Abstract
Background
Understanding the burden and clinical presentation of tuberculosis in patients with severe respiratory illness (SRI) has important implications for anticipating treatment requirements.
Methods
Hospitalized patients aged ≥15 years with SRI at 2 public teaching hospitals in periurban areas in 2 provinces (Edendale Hospital in Pietermaritzburg, KwaZulu-Natal Province and Tshepong Hospital in Klerksdorp, North West Province) were enrolled prospectively from 2012 to 2014. Tuberculosis testing included smear microscopy, culture, or Xpert MTB/Rif.
Results
We enrolled 2486 individuals with SRI. Of these, 2097 (84%) were tested for tuberculosis, 593 (28%) were positive. Tuberculosis detection rate was 18% (133 of 729) in individuals with acute (≤14 days) presentation and 34% (460 of 1368) in those with chronic (>14 days) presentation. Among laboratory-confirmed tuberculosis cases, those with acute presentation were less likely to present with cough (88% [117 of 133] vs 97% [447 of 460]; ajusted odds ratio [aOR] = 0.2, 95% confidence interval [CI] = 0.1–0.5), night sweats (57% [75 of 132] vs 73% [337 of 459]; aOR = 0.4, 95% CI = 0.3–0.7), or be started on tuberculosis treatment on admission (63% [78 of 124] vs 81% [344 of 423]; aOR = 0.4, 95% CI = 0.3–0.7), but they were more likely to be coinfected with pneumococcus (13% [16 of 124] vs 6% [26 of 411]; aOR 2.3, 95% CI 1.3–5.3) than patients with chronic presentation. Annual incidence of acute and chronic tuberculosis-associated SRI per 100000 population was 28 (95% CI = 22–39) and 116 (95% CI = 104–128), respectively.
Conclusions
In this setting, tuberculosis, including acute presentation, is common in patients hospitalized with SRI.
Keywords: HIV, severe respiratory illness, South Africa, tuberculosis
Tuberculosis is an important cause of severe respiratory illness (SRI) causing significant morbidity and mortality globally. In 2015, an estimated 10.4 million people developed tuberculosis and 1.8 million died from the disease, 0.4 million (22%) of whom were infected with human immunodeficiency virus (HIV) [1]. In South Africa in the same year, there were an estimated 454000 incident cases of tuberculosis and 258000 (57%) were infected with HIV [1].
The 2014, South African national guidelines for tuberculosis management recognized the importance of early diagnosis and treatment of tuberculosis and advocated that sputa be tested in patients with symptoms of tuberculosis, especially those who are infected with HIV irrespective of the duration of cough. However, for HIV-uninfected patients, these guidelines advise testing only patients with chronic duration of symptoms (ie, cough or fever >14 days) [2]. The World Health Organization (WHO) guidelines also call for intensified case finding among HIV-infected individuals and recommend screening for tuberculosis in patients with any current symptoms of tuberculosis [3]. In an earlier publication including data from the same hospitals as the current study, we found that only 38% of individuals admitted for lower respiratory tract infection (LRTI) with any symptom duration were tested for tuberculosis, whereas 80% of admitted patients ≥15 years with LRTI were infected with HIV [4]. In addition, clinicians were significantly less likely to test patients with acute symptoms for tuberculosis and to start them on tuberculosis treatment [4]. Understanding the spectrum of clinical presentation of tuberculosis may contribute to timely diagnosis and treatment and subsequent better control of the tuberculosis epidemic. Among HIV-infected and -uninfected persons aged ≥15 years hospitalized with SRI in South Africa during 2012–2014, we aimed to describe the following: (1) factors associated with acute compared with chronic presentation of SRI irrespective of laboratory diagnosis; (2) the incidence of laboratory-confirmed acute and chronic presentation of pulmonary tuberculosis; (3) factors associated with laboratory-confirmed pulmonary tuberculosis among patients tested for tuberculosis; and (4) factors associated with acute and chronic presentation among patients with laboratory-confirmed tuberculosis.
METHODS
Study Design
This study used data collected as part of surveillance for SRI [5]. Prospective hospital-based active surveillance was conducted for SRI at 2 sentinel surveillance sites (Edendale Hospital in Pietermaritzburg, Umgungundlovu District, KwaZulu-Natal Province and Tshepong Hospital in Klerksdorp, Matlosana District, North West Province) from July 2012 to August 2014.
Case Definitions
A case of SRI was defined as admission with a physician diagnosis of LRTI (eg, pneumonia, bronchiolitis, bronchitis, and pleural effusion, suspected or confirmed tuberculosis) irrespective of symptom duration and included individuals who met the WHO severe acute respiratory illness (SARI) case definition of cough and fever presenting within 10 days of onset of illness [6].
Study Procedures
Patients admitted from Sunday at 5:00 pm through to 1:00 pm on Fridays were screened for inclusion. Patients who refused or were unable to give consent and patients from outside the catchment area of the respective hospitals were excluded. Data on clinical presentation, previous medical history, antiviral therapy for HIV-infected individuals, inpatient investigations, and management and outcome were collected by interview and medical record review. Patients were followed-up until discharge. Treatment decisions, including initiation of tuberculosis treatment based on laboratory confirmation or empirically, and diagnostic tests were performed according to the attending physician.
In addition, combined nasopharyngeal (NP) and oropharyngeal (OP) swabs, expectorated or induced sputum, and blood specimens were collected from consenting patients. We instituted testing for tuberculosis for all consenting patients, and details of testing for tuberculosis are included in the Supplementary Material.
Laboratory Methods
Nasopharyngeal and OP swabs were transported in universal transport medium at 4–8°C to the National Institute for Communicable Diseases (NICD) within 72 hours of collection. Combined NP and OP swabs were tested for influenza A and B and 8 other respiratory viruses (parainfluenza virus types 1, 2, and 3; respiratory syncytial virus; adenovirus; rhinovirus; human metapneumovirus; and enterovirus) by real-time, reverse-transcription polymerase chain reaction (PCR) [7]. Sputum samples were frozen at −20°C and shipped on dry ice weekly to NICD. Testing for tuberculosis at the hospital laboratory was performed by smear microscopy, culture, and/or XpertMTB/Rif based on the hospital testing algorithm. At NICD, in 2012 testing only included culture and microscopy was added in 2013. Details of tuberculosis testing are included in Supplementary Material. Determination of HIV status is described in detail in Supplementary Material. In brief, HIV testing was not required for participation, and HIV results were obtained from clinical records when available. For consenting patients with unavailable results, either bedside testing was done or a dried blood spot was tested as part of surveillance. Testing included HIV enzyme-linked immunosorbent assay (ELISA) for patients aged ≥18 months and PCR for children aged <18 months if the ELISA was reactive.
Tuberculosis Status
Tuberculosis status was determined using tuberculosis results from specimens tested at the site laboratory and at NICD. A laboratory-confirmed tuberculosis case was defined as an individual with any positive result for acid-fast bacilli on microscopy, Mycobacterium tuberculosis on culture, or PCR (Xpert MTB/Rif or MTBDRplus) from the current hospital admission.
Symptom Duration
For duration of symptoms, we used the date of onset of symptoms as reported by the patient and considered cough and fever as the main variables to categorize duration of symptoms as either an acute (≤14 days) or chronic (>14 days) presentation. The cutoff of 14 days was chosen a priori based on South African guidelines for tuberculosis screening for HIV-negative individuals.
Incidence Calculation
Incidence estimates of acute and chronic tuberculosis-associated SRI hospitalization (per 100000 population) for Pietermaritzburg and Klerksdorp were calculated for 2013. Population denominators for the hospital catchment population were accessed from the 2011 census data as collected by Statistics South Africa [8]. Mid-year population figures by age group were estimated for the districts served by the hospitals. These population numbers were adjusted for proportions of people in the hospital catchment area that attended the surveillance hospital for care for respiratory illness using information from healthcare utilization surveys previously conducted at each site (Karen Wong and Jo McAnerney, Oral communication, May 2016). Data on all admissions during 2013, including nonenrolled individuals, were collected and used to estimate the number of SRI cases missed during the study period due to nonenrollment over weekends and refusal. The incidence of acute and chronic tuberculosis-associated SRI hospitalizations/100000 population for 2013 was estimated using the number of patients with acute and chronic presentation testing positive for tuberculosis, adjusting for nonenrollment (due to refusal and over weekend admissions) and patients who did not seek care by age groups divided by the mid-year population estimates for 2013 and multiplied by 100000. Confidence intervals (CIs) were calculated using the Poisson distribution.
Data Analysis
To identify factors associated with tuberculosis positivity and acute compared with chronic presentation of tuberculosis, we included potential determinants for, as well as outcomes or characteristics of, the primary endpoints of the analysis. We implemented 3 multivariable logistic regression models to identify factors associated with the following: (1) acute compared with chronic presentation among patients hospitalized with SRI irrespective of laboratory diagnosis; (2) laboratory confirmation of tuberculosis among patients admitted with SRI with symptoms of any duration and with available tuberculosis results; and (3) acute compared with chronic presentation among individuals with laboratory-confirmed tuberculosis to assess whether cases with an acute presentation differed in presentation and outcome because they would likely not have been tested for tuberculosis especially if they were HIV negative. We repeated model (3) among HIV-infected and HIV-uninfected individuals separately.
For the multivariate model, all factors significant at P < .1 on univariate analysis were evaluated, and nonsignificant factors at P < .05 were then dropped from the multivariable model using stepwise forward selection. All 2-way interactions in the final multivariable additive model were evaluated. Two-sided P values <.05 were considered significant throughout. Stata version 13.1 (StataCorp Limited, College Station, TX) was used for the analysis.
RESULTS
From July 2012 through August 2014, 4045 patients fulfilling the SRI case definition were enrolled; 2486 (61%) were aged ≥15 years and were included in the analysis. Of these, 866 (35%) had an acute presentation. A total of 2097 of 2486 (84%) patients were tested for tuberculosis, with equal proportions tested among those with an acute (84%; 729 of 866) and chronic (84%; 1368 of 1620) presentation (Figure 1). Tuberculosis was detected in 28% (593 of 2097) of enrolled individuals, 18% (133 of 729) and 34% (460 of 1368) in those with an acute and chronic presentation, respectively (P < .001). Results of different tuberculosis microbiologic tests are included in the Supplementary Material.
Figure 1.
Individuals admitted with severe respiratory illness and consented for enrollment by symptom duration and laboratory-confirmed tuberculosis (TB) status, South Africa, July 2012–August 2014.
NOTE: Reasons for not testing for TB (n = 389): 54% (210) not able to expectorate and sputum induction contraindicated or too sick or weak to cough up induced sputum, 30% (n = 117) induction not successful, 6% (n = 23) refused, 10% (n = 39) reason not recorded.
Compared with patients tested for tuberculosis, controlling for hospital, patients not tested were less likely to present with cough (ajusted odds ratio [aOR], 0.3; 95% CI, 0.2–0.5) or night sweats (aOR, 0.7; 95% CI, 0.6–0.9) but were more likely to be admitted for >7 days compared with <3 days (aOR, 1.7; 95% CI, 1.1–2.4) and to die (aOR, 3.1; 95% CI, 2.3–4.1) (Supplementary Table 1). There was no significant difference in HIV status among patients tested (77% [1438 of 1877]) and not tested (72% [247 of 344]) for tuberculosis (P = .06).
The overall HIV prevalence in patients with SRI was 76% (1685 of 2221) and varied by age group: 72% (134 of 186) in those 15–24 years, 91% (1071 of 1179) in those 25–44 years, 66% (437 of 665) in those 45–64 years, and 23% (43 of 191) in those ≥65 years (P < .001). Human immunodeficiency virus status also varied by tuberculosis disease: 75% (1014 of 1355) and 81% (424 of 522) in tuberculosis-uninfected and -infected individuals, respectively (P = .003). Of the 1685 HIV-infected SRI patients, 389 (23%) were diagnosed with HIV at the current admission. Of 1482 HIV-infected SRI patients with information on HIV treatment, 45% (674) were receiving HIV treatment at time of admission.
Comparison of Characteristics of Individuals With Acute Presentation to Individuals With Chronic Presentation Hospitalized With Severe Respiratory Illness
Of the patients with acute presentation, 95% (820 of 866) had symptom duration ≤10 days, and 28% (231 of 820) of these met the WHO SARI case definition of LRTI with reported or documented fever, cough, and symptoms ≤10 days [6]. On multivariable analysis, compared with patients with a chronic presentation, patients with an acute presentation were less likely to present with cough or night sweats, to have laboratory-confirmed tuberculosis, to be started on treatment for tuberculosis on current admission, to be hospitalized for a longer duration ≥3 days, and to die (Table 1). The HIV prevalence was similar (73% [568 of 773] vs 77% [1117 of 1448]) in patients with acute and chronic presentation, respectively.
Table 1.
Demographic and Clinical Characteristics of Individuals ≥15 Years Admitted With Severe Respiratory Illness by Symptom Duration (≤ or >14 days), in South Africa, July 2012–August 2014 (N = 2486)
| Variables |
Acute Presentation (≤14 Days) n/N (%) |
Chronic Presentation (>14 Days) n/N (%) |
Univariate Analysis (Acute Compared With Chronic Presentation) | Multivariable Analysis (Acute Compared With Chronic Presentation) | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |||
| Age | ||||||
| 15–24 | 69/866 (8) | 137/1620 (8) | Reference | Reference | ||
| 25–44 | 459/866 (53) | 860/1620 (53) | 1.1 (0.8–1.4) | .715 | 1.3 (0.8–2.0) | .221 |
| 45–64 | 244/866 (28) | 500/1620 (31) | 1.0 (0.7–1.3) | .850 | 1.2 (0.7–1.9) | .514 |
| 65+ | 94/866 (11) | 123/1620 (8) | 1.5 (1.1–2.3) | .038 | 1.1 (0.6–2.0) | .721 |
| Female sex | 508/866 (57) | 816/1619 (50) | 1.4 (1.2–1.6) | <.001 | 1.3 (1.0–1.6) | .027 |
| Tshepong Hospital | 547/866 (63) | 1069/1620 (66) | 0.9 (0.7–1.1) | .160 | ||
| History of cough | 748/865 (84) | 1564/1620 (96) | 0.2 (0.1–0.3) | <.001 | 0.2 (0.1–0.4) | <.001 |
| History of fever | 374/861 (43) | 551/1608 (34) | 1.5 (1.2–1.7) | <.001 | 1.4 (1.1–1.8) | .004 |
| Night sweats | 444/864 (54) | 1111/1619 (67) | 0.5 (0.4 -0.6) | <.001 | 0.5 (0.4–0.6) | <.001 |
| Underlying medical conditionsa | 88/866 (10) | 134/1619 (8) | 1.3 (0.9–1.7) | .117 | ||
| Diabetes | 40/865 (5) | 37/1619 (2) | 2.1 (1.3–3.3) | .002 | 2.1 (1.1–3.8) | .028 |
| Mine exposurec | 83/857 (10) | 189/1594 (12) | 0.8 (0.6–1.0) | .103 | ||
| History of smokingb | 169/856 (20) | 273/1602 (17) | 1.2 (1.0–1.5) | .097 | ||
| History of alcoholb | 214/858 (25) | 349/1603 (22) | 1.2 (1–1.5) | .075 | ||
| Tested for TB | 729/866 (84) | 1368/1620 (84) | 1.0 (0.8–1.2) | .863 | ||
| Laboratory confirmed TBg | 133/729 (18) | 460/1368 (34) | 0.4 (0.3–0.5) | <.001 | 0.7 (0.5–0.9) | .004 |
| History of TB treatmente | 73/863 (8) | 189/1612 (12) | 0.7 (0.5–0.9) | .012 | ||
| HIV infected | 568/773 (73) | 1117/1448 (77) | 0.8 (0.7–1.0) | .055 | ||
| Influenza-positive | 59/842 (7) | 72/1587 (4) | 0.6 (0.4–0.9) | .011 | ||
| Pneumococcal coinfectiond | 144/790 (18) | 144/1457 (10) | 2.0 (1.6–2.6) | <.001 | 1.9 (1.5–2.6) | <.001 |
| Viral coinfection | 231/842 (27) | 429/1587 (27) | 1.0 (0.8–1.2) | .832 | ||
| Required oxygen | 300/836 (36) | 569/1570(36) | 1.0 (0.8–1.2) | .862 | ||
| Antibiotics on admission | 823/850 (97) | 1509/1591 (95) | 1.7 (1.1–2.6) | .026 | ||
| Started on TB treatmentf | 247/803 (31) | 809/1457 (56) | 0.3 (0.3–0.4) | <.001 | 0.4 (0.3–0.6) | <.001 |
| Duration of hospitalization | ||||||
| <3 days | 168/788 (21) | 226/1484 (15) | Reference | Reference | ||
| 3–7 days | 337/788 (43) | 605/1484 (41) | 0.7 (0.6–0.9) | .018 | 0.7 (0.5–0.9) | .013 |
| 8+ days | 283/788 (34) | 653/1484 (44) | 0.6 (0.5–0.7) | <.001 | 0.6 (0.4–0.8) | .001 |
| Died during admission | 91/824 (11) | 228/1546 (15) | 0.7 (0.5–0.9) | .012 | 0.7 (0.5–0.97) | .038 |
Bold indicates significant variables.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio; PCR, polymerase chain reaction; TB, tuberculosis.
aUnderlying conditions included any of the following: asthma, other chronic lung disease, chronic heart disease (valvular heart disease, coronary artery disease, or heart failure excluding hypertension), liver disease (cirrhosis or liver failure), renal disease (nephrotic syndrome, chronic renal failure), immunocompromising conditions excluding HIV infection (organ transplant, immunosuppressive therapy, immunoglobulin deficiency, malignancy), neurological disease (cerebrovascular accident, spinal cord injury, seizures, neuromuscular conditions), or pregnancy. Comorbidities were considered absent in cases for which the medical records stated that the patient had no underlying medical condition or when there was no direct reference to that condition.
bCurrent history.
cAny history of having worked in the mine.
d lytA PCR positive for Streptococcus pneumoniae on blood specimen.
eHistory of TB treatment within the last 12 months of current admission.
fStarted on TB treatment at current admission; excludes those already on TB treatment at time of admission (n = 144). Of the 1056 patients started on TB treatment, 42% (441 of 1056) had a negative TB result, 36% (376 of 1056) tested positive, and 23% (239 of 1056) had missing/pending results at the time of initiation of TB treatment.
g6% (38/593) only detected on culture.
Factors Associated With Laboratory Confirmation of Tuberculosis Among Patients Admitted With Severe Respiratory Illness of Any Duration and Tested for Tuberculosis
Among 2097 patients with available tuberculosis results, 28% (593 of 2097) tested positive for tuberculosis at the current admission. Eighteen percent (33 of 183) of those who met the WHO SARI case definition tested positive for tuberculosis.
On multivariable analysis, compared with tuberculosis-negative patients, patients with laboratory-confirmed tuberculosis of any symptom duration were less likely to be in older age groups >45 year compared with the 15–24 age group, to be coinfected with pneumococcus, and to have received oxygen therapy. They were more likely to present with symptoms >14 days (Table 2). The prevalence of influenza (4% vs 6%, P = .09) was similar in patients with and without tuberculosis disease of any duration.
Table 2.
Demographic and Clinical Characteristics Associated With Laboratory-Confirmed Tuberculosis Among Cases Admitted With Severe Respiratory Illness of Any Duration and Tested for Tuberculosis at Two Sites in South Africa, 2012–2014 (N = 2097)
| Variables |
Tuberculosis Negative
n/N (%) |
Tuberculosis Positive
n/N (%) |
Univariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |||
| Age | ||||||
| 15–24 | 107/1504 (7) | 63/593 (11) | Reference | Reference | ||
| 25–44 | 780/1504 (52) | 365/593 (62) | 0.8 (0.6–1.1) | .179 | 0.7 (0.5–1.1) | .114 |
| 45–64 | 475/1504 (32) | 144/593 (24) | 0.5 (0.4–0.7) | <.001 | 0.6 (0.4–0.9) | .007 |
| 65+ | 142/1504 (9) | 21/593 (3) | 0.3 (0.1–0.4) | <.001 | 0.3 (0.1–0.5) | <.001 |
| Female gender | 801/1503 (53) | 397/593 (51) | 0.9 (0.7–1.1) | .295 | ||
| Klerksdorp Tshepong | 991/1504 (66) | 397/593 (67) | 1.04 (0.9–1.3) | .645 | ||
| Duration of symptoms >14 days | 908/1504 (60) | 460/593 (76) | 2.3 (1.8–2.8) | <.001 | 1.6 (1.2–2.0) | .001 |
| History of cough (any duration) | 1416/1503 (94) | 564/593 (95) | 1.2 (0.8–1.8) | .419 | ||
| Chronic cough >14 days | 833/1503 (55) | 833/592 (73) | 2.2 (1.8–2.7) | <.001 | ||
| History of fever | 544/1493 (37) | 239/590 (41) | 1.2 (0.9–1.4) | .084 | ||
| Night sweats | 927/1503 (62) | 412/591 (70) | 1.4 (1.2–1.8) | <.001 | ||
| Underlying medical conditiona | 161/1503 (11) | 27/593 (5) | 0.4 (0.3–0.6) | <.001 | ||
| Diabetes | 48/1502 (3) | 12/593 (2) | 0.6 (0.3–1.2) | .151 | ||
| History of TB treatmente | 142/1499 (9) | 68/589 (28) | 1.2 (0.9–1.7) | .157 | ||
| HIV infected | 1014/1355 (75) | 424/522 (81) | 1.5 (1.1–1.9) | .003 | ||
| Worked in minec | 173/1484 (12) | 68/583 (12) | 1.0 (0.7–1.3) | .997 | ||
| History of smokingb | 281/1488 (19) | 113/586 (19) | 1.0 (0.8–1.3) | .835 | ||
| History of alcoholb | 374/1489 (25) | 128/586 (22) | 0.8 (0.6–1.0) | .117 | ||
| Influenza-positive | 89/1471 (6) | 24/582 (4) | 0.7 (0.4–1.06) | .086 | ||
| Pneumococcal coinfectiond | 212/1366 (16) | 42/535 (8) | 0.5 (0.3–0.7) | <.001 | 0.6 (0.4–0.9) | .024 |
| Viral coinfection | 419/1471 (28) | 169/582 (29) | 1.03 (0.8–1.3) | .802 | ||
| Invasive bacterial infection on cultureg | 7/251 (3) | 2/122 (2) | 0.6 (0.1–2.8) | .502 | ||
| Required oxygen | 547/1445 (38) | 171/584(29) | 0.7 (0.5–0.8) | <.001 | 0.7 (0.6–0.9) | .005 |
| Antibiotics on admission | 1421/1468 (97) | 554/588 (94) | 0.5 (0.3–0.8) | .007 | ||
| TB treatment startedf | 470/1371 (34) | 422/547 (77) | 6.5 (5.1–8.1) | <.001 | 5.6 (4.-7.2) | <.001 |
| Duration of hospitalization | ||||||
| <3 days | 247/1364 (18) | 102/554 (18) | Reference | |||
| 3–7 days | 557/1364 (41) | 248/554 (45) | 1.1 (0.8–1.4) | .592 | ||
| 8+ days | 560/1364 (41) | 204/554 (37) | 0.9 (0.7–1.2) | .382 | ||
| Died during admission | 152/1423 (11) | 67/578 (12) | 1.1 (0.8–1.5) | .555 | ||
Bold indicates significant variables.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio; PCR, polymerase chain reaction; TB, tuberculosis.
aUnderlying conditions included any of the following: asthma, other chronic lung disease, chronic heart disease (valvular heart disease, coronary artery disease, or heart failure excluding hypertension), liver disease (cirrhosis or liver failure), renal disease (nephrotic syndrome, chronic renal failure), immunocompromising conditions excluding HIV infection (organ transplant, immunosuppressive therapy, immunoglobulin deficiency, malignancy), neurological disease (cerebrovascular accident, spinal cord injury, seizures, neuromuscular conditions), or pregnancy. Comorbidities were considered absent in cases for which the medical records stated that the patient had no underlying medical condition or when there was no direct reference to that condition.
bCurrent history.
cAny history of having worked in the mine.
d lytA PCR positive for Streptococcus pneumoniae on blood specimen.
eHistory of TB treatment within the last 12 months of current admission.
fStarted on TB treatment at current admission; excludes those already on TB treatment at time of admission (n = 108).
gInvasive isolates were defined as a bacterial pathogen isolated from blood or pleural fluid from a specimen taken within 48 hours of hospitalization; organisms viewed as likely contaminants were excluded. Two percent (9 of 373) of patients had a positive blood culture (5 S pneumoniae, 2 Staphylococcus aureus, and 1 Escherischia coli). Two percent (2 of 122) of laboratory-confirmed TB patients had a positive blood culture (1 E coli and 1 S aureus).
Factors Associated With Acute Presentation Compared to Chronic Presentation in Cases With Laboratory-Confirmed Tuberculosis
Of the 593 laboratory-confirmed tuberculosis cases, 22% (133) and 78% (460) had an acute and chronic presentation, respectively. Human immunodeficiency virus prevalence among laboratory-confirmed cases was 81% (424 of 522), and it was similar in those with acute (83%, 102 of 123) and chronic (81%, 322 of 399) presentation (P = .51). Among HIV-uninfected laboratory-confirmed cases, 21% (21 of 98) had an acute presentation. On multivariable analysis, among patients with laboratory-confirmed tuberculosis, cases with acute presentation were less likely to present with cough (aOR, 0.2; 95% CI, 0.1–0.5) or night sweats (aOR, 0.4; 95% CI, 0.3–0.7) or be started on treatment for tuberculosis at current admission (aOR, 0.4; 95% CI, 0.3–0.7) than cases with chronic presentation. In contrast, they were more likely to have pneumococcal coinfection (aOR, 2.6; 95% CI, 1.3–5.3). The case-fatality ratio was not statistically different between laboratory-confirmed tuberculosis patients with acute (8%, 11 of 131) and chronic (13%, 56 of 447) presentation (P = .194) (Table 3). Similar results were seen when restricting analysis to HIV-infected patients with laboratory-confirmed tuberculosis (Supplementary Material).
Table 3.
Demographic and Clinical Factors Associated With Acute (≤14 Days) vs Chronic (>14 Days) Presentation Among Individuals ≥15 Years With Laboratory-Confirmed Tuberculosis, July 2012–August 2014 (N = 593)
| Variables |
Acute Presentation
n/N (%) |
Chronic Presentation
n/N (%) |
Univariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |||
| Age | ||||||
| 15–24 | 11/133 (8) | 52/460 (11) | Reference | Reference | ||
| 25–44 | 82/133 (62) | 283/460 (62) | 1.4 (0.7–2.7) | .375 | ||
| 45–64 | 33/133 (24) | 111/460 (24) | 1.4 (0.7–2.9) | .379 | ||
| 65+ | 7/133 (5) | 14/460 (3) | 2.4 (0.8–7.2) | .131 | ||
| Female gender | 73/133 (55) | 228/460 (50) | 1.2 (0.8–1.8) | .280 | ||
| Tshepong Hospital | 89/133 (67) | 308/460 (67) | 1.0 (0.7–1.5) | .993 | ||
| History of any cough | 117/133 (88) | 447/460 (97) | 0.2 (0.1–0.5) | <.001 | 0.2 (0.1–0.5) | <.001 |
| History of fever | 60/133 (45) | 179/457 (41) | 1.3 (0.9–1.9) | .220 | ||
| Night sweats | 75/132 (57) | 337/459 (73) | 0.5 (0.3–0.7) | <.001 | 0.4 (0.3–0.7) | <.001 |
| Underlying medical conditiona | 6/133 (5) | 21/460 (5) | 0.98 (0.4–2.5) | .979 | ||
| History of TB treatmentc | 51/4457(11) | 17/132 (13) | 1.2 (0.7–2.1) | .586 | ||
| Diabetes | 5/433 (5) | 7/460 (2) | 2.5 (0.8–8.1) | .118 | ||
| HIV infected | 102/123 (83) | 322/399 (81) | 1.2 (0.7–1.9) | .581 | ||
| Influenza positive | 9/130 (7) | 15/452 (3) | 2.2 (0.9–5.1) | .075 | ||
| Pneumococcal coinfectionb | 16/124 (13) | 26/411 (6) | 2.2 (1.1–4.2) | .019 | 2.6 (1.3–5.3) | .008 |
| Viral coinfection | 40/130 (31) | 129/452 (29) | 1.1 (0.7–1.7) | .622 | ||
| Required oxygen | 48/131 (37) | 123/453 (27) | 1.6 (1.1–2.3) | .036 | ||
| Started on antibiotics | 125/131 (95) | 429/457 (94) | 1.4 (0.6–3.4) | .505 | ||
| Started on TB treatmentd | 78/124 (63) | 344/423 (81) | 0.4 (0.3–0.6) | <.001 | 0.4 (0.3–0.7) | .001 |
| Duration of hospitalization | ||||||
| <3 | 21/124 (21) | 89/430 (19) | Reference | |||
| 3–7 | 54/124 (42) | 194/430 (45) | 1.1 (0.6–1.9) | .806 | ||
| 8+ | 49/124 (37) | 155/430 (36) | 1.2 (0.7–2.2) | .501 | ||
| Died during admission | 11/131 (8) | 56/447 (13) | 0.6 (0.3–1.3) | .197 | ||
Bold indicates significant variables.
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; OR, odds ratio; PCR, polymerase chain reaction; TB, tuberculosis.
aUnderlying conditions included any of the following: Asthma, other chronic lung disease, chronic heart disease (valvular heart disease, coronary artery disease, or heart failure excluding hypertension), liver disease (cirrhosis or liver failure), renal disease (nephrotic syndrome, chronic renal failure), immunocompromising conditions excluding HIV infection (organ transplant, immunosuppressive therapy, immunoglobulin deficiency, malignancy), neurological disease (cerebrovascular accident, spinal cord injury, seizures, neuromuscular conditions) or pregnancy. Comorbidities were considered absent in cases for which the medical records stated that the patient had no underlying medical condition or when there was no direct reference to that condition.
b lytA PCR positive for Streptococcus pneumoniae on blood specimen.
cHistory of TB treatment within the last 12 months of current admission.
dStarted on TB treatment at current admission; excludes those already on TB treatment at time of admission (n = 39).
Incidence of Hospitalization for Tuberculosis-Associated Severe Respiratory Illness
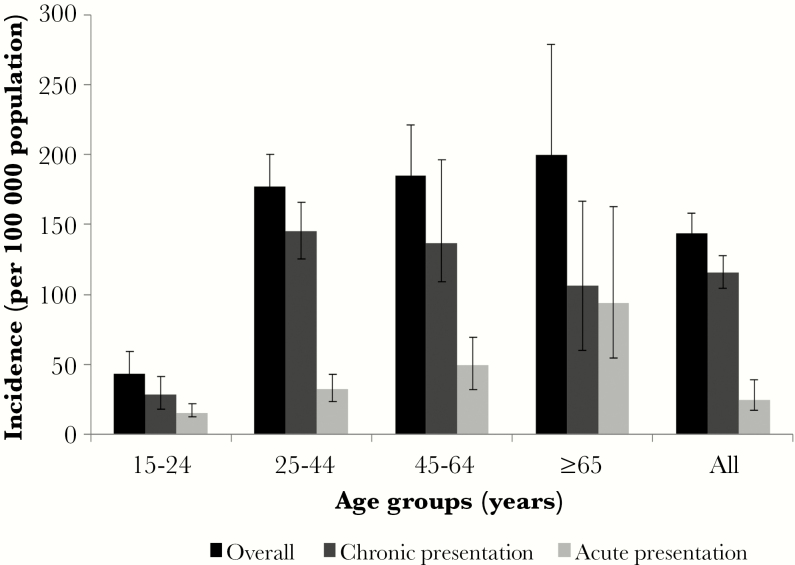
The estimated overall incidence (per 100000 population) of hospitalization for tuberculosis-associated SRI was 144 (95% CI, 131–158), ranged from 43 to 200 per 100000 among age groups, and was significantly higher in Klerksdorp than in Pietermaritzburg (181 [95% CI, 162–201] vs 93 [95% CI, 77–111]). The annual incidence of tuberculosis-associated SRI in individuals hospitalized with acute presentation was 28 per 100000 population (95% CI, 22–39), increased with increasing age, and was highest in individuals ≥65 years (94 cases per 100000 population; 95% CI, 54–153). The incidence of tuberculosis-associated SRI in individuals with chronic presentation was 116 per 100000 population per annum (95% CI, 104–128) and was highest in individuals aged 25–44 years (145 cases per 100000 population; 95% CI, 125–166) (Figure 2). The incidence of tuberculosis-associated SRI was lower in individuals with acute compared with chronic presentation, except in individuals aged ≥65 years where there was no significant difference (0.8; 95% CI, 0.4–1.8) (Figure 2).
Figure 2.
Incidence of tuberculosis-associated severe respiratory illness in acute (≤14 days) vs chronic (>14 days) presentation per 100000 population, South Africa 2013.
DISCUSSION
We found that in our setting, a high proportion of adolescents and adults admitted with SRI regardless of symptom duration had active tuberculosis. For patients with acute presentation, 1 in 5 (18%) were found to have active tuberculosis, whereas among those with longer duration of symptoms, 1 in 3 (34%) had active tuberculosis. Although the likelihood of tuberculosis disease was higher if symptom duration was >14 days, additional numbers of cases will be identified if laboratory testing for tuberculosis is conducted for adults with SRI irrespective of symptom duration or HIV status. In particular, 21% of HIV-uninfected patients with laboratory-confirmed tuberculosis had an acute presentation, and these would have been missed by the South African tuberculosis testing guidelines [2]. Although the HIV-uninfected tuberculosis cases with acute presentation constituted 3.5% of all tuberculosis cases in this study, in a setting with high tuberculosis prevalence and where tuberculosis is the leading cause of death, any opportunity to diagnose tuberculosis should be used. Studies have reported a high prevalence or incidence of active tuberculosis in South African communities including in cases where tuberculosis was not suspected [9–11]. In a postmortem study conducted in a community served by one of the hospitals included in our study, 31% of patients who died at home with uncategorized cause of death had tuberculosis [10]. Wong et al [11] reported that among HIV-positive individuals, ~30% of microbiologically and histologically proven tuberculosis infections on postmortem were clinically not suspected at the time of death.
In our study, 59% and 27% of laboratory-confirmed tuberculosis cases did not present with fever and chronic cough, respectively, potentially reducing the clinician’s suspicion of tuberculosis even further. The atypical clinical presentation of tuberculosis in our study is likely to be related to HIV infection because 81% of patients in our study were infected with HIV. Symptom screening has been reported to perform poorly in HIV-infected patients, particularly in those on antiretroviral therapy [12, 13]. Early detection and prompt treatment are key to the success of tuberculosis infection control [14]. To facilitate diagnosis, minimize delays to treatment initiation, interrupt transmission, and improve patient outcomes, studies have advocated for rapid microbiological diagnosis of tuberculosis, which could be achieved through using rapid diagnostic tests including Xpert and urine assay for lipoarabinomannan (LAM) [15–20]. Urine LAM tests, although not currently part of standard of care in our setting, have been shown to be beneficial especially among HIV-infected individuals with advanced disease where systematic screening of admitted individuals is advised [16–19, 21].
Tuberculosis patients with acute presentation compared with chronic presentation were less likely to present with typical symptoms of tuberculosis such as night sweats and cough and were less likely to be started on tuberculosis treatment by the attending clinician. The delay in initiating tuberculosis treatment, especially among patients with acute presentation, may reflect the clinicians’ level of suspicion for tuberculosis in this group of patients. Patients with undiagnosed tuberculosis may continue to spread tuberculosis to other patients and healthcare workers [22–24]. Healthcare service delays have been cited as major contributors to total delay to initiation of tuberculosis treatment [25–27]. A postmortem study in patients dying in hospital found that among patients not suspected of having tuberculosis at the time of death, 42% (40 of 96) were culture positive for tuberculosis and that pneumonia was the leading admission diagnosis (25%) in those not suspected of tuberculosis [28]. This study supports the importance of maintaining a high clinical index of suspicion for tuberculosis in patients presenting with acute SRI symptoms in high HIV and tuberculosis burden settings.
Coinfection with respiratory viruses was common (29%) among tuberculosis-confirmed cases. Four percent were coinfected with influenza: 7% and 3% in patients with an acute and chronic presentation, respectively. This may be an important group to target for influenza vaccination because increased risk of influenza-associated mortality in patients with tuberculosis has been reported [4, 29–31]. Pneumococcal coinfection was identified in 8% of tuberculosis cases and more commonly identified in tuberculosis cases with an acute than chronic presentation. Among cases with an acute presentation, pneumococcus was the most commonly detected copathogen. Studies have postulated an interaction between tuberculosis and pneumococcus [32–36]. This may suggest severe comorbidity as the reason for admission to hospital in patients with tuberculosis. Blood cultures were performed in <20% of enrolled patients with tuberculosis results, which limited our ability to comment on the proportion with bacteremic pneumonia.
The overall incidence of hospitalized tuberculosis-associated SRI was high (144; 95% CI, 131–158), and it was twice as high in the Matlosana district compared with areas around Pietermaritzburg (181 [95% CI, 162–201] vs 93 [95% CI, 77–111]). Because gold mining is a major industry in Klerksdorp, tuberculosis incidence may be higher compared with Pietermaritzburg, which is a nonmining town. Silicosis is a frequent occurrence among miners, and gold miners are at higher risk for acquiring tuberculosis [37]. In addition, mining areas could fuel transmission among general population. Both Matlosana and UMgungundlovu Districts are high HIV-prevalence areas, and SRI cases at Tshepong and Edendale hospital had a similar high HIV prevalence. Additional results on incidence are discussed in the Supplementary Material.
Our study has a number of limitations. This study included hospitalized individuals only and may not be reflective of patients with respiratory symptoms treated in outpatient facilities. Although our study aimed to test all patients admitted with SRI for tuberculosis, only 84% of patients were tested. Tested patients were less likely to die, and were more likely to die, and this could have biased our study. More than half (54%) of patients that were not tested were not able to expectorate and induced sputum was contraindicated or they were either too sick or weak to cough up sputum. In addition, 18% of patients in our study were tested on tuberculosis PCR only and 6% were tested on smear only. It is possible that patients diagnosed as not having tuberculosis did actually have tuberculosis because the sensitivity of diagnostic tests is <100%. Guidelines for testing with the Xpert MTB/Rif advise that a second sample be tested with microscopy and culture especially in HIV-infected patients who test negative for tuberculosis on PCR. For tuberculosis testing at site, we included testing conducted as part of clinical care, and additional testing was conducted as part of surveillance. Surveillance testing initiated in July 2012 used the same systems as clinician-initiated testing and coincided with a campaign to encourage clinician testing because we wanted to make sure that all results would be available to clinicians. For this reason we were not able to tell whether the proportion of clinician-initiated testing for individual cases differed between patients with acute and chronic presentation. In a previous study that included the same sites from 2010 to 2011 when no systematic testing was implemented, we found that testing for tuberculosis was less common in individuals with a duration of symptoms <7 days [4].
Data on cryptococcosis was not collected as part of the main study; therefore, we were not able to assess its contribution. For mortality, we used in-hospital outcome, and the 12% case-fatality ratio reported in our study would likely be higher if patients were followed up after discharge from hospital. According to death notification forms in South Africa, of the 458 933 tuberculosis deaths that occurred in 2013, 106 554 (23%) occurred at home [38]. For incidence calculation, uncertainty may have been introduced through adjustment for nonenrollment, and this was not included in the estimation of CIs. Finally, we only included patients aged ≥15 years in our study and therefore cannot comment on acute and chronic presentation of tuberculosis in children.
CONCLUSIONS
Tuberculosis should be considered in a differential diagnosis of patients presenting with SRI irrespective of the duration of symptoms in high HIV and tuberculosis burden settings such as South Africa. The absence of classic tuberculosis symptoms and an acute presentation should not preclude the possibility of a diagnosis of tuberculosis.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
Disclaimer. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the Centers for Disease Control and Prevention.
Financial support. This work was funded by the National Institute for Communicable Diseases of the National Health Laboratory Service and supported in part by funds from the US Centers for Disease Control and Prevention (Atlanta, GA) Preparedness and Response to Avian and Pandemic Influenza in South Africa (Cooperative Agreement Number U51/IP000155-04).
Potential conflicts of interest. All authors: No reported conflicts of interest.All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization. Global tuberculosis report 2016 World Health Organization; Available at: http://apps.who.int/iris/bitstream/10665/250441/1/9789241565394-eng.pdf?ua=1. Accessed 20 December 2016. [Google Scholar]
- 2. Department of Health Republic of South Africa. National Tuberculosis Management Guidelines Available at: https://www.idealclinic.org.za/docs/National-Priority-Health-Conditions/National%20TB%20management%20guidelines%202014.pdf. Accessed 20 December 2016.
- 3. World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settingsWorld Health Organization. Report No.: ISBN 978 92 4 150070 8. [Google Scholar]
- 4. Walaza S, Tempia S, Dawood H et al. Influenza virus infection is associated with increased risk of death amongst patients hospitalized with confirmed pulmonary tuberculosis in South Africa, 2010–2011. BMC Infect Dis 2015; 15:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walaza S, Cohen C, Treurnicht F et al. Burden of respiratory pathogens from influenza-like illness and pneumonia surveillance programmes, South Africa, 2015. Communicable Diseases Surveillance Bulletin: National Intitute for Communicable Diseases; 2016 Available at: http://www.nicd.ac.za/assets/files/Burden%20of%20respiratory%20pathogens%20frm%20influenza.pdf. Accessed 14 July 2017. [Google Scholar]
- 6. World Health Organization. Global epidemiological surveillance standards for influenza. World Health Organization; 2014. Available at: http://www.who.int/influenza/resources/documents/WHO_Epidemiological_Influenza_Surveillance_Standards_2014.pdf?ua=1. Accessed 20 December 2016. [Google Scholar]
- 7. Pretorius MA, Madhi SA, Cohen C et al. Respiratory viral coinfections identified by a 10-plex real-time reverse-transcription polymerase chain reaction assay in patients hospitalized with severe acute respiratory illness–South Africa, 2009–2010. J Infect Dis 2012; 206Suppl 1:S159–65. [DOI] [PubMed] [Google Scholar]
- 8. Statistics South Africa. Mortality and cause of death in South Africa, 1998 through 2009. Available at: http://www.statssa.gov.za/publications/P03093/P030932007.pdf. Accessed 14 July 2017. [Google Scholar]
- 9. van Schalkwyk C, Variava E, Shapiro AE et al. Incidence of TB and HIV in prospectively followed household contacts of TB index patients in South Africa. PLoS One 2014; 9:e95372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Omar T, Variava E, Moroe E et al. Undiagnosed TB in adults dying at home from natural causes in a high TB burden setting: a post-mortem study. Int J Tuberc Lung Dis 2015; 19:1320–5. [DOI] [PubMed] [Google Scholar]
- 11. Wong EB, Omar T, Setlhako GJ et al. Causes of death on antiretroviral therapy: a post-mortem study from South Africa. PLoS One 2012; 7:e47542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahmad Khan F, Verkuijl S, Parrish A et al. Performance of symptom-based tuberculosis screening among people living with HIV: not as great as hoped. AIDS 2014; 28:1463–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoffmann CJ, Variava E, Rakgokong M et al. High prevalence of pulmonary tuberculosis but low sensitivity of symptom screening among HIV-infected pregnant women in South Africa. PLoS One 2013; 8:e62211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization. WHO policy on TB infection control in health-care facilities, congregate settings and households. World Health Organization; Available at: http://apps.who.int/iris/bitstream/10665/44148/1/9789241598323_eng.pdf. Accessed 14 July 2017. [PubMed] [Google Scholar]
- 15. Lawn SD, Kerkhoff AD, Vogt M, Wood R. HIV-associated tuberculosis: relationship between disease severity and the sensitivity of new sputum-based and urine-based diagnostic assays. BMC Med 2013; 11:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lawn SD, Kerkhoff AD, Vogt M, Wood R. Clinical significance of lipoarabinomannan detection in urine using a low-cost point-of-care diagnostic assay for HIV-associated tuberculosis. AIDS 2012; 26:1635–43. [DOI] [PubMed] [Google Scholar]
- 17. Boehme CC, Nabeta P, Hillemann D et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010; 363:1005–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dheda K, Davids V, Lenders L et al. Clinical utility of a commercial LAM-ELISA assay for TB diagnosis in HIV-infected patients using urine and sputum samples. PLoS One 2010; 5:e9848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shah M, Variava E, Holmes CB et al. Diagnostic accuracy of a urine lipoarabinomannan test for tuberculosis in hospitalized patients in a High HIV prevalence setting. J Acquir Immune Defic Syndr 2009; 52:145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Steingart KR, Schiller I, Horne DJ et al. Xpert(R) MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2014; 1:Cd009593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Peter JG, Zijenah LS, Chanda D et al. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: a pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet 2016; 387:1187–97. [DOI] [PubMed] [Google Scholar]
- 22. Joshi R, Reingold AL, Menzies D, Pai M. Tuberculosis among health-care workers in low- and middle-income countries: a systematic review. PLoS Med 2006; 3:e494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grobler L, Mehtar S, Dheda K et al. The epidemiology of tuberculosis in health care workers in South Africa: a systematic review. BMC Health Serv Res 2016; 16:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Menzies D, Joshi R, Pai M. Risk of tuberculosis infection and disease associated with work in health care settings. Int J Tuberc Lung Dis 2007; 11:593–605. [PubMed] [Google Scholar]
- 25. Qureshi SA, Morkve O, Mustafa T. Patient and health system delays: health-care seeking behaviour among pulmonary tuberculosis patients in Pakistan. J Pak Med Assoc 2008; 58:318–21. [PubMed] [Google Scholar]
- 26. Uys PW, Warren RM, van Helden PD. A threshold value for the time delay to TB diagnosis. PLoS One 2007; 2:e757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meintjes G, Schoeman H, Morroni C et al. Patient and provider delay in tuberculosis suspects from communities with a high HIV prevalence in South Africa: a cross-sectional study. BMC Infect Dis 2008; 8:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cohen T, Murray M, Wallengren K et al. The prevalence and drug sensitivity of tuberculosis among patients dying in hospital in KwaZulu-Natal, South Africa: a postmortem study. PLoS Med 2010; 7:e1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Noymer A. The 1918 influenza pandemic hastened the decline of tuberculosis in the United States: an age, period, cohort analysis. Vaccine 2011; 29Suppl 2:B38–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oei W, Nishiura H. The relationship between tuberculosis and influenza death during the influenza (H1N1) pandemic from 1918–19. Comput Math Methods Med 2012; 2012:124861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Walaza S, Cohen C, Nanoo A et al. Excess mortality associated with influenza among tuberculosis deaths in South Africa, 1999–2009. PLoS One 2015; 10:e0129173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moore DP, Klugman KP, Madhi SA. Role of Streptococcus pneumoniae in hospitalization for acute community-acquired pneumonia associated with culture-confirmed Mycobacterium tuberculosis in children: a pneumococcal conjugate vaccine probe study. Pediatr Infect Dis J 2010; 29:1099–04. [DOI] [PubMed] [Google Scholar]
- 33. Scott JA, Hall AJ, Muyodi C et al. Aetiology, outcome, and risk factors for mortality among adults with acute pneumonia in Kenya. Lancet 2000; 355:1225–30. [DOI] [PubMed] [Google Scholar]
- 34. Ansari NA, Kombe AH, Kenyon TA et al. Pathology and causes of death in a group of 128 predominantly HIV-positive patients in Botswana, 1997–1998. Int J Tuberc Lung Dis 2002; 6:55–63. [PubMed] [Google Scholar]
- 35. Chintu C, Mudenda V, Lucas S et al. Lung diseases at necropsy in African children dying from respiratory illnesses: a descriptive necropsy study. Lancet 2002; 360:985–90. [DOI] [PubMed] [Google Scholar]
- 36. Martinson NA, Karstaedt A, Venter WD et al. Causes of death in hospitalized adults with a premortem diagnosis of tuberculosis: an autopsy study. AIDS 2007; 21:2043–50. [DOI] [PubMed] [Google Scholar]
- 37. Stuckler D, Basu S, McKee M, Lurie M. Mining and risk of tuberculosis in sub-Saharan Africa. Am J Public Health 2011; 101:524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Statistics South Africa. Mortality and causes of death in South Africa, 2013: Findings from death notification: Statistics South Africa; 2013. Report No.: P0309.3. Available at: http://www.statssa.gov.za/publications/P03093/P030932013.pdf. Accessed 14 July 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.