immunogenic cancer cell death. Cell Death Differ 2008;15:3–12. [DOI] [PubMed] [Google Scholar]
- 13. Obeid M, Tesniere A, Ghiringhelli F, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med 2007;13:54–61. [DOI] [PubMed] [Google Scholar]
- 14. Tesniere A, Apetoh L, Ghiringhelli F, et al. Immunogenic cancer cell death: a key–lock paradigm. Curr Opin Immunol 2008;20:504–11. [DOI] [PubMed] [Google Scholar]
- 15. Dalod M, Chelbi R, Malissen B, et al. Dendritic cell maturation: functional specialization through signaling specificity and transcriptional programming. EMBO J 2014;33:1104–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mogi S, Sakurai J, Kohsaka T, et al. Tumour rejection by gene transfer of 4-1BB ligand into a CD80+ murine squamous cell carcinoma and the requirements of co-stimulatory molecules on tumour and host cells. Immunology 2000;101:541–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kitamura A. Bleomycin-mediated electrochemotherapy in mouse NR-S1 carcinoma. Cancer Chemother Pharmacol 2003;51:359–62. [DOI] [PubMed] [Google Scholar]
- 18. Fujita S, Seino K, Sato K, et al. Regulatory dendritic cells act as regulators of acute lethal systemic inflammatory response. Blood 2006;107:3656–64. [DOI] [PubMed] [Google Scholar]
- 19. Granucci F, Ferrero E, Foti M, et al. Early events in dendritic cell maturation induced by LPS. Microbes Infect 1999;1:1079–84. [DOI] [PubMed] [Google Scholar]
- 20. Kanai T, Endo M, Minohara S, et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int J Radiat Oncol Biol Phys 1999;44:201–10. [DOI] [PubMed] [Google Scholar]
- 21. Ando K, Koike S, Ohira C, et al. Accelerated reoxygenation of a murine fibrosarcoma after carbon-ion radiation. Int J Radiat Biol 1999;75:505–12. [DOI] [PubMed] [Google Scholar]
- 22. Yamada S, Yakabe K, Ishii J, et al. New high mobility group box 1 assay system. Clin Chim Acta 2006;372:173–8. [DOI] [PubMed] [Google Scholar]
- 23. Imadome K, Iwakawa M, Nojiri K, et al. Upregulation of stress-response genes with cell cycle arrest induced by carbon ion irradiation in multiple murine tumors models. Cancer Biol Ther 2008;7:208–17. [DOI] [PubMed] [Google Scholar]
- 24. Shimokawa T, Ma L, Ando K, et al. The future of combining carbon-ion radiotherapy with immunotherapy: evidence and progress in mouse models. Int J Particle Ther 2016;3:23–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sharon E, Polley MY, Bernstein MB, et al. Immunotherapy and radiation therapy: considerations for successfully combining radiation into the paradigm of immuno-oncology drug development. Radiat Res 2014;182:252–7. [DOI] [PubMed] [Google Scholar]
- 26. Chen Z, Xia D, Bi X, et al. Combined radiation therapy and dendritic cell vaccine for treating solid tumors with liver micro-metastasis. J Gene Med 2005;7:506–17. [DOI] [PubMed] [Google Scholar]
- 27. Lappin MB, Weiss JM, Delattre V, et al. Analysis of mouse dendritic cell migration in vivo upon subcutaneous and intravenous injection. Immunology 1999;98:181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eggert AA, Schreurs MW, Boerman OC, et al. Biodistribution and vaccine efficiency of murine dendritic cells are dependent on the route of administration. Cancer Res 1999;59:3340–5. [PubMed] [Google Scholar]
- 29. Teitz-Tennenbaum S, Li Q, Rynkiewicz S, et al. Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Res 2003;63:8466–75. [PubMed] [Google Scholar]
- 30. Celluzzi CM, Mayordomo JI, Storkus WJ, et al. Peptide-pulsed dendritic cells induce antigen-specific CTL-mediated protective tumor immunity. J Exp Med 1996;183:283–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Komaru A, Ueda Y, Furuya A, et al. Sustained and NK/CD4+ T cell-dependent efficient prevention of lung metastasis induced by dendritic cells harboring recombinant sendai virus. J Immunol 2009;183:4211–9. [DOI] [PubMed] [Google Scholar]
- 32. Van Gool SW, Vandenberghe P, de Boer M, et al. CD80, CD86 and CD40 provide accessory signals in a multiple-step T-cell activation model. Immunol Rev 1996;153:47–83. [DOI] [PubMed] [Google Scholar]
- 33. Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Semin Immunol 2009;21:265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lanzavecchia A. Immunology. Licence to kill. Nature 1998;393:413–4. [DOI] [PubMed] [Google Scholar]
- 35. Melief CJ. “License to kill” reflects joint action of CD4 and CD8 T cells. Clin Cancer Res 2013;19:4295–6. [DOI] [PubMed] [Google Scholar]
- 36. Cella M, Scheidegger D, Palmer-Lehmann K, et al. Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med 1996;184:747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gameiro SR, Jammeh ML, Wattenberg MM, et al. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget 2014;5:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol 2002;2:116–26. [DOI] [PubMed] [Google Scholar]
- 39. Galluzzi L, Kepp O, Kroemer G. Immunogenic cell death in radiation therapy. Oncoimmunology 2013;2:e26536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yoshimoto Y, Oike T, Okonogi N, et al. Carbon-ion beams induce production of an immune mediator protein, high mobility group box 1, at levels comparable with x-ray irradiation. J Radiat Res 2015;56:509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Obeid M, Tesniere A, Panaretakis T, et al. Ecto-calreticulin in immunogenic chemotherapy. Immunol Rev 2007;220:22–34. [DOI] [PubMed] [Google Scholar]
- 42. Zitvogel L, Kepp O, Senovilla L, et al. Immunogenic tumor cell death for optimal anticancer therapy: the calreticulin exposure pathway. Clin Cancer Res 2010;16:3100–4. [DOI] [PubMed] [Google Scholar]
- 43. Koo T, Kin IA. Radiotherapy and immune checkpoint blockades: a snapshot in 2016. Radiat Oncol J 2016;34:250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Neller MA, López JA, Schmidt CW. Antigens for cancer immunotherapy. Semin Immunol 2008;20:286–95. [DOI] [PubMed] [Google Scholar]