Abstract
Patterns of woody-plant mortality often reflect tradeoffs associated with resource allocation. Plants that allocate a high proportion of carbon acquired from photosynthesis to non-structural carbohydrate storage may be buffered from the synergistic effects of climate change and episodic disturbance.
Keywords: carbon allocation, climate change, Diorhabda carinulata, local adaptation, non-structural carbohydrates, Tamarix
Abstract
Patterns of woody-plant mortality have been linked to global-scale environmental changes, such as extreme drought, heat stress, more frequent and intense fires, and episodic outbreaks of insects and pathogens. Although many studies have focussed on survival and mortality in response to specific physiological stresses, little attention has been paid to the role of genetic heritability of traits and local adaptation in influencing patterns of plant mortality, especially in non-native species. Tamarix spp. is a dominant, non-native riparian tree in western North America that is experiencing dieback in some areas of its range due to episodic herbivory by the recently introduced northern tamarisk leaf beetle (Diorhabda carinulata). We propose that genotype × environment interactions largely underpin current and future patterns of Tamarix mortality. We anticipate that (i) despite its recent introduction, and the potential for significant gene flow, Tamarix in western North America is generally adapted to local environmental conditions across its current range in part due to hybridization of two species; (ii) local adaptation to specific climate, soil and resource availability will yield predictable responses to episodic herbivory; and (iii) the ability to cope with a combination of episodic herbivory and increased aridity associated with climate change will be largely based on functional tradeoffs in resource allocation. This review focusses on the potential heritability of plant carbon allocation patterns in Tamarix, focussing on the relative contribution of acquired carbon to non-structural carbohydrate (NSC) pools versus other sinks as the basis for surviving episodic disturbance. Where high aridity and/or poor edaphic position lead to chronic stress, NSC pools may fall below a minimum threshold because of an imbalance between the supply of carbon and its demand by various sinks. Identifying patterns of local adaptation of traits related to resource allocation will improve forecasting of Tamarix population susceptibility to episodic herbivory.
Introduction
Plant ecologists have recently paid considerable attention to woody-plant mortality because of continental-scale die-offs of woody plants across the globe (Allen et al., 2010). Rapid increases in mortality rates have been largely attributed to global environmental changes that have resulted in extreme droughts, heat waves, increased episodic insect and pathogen outbreaks and a measurable increase in forest fire frequency and intensity (Allen et al., 2010; van der Werf et al., 2010; Carnicer et al., 2011). Recent research has addressed the combined impacts of warming temperatures and water deficits on plant survival and there is now a wealth of data on the physiological mechanisms that underpin mortality surges in many regions (McDowell et al., 2008; Plaut et al., 2012; Sevanto et al., 2014). However, a clear genetic basis underlying reductions in plant fitness is still lacking for the expression of traits such as phenology, resource allocation or susceptibility to cavitation that are related to plant tolerance and resistance to environmental change. Understanding genetic variation in response to environmental change will improve predictions of future patterns of mortality across broad spatial scales.
A primary hurdle for addressing genetic versus environmental contributions to trait expression in plant mortality studies is that heritability is often difficult to measure in field settings. Common gardens provide excellent opportunities for testing hypotheses about traits that are favoured under specific environmental conditions. Numerous studies using common gardens of various species that incorporate genotypes from several source populations have yielded a broad range of information on genotype- and population-level patterns of net primary productivity (NPP), biomass allocation, water use efficiency, nutrient fertilization impacts on NPP and hydrologic processes (Zhang et al., 1993; Powers and Reynolds, 1999; Savolainen et al., 2007; Grady et al., 2011; Gray et al., 2016). Some of the strongest evidence that specific phenotypic traits are locally adapted to environmental conditions has emerged from common garden studies (Clausen et al., 1941; Savolainen et al., 2007; Grady et al., 2011). However, the relationship between local adaptation to a given stress and patterns of whole-plant mortality and canopy dieback under changing environmental conditions is largely unstudied (but see Yanchuk et al., 2008; Williams et al., 2014). This is particularly true for non-native plant taxa where few experimental common gardens have been established to address the potential for rapid selection on recently established populations (but see Alexander et al. 2012, Liao et al. 2016).
The genus Tamarix comprises a group of riparian woody species and their hybrids from Eurasia introduced to, and now distributed broadly across arid- and semi-arid regions of western North America. As with native woody plants in these regions, Tamarix spp. are experiencing moderate to extreme drought, and in addition exhibit dieback from episodic defoliation by a foliage-feeding beetle, Diorhabda spp. (Chrysomelidae), released for biological control of this genus (Bean et al. 2012). The northern tamarisk leaf beetle, Diorhabda carinulata, also native to Eurasia, was released more than a decade ago and has since affected thousands of hectares across the southwestern USA. The beetle produces two or more generations in a season and can develop high population densities that can completely defoliate Tamarix stands in <2 weeks. Repeated defoliation events eventually result in significant stand dieback and mortality (Pattison et al. 2011), but individual susceptibility to dieback can vary dramatically. Recent surveys across Tamarix populations have found that dieback can range from near 0% to >80%, after 2–5 years of repeated herbivory (Hultine et al., 2015a; Kennard et al., 2016). These disparate responses to repeated herbivory events invite many questions such as whether some Tamarix populations express phenotypic traits that make them more tolerant to episodic canopy disturbances than other populations. If so, two important follow-up questions (i) are there fundamental plant tradeoffs in carbon allocation patterns associated with herbivory tolerance? and (ii) is herbivory tolerance/sensitivity tied to adaptation to local environmental conditions? Although considerable research has been undertaken on Tamarix invasion into arid riparian ecosystems of North America, including identification of the hybrid nature of the genus in the western USA (Gaskin and Kazmer, 2009), information is lacking on the potential adaptive evolution of this highly successful non-native plant. Nevertheless, as beetle populations continue to disperse into broader geographic locations, clues are beginning to emerge on the extent to which Tamarix genotypes vary in their ability to cope with episodic foliage herbivory.
This paper synthesizes ongoing research on the patterns and mechanisms of Tamarix canopy dieback and mortality in response to intense episodic herbivory by D. carinulata. We focus on Tamarix/Diorhabda interactions as a model system to test hypotheses related to plant resource allocation, local adaptation and the impacts of multiple stressors on plant mortality and fitness. We present Tamarix/Diorhabda as a model system because of the intense episodic patterns of foliage herbivory by Diorhabda, coupled with the wide geographic distribution of Tamarix across broad environmental gradients and potential stressors. Together, these provide a system to investigate variation in traits associated with survival in response to defoliation events under a wide range of stressors. First, we provide a brief overview of the history of Tamarix in North America, followed by a review of recent research on the genetic diversity and evidence of local adaptation of Tamarix in its novel environment. We then propose potential tradeoffs associated with physiological traits related to carbon allocation. Here, we specifically address cases in which the expression of a given trait leads to resistance to one stress mechanism at the cost of reduced resistance to another stress mechanism, including stressors that are introduced or occur episodically, such as defoliation by D. carinulata. We pay special attention to the impacts that changes in mortality pressures can have on directional selection resulting in reduced genetic and phenotypic diversity, and potentially reduced tolerance of other stressors or competition. Finally, we summarize experiments that we believe are critical to merge studies of Tamarix mortality with those that focus on patterns of local adaptation, including the construction of common gardens and reciprocal transplant experiments. The specific hypotheses that are advanced here include: (i) despite its recent introduction, and the potential for significant gene flow, Tamarix in western North America is generally adapted to local environmental conditions across its current range in part due to hybridization of two species, (ii) local adaptation to specific climate, soil and resource availability conditions will yield predictable responses to episodic herbivory and (iii) the ability to cope with a combination of episodic herbivory and increased aridity associated with climate change will be largely based on functional tradeoffs in resource allocation that fall along a predictable trait spectrum.
History and ecology of Tamarix
Tamarix has become one of the most successful non-native woody plants in the western USA, covering nearly 500 000 hectares (Friedman et al., 2005; Nagler et al., 2011), with a range that spans much of North America. Trees in this genus were introduced to the western states in the mid-19th century as ornamentals and for erosion control by governmental agencies due to their ability to thrive in xeric and saline environments (Horton, 1977). The genus was identified as a threat to native ecosystems in the 1930s (Robinson, 1965). Previously, the two most widely distributed species in North America, T. ramosissima and T. chinensis, were treated either as two separate taxa (Baum, 1978; Gaskin, 2003) or as a single aggregate species (Allred, 2002). These conflicting classifications are partly explained by results from molecular analyses that revealed that as much as 85% of Tamarix sampled from populations in the USA were a mosaic of hybrids between T. ramosissima and T. chinensis (Gaskin and Kazmer, 2009). While some hybridization of Tamarix species had been recognized in previous studies (Gaskin and Schaal, 2003; Gaskin and Shafroth, 2005), these two species and their related hybrids (hereafter referred to as Tamarix) are now recognized to dominate desert riparian habitats (Gaskin and Schaal, 2002; Sher, 2013). In addition to T. ramosissima and T. chinensis there are six other species of Tamarix in North America, and some of these hybridize with T. ramosissima and T. chinensis (i.e. T. gallica, T. canariensis, and T. aphylla), but these are less common species and even rarer as parents of hybrids (Gaskin and Schaal, 2003; Gaskin and Shafroth, 2005). Tamarix has had substantial impacts on hydrological function, the occurrence of fire and food web structure in riparian ecosystems in the southwestern USA and northern Mexico, in part due to the initial widespread planting of diverse Tamarix species (Friedman et al., 2005; Sher, 2013), as well as a suite of traits that allow Tamarix to be a rapid post-disturbance colonizer, a strong competitor and/or capable of tolerating considerable stress (Hultine and Dudley, 2013).
Tamarix has been targeted for large-scale removal projects in attempts to conserve water and maintain flows in arid regions based on assumptions that replacement of native taxa by Tamarix resulted in an overall increase of transpiration across riparian land areas (Shafroth et al., 2005). However, Tamarix removal from invaded systems has proved difficult, and control efforts using fire, mechanical removal at ground level, and herbicide treatments have mostly been proven to be ineffective or unsustainable (Gaskin, 2003). Tamarix resprouts from underground storage tissues following these eradication techniques, necessitating repeated treatments and sometimes soil reclamation to establish native species. The cost of removal alone is often prohibitive, around USD 1500–1700 ha−1 (Shafroth et al., 2005), and for successful eradication of established stands where revegetation and restoration efforts have been undertaken the cost can be upwards of USD 12 000 ha−1 (Zavaleta, 2000).
The USDA Agricultural Research Service began investigating the use of a biological control for Tamarix in the 1960s (Deloach et al., 2003). Tamarix seemed to be an ideal candidate for a biocontrol program, since there are no native congeners of Tamarix in North America (Gaskin, 2003). Three specialist insects from the Tamarix native range were approved by regulatory agencies for release, and beginning in 1998 controlled field trials began for the northern tamarisk leaf beetle (D. carinulata) to determine its suitability as a biocontrol agent (DeLoach et al., 2003). Open field releases followed successful cage trials in Colorado, Nevada and Utah that resulted in establishment and associated defoliation of Tamarix stands by D. carinulata (Dudley and Bean, 2012; Dudley et al., 2012); three other species of Diorhabda were subsequently released, primarily in Texas (Knutson et al., 2012). Multiple defoliation events typically occur over a single season, with up to three events at warmer sites (with longer growing season), and tree mortality has been documented after multiple years of defoliation (Bean et al., 2012). Some populations appear to be more tolerant to defoliation. For example, sites along the Humboldt River in Nevada tolerated three defoliation events per year for at least 3 years before any mortality was observed (Pattison et al. 2011). Likewise, sites along the Virgin River, a tributary of the Colorado River, have also exhibited low mortality rates after as many as seven defoliation events (Hultine et al., 2015a). By contrast, mortality of approximately half of individual plants has been documented at some sites along the Colorado River near Moab, Utah 3 years after the first observations of defoliation associated with Diorhabda feeding (Hultine et al., 2013). To date, the range of D. carinulata continues to expand southward, despite initial projections that physiological constraints would inhibit establishment south of the 38th parallel (Bean et al., 2012).
Hybridization and the potential for local adaptation
Early investigators (Baker, 1965) suggested that invaders were successful because they had a ‘general purpose genotype’ that would perform well across a range of environmental conditions (Dlugosch and Parker, 2008). This hypothesis was grounded in the idea that founder effects reduce genetic variation in a species’ introduced range compared with its native range, and that populations of non-native species that become established would be composed of highly plastic individuals that could be successful across a range of environmental conditions (Baker, 1974). However, even early supporters of the ‘general purpose genotype’ hypothesis recognized that it could be offset by multiple introductions, or by plants with putative adaptive traits that are specialized to specific environments. It is believed that after initial colonization by a non-native species, local adaptation may play an important role in continued existence (Baker, 1974; Liao et al., 2016). There is building evidence that the hybridization of Tamarix may provide variation in traits that could promote local adaptation.
The hybridization of T. ramosissima with T. chinensis is widespread in North America (and southern Africa where the two species have also been introduced), but not in Eurasia in part because of the allopatric distributions in their native ranges (Gaskin and Schaal, 2002; Mayonde et al., 2016). Hybridization can also provide novel gene combinations that may promote adaptations to specific ecological problems in the new range that were not expressed by either parental lineage, as well as help overcome genetic bottlenecks that arise from founder effects (Anderson, 1953; Dlugosch and Parker, 2008). Furthermore, studies have shown that most naturalized Tamarix are more closely related to other wild naturalized Tamarix than they are to nearby ornamental populations, suggesting that most recent recruitment has occurred from naturalized individuals, despite potential gene flow between ornamental and naturalized populations (Gaskin and Kazmer, 2006). This suggests that naturalized genetic sources may be better adapted for successful invasion into these ecosystems than cultivated genetic lineages. Since Tamarix plants are fairly long lived (>70 years), and there has been extensive backcrossing, it is likely that hybridization occurred soon after introductions into western North America (Gaskin et al., 2012). Two pieces of evidence support the early hybridization hypothesis: (i) most individuals that have been sampled for molecular analysis from naturalized populations are the product of multiple generations of hybridization, as exhibited by varying levels of introgression (Gaskin and Kazmer, 2009); and (ii) studies of populations in southern Utah showed that the plants established there in the 1930s were T. ramosissima × chinensis hybrids (Gaskin et al., 2012). Nevertheless, information is generally lacking on whether breeding efforts by nurseries played a significant role in the observed patterns of hybridization in North America.
Molecular studies conducted across a latitudinal gradient from Texas to Montana showed that plants from lower latitudes were most representative of genetic material from the T. chinensis parental strain and that the higher latitude plants were most representative of T. ramosissima parents (Friedman et al., 2008; Gaskin and Kazmer, 2009). The climates in the extreme northern and southern ranges of the study reflect some of the differences between the climates of T. ramosissima and T. chinensis in their respective native ranges. Specifically, T. ramosissima occurs in the Eurasian interior where minimum temperatures are typically much lower than the thermal range of T. chinensis (Baum, 1978; Friedman et al., 2011). A common garden investigation of cold hardiness in Tamarix across this latitudinal gradient showed that plants from Montana were able to survive temperatures 21°C lower than the genotypes from Texas, and that there was a correlation between the latitude of origin and overwinter survival (Friedman et al., 2008). These data suggest that there has been local adaptation to climatic pressures (described in greater detail in the next section), and that hybridization of the two parental species may play a role in these adaptive traits as increased introgression towards T. ramosissima increased the cold hardiness of plants.
A recent genetic survey using microsatellites as described by Gaskin et al. (2006) and Friedman et al. (2008) was completed in 2016 from 18 Tamarix populations sampled across an elevation gradient in Arizona and southern Utah and showed that intra-population diversity was higher than inter-population diversity (Fehlberg, unpublished data). The amount of introgression towards T. ramosissima in these Arizona/southern Utah populations matched the levels of hybridization reported earlier from other locations in western North America (Gaskin and Schaal, 2002; Gaskin and Kazmer, 2009) and supports evidence that the majority of naturalized plants are hybrids of the two species. The novel genotypes created in such hybrid swarms could increase the capacity for emergence of a variety of genotype × environment combinations. Taken together, results from these genetic studies indicate that hybridization may explain the wide distribution and high abundance of Tamarix in the United States. Intermediate traits between the two parental species and novel gene combinations could provide the material for adaptation to local climatic and edaphic conditions or increase plastic expression of traits that allow for acclimatization to a range of sites by individuals. Variation in tolerance to cold, salinity and herbivory all indicate that hybridization could play an important role in the propensity of Tamarix to dominate riparian habitats.
Evidence for local adaptation in Tamarix common garden studies
Local adaptation is driven by divergent natural selection on genotypes for traits that favour fitness in any given genotype × environment combination and should result in local populations that are more fit in their local habitat, defined by the suite of environmental factors, compared with populations from other habitats (Williams, 1966; Kawecki and Ebert, 2004). Gene flow, lack of genetic variation, extinction and environmental variability all have the potential to slow the emergence of local adaptation. Evidence has shown that gene flow can be maladaptive for populations, specifically away from the core of a species distribution range (Kirkpatrick and Barton, 1997), yet in small populations, increases in genetic variation resulting from gene flow can favour local adaptations (Moore and Hendry, 2009). Even in areas with high rates of gene flow, local adaptation may arise when there is strong selection due to high spatial heterogeneity in the environment (Macnair, 1991; Kawecki and Ebert, 2004) Multiple introductions may promote local adaptation by increasing the genetic diversity of a species in its introduced range, even potentially increasing diversity beyond that found in native ranges (Kolbe et al., 2004). This is especially true when introductions occur from across a large geographical distribution where plants may have low genetic diversity within a population but high diversity across populations (Dlugosch and Parker, 2008).
Native species have been shown to inherit traits linked to variation in cold hardiness and senescence periods, where selection over long periods of time have resulted in local populations that are more fit than conspecific individuals from other habitats in response to different photoperiods across latitudinal gradients and minimum winter temperatures in local climates (Howe et al., 1995). Similarly, introduced species can also express significant levels of local adaptation in their novel ranges (Rice et al., 1992; Dlugosch and Parker, 2008; Oduor et al., 2016). It has been shown that local adaptation can sometimes be as important as phenotypic plasticity in the capacity of some invasive plants to occupy a broad range of environments (Liao et al. 2016; Oduor et al. 2016). The bulk of literature available regarding local adaptation of non-native plants, however, has been based on annuals or herbaceous perennial species, and a paucity of work has been conducted with invasive perennial woody species.
Common garden studies involving Tamarix from populations exhibiting varying amounts of T. ramosissima introgression show that there is a range of traits that are expressed across a climate gradient (Friedman et al., 2008; Williams et al., 2014). A common garden study in Ft. Collins, Colorado compared characteristics of a native riparian tree species, Populus deltoides (Plains cottonwood), and Tamarix across a latitudinal gradient to investigate variations in cold hardiness and phenology (Friedman et al. 2008). As expected, the northern P. deltoides populations entered dormancy earlier and had higher rates of survival over the winter when compared with the southern populations. Tamarix populations showed similar results, with fewer individuals from the southern populations surviving through the winter (Friedman et al., 2008). Likewise, the date of leaf senescence was correlated with latitude for both Tamarix and P. deltoides genotypes in the common garden (Friedman et al., 2011). Studies have also shown that northern populations of Tamarix have a larger root to shoot ratio, indicating that northern populations allocate more biomass to belowground tissues than southern populations (Sexton et al., 2002; Williams et al., 2014). For populations experiencing higher rates of freeze-induced dieback, it may be adaptive to have larger pools of carbon in the form of belowground biomass for replacement growth of dead tissues when the growing season resumes. Similar trends of increased cold hardiness and early leaf senescence in Tamarix when compared with P. deltoides suggest that there may be rapid acclimatization of the introduced species to local environments.
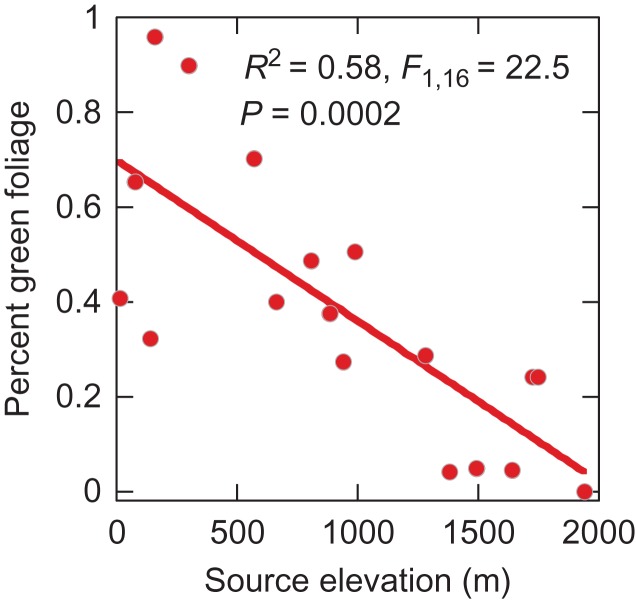
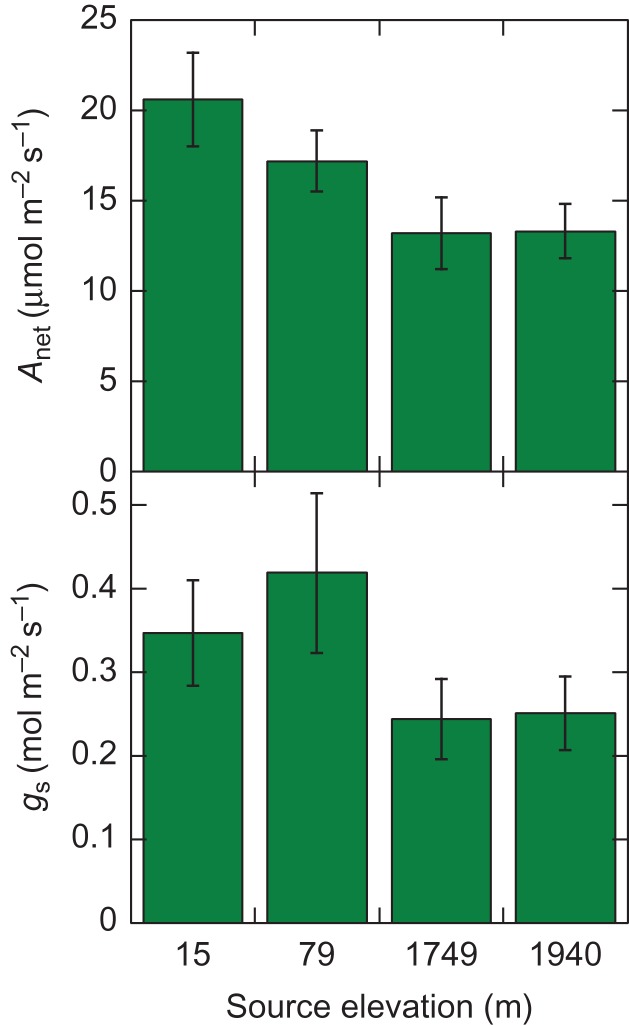
Preliminary work from a Tamarix common garden study in Yuma, AZ also supports evidence of variation in phenology and foliar gas-exchange rates across populations. Phenology and leaf-level gas exchange patterns tend to correlate with minimum winter and maximum July/August temperatures of source populations. The common garden study conducted at the University of Arizona Mesa Farm (lat. 32.6151°N, long. −114.6365°W, elev. 60 m) involves 18 populations derived from an elevation gradient from 15 to 1940 m and a latitudinal range of 32.0° to 37.3°N. Beginning in January 2016, bimonthly phenological observations of floral characteristics were made on at least 36 individual plants from each population. Each individual was assigned a score for foliage greenness on a scale from 0 to 5, with a score of 0 indicating that the plants were still dormant, and a 5 indicating that the leaves were fully flushed out. Low elevation populations broke dormancy and were fully leafed out earlier than those that originated from mid- and high-elevation sites (Fig. 1). Likewise, leaf gas exchange measurements conducted in mid-June 2016 revealed that genotypes collected from low elevations sites with similar climate means as the common garden location in Yuma exhibited higher rates of midday net photosynthesis (Anet) than genotypes collected from higher elevations with cooler climate means than in Yuma (upper panel of Fig. 2). The low-elevation populations also had higher rates of stomatal conductance (gs) when compared with high-elevation populations (lower panel of Fig. 2). Although the physiological mechanism that allows genotypes originating from warmer climate regions to maintain high levels of leaf-level photosynthetic rates is not known, the fact that phenology and physiology varied among provenances provides evidence that low-elevation genotypes may be adapted to the extreme heat that typifies low deserts of North America. Consequently, locally adapted Tamarix plants could become locally maladapted under projected future climate conditions.
Figure 1:
The correlation of percent green foliage versus population source elevation for Tamarix branches in January 2016 at a common garden in Yuma, AZ. Eighteen populations were represented at the common garden, collected across an elevation gradient from Arizona and Southern Utah. Populations originating from sites of lower elevation and correspondingly higher minimum winter temperature showed earlier leaf out relative to high elevation populations.
Figure 2:
A comparison of mean ± SE net photosynthesis (Anet, µmol CO2 m−2 s−1; upper panel) and mean ± SE stomatal conductance (gs, mol H2O m−2 s−1; lower panel) of four populations at a progeny study of Tamarix in Yuma, AZ. The common garden is located at an elevation of 56 m. Populations that were from nearby sites exhibited higher rates of photosynthesis and conductance compared with populations that were from higher elevations, showing some evidence for local adaptation based on differences in expressed traits in local and foreign populations.
Fine-tuning carbon allocation to cope with multiple stressors
Plants face many challenges for avoiding mortality and maximizing fitness including competition, drought, disease and episodic disturbance from herbivory, fire and flooding. Compounding these challenges are changes in climate that are predicted to bring warmer temperatures, increased water deficits, alterations of fire regime and insect outbreaks, particularly in arid regions over the next century (Seager et al., 2007; IPCC, 2013). For trees, and other perennial plants, mortality is often not caused by a single stressor, but by multiple stressors interacting to reduce resource uptake and metabolic function below a minimum threshold for survival. For example, surges in forest mortality have been attributed to climate-induced stress coupled with insect outbreaks and fire (Kurz et al., 2008; Allen et al., 2010; Carnicer et al., 2011; Andregg et al., 2015). For many plants, the interaction between chronic drought and episodic disturbance leads to a cumulative reduction in resource supply relative to demand, particularly if already exposed to a long-term stressor such as poor edaphic conditions (Manion, 1991; Levanic et al., 2011). Therefore, persistence of individual plants and populations may depend on prior adaptation to stress, acclimation to edaphic and topographic location, as well as exposure to episodic disturbance events.
Regardless of edaphic location, disturbance and life history stage, plants must find ways to optimize carbon uptake and allocation by acquiring limited resources—mainly water, nutrients sunlight—under varying environmental conditions (Bloom et al., 1985). When resources are available above a minimum threshold, plants acquire carbon through photosynthesis and the products of photosynthesis (i.e. sugars) are used to maximize plant fitness—defined here as fecundity multiplied by life span. Maximizing fitness requires sugars to be allocated into a complex suite of carbon sinks, including tissue growth, reproduction, cellular respiration, defense chemistry (i.e. secondary metabolites and phenolics) and the storage of non-structural carbohydrates (NSCs) for subsequent utilization. Most fundamentally, osmotically active NSC pools maintain cell turgor and serve as a reservoir to buffer imbalances between carbon supply and demand (Chapin et al., 1990). Recently, particular attention has been given to the role NSC storage plays in overall plant function, fitness and capacity to withstand stress. These storage pools generally increase with plant size, which may serve as a major benefit since larger plants require larger buffers to cope with carbon imbalances in response to stress or episodic disturbance (Hoch et al., 2002; Sala and Hoch, 2009; Woodruff and Meinzer, 2011). Likewise, NSC pools are critical for vascular function (phloem and xylem) in response to varying environmental conditions. In fact, several studies have documented that NSC pools can play a primary role in maintaining plant water balance and long-distance water transport in the xylem by osmotically repairing xylem conduits following either drought-induced (Bucci et al., 2003; Nardini et al., 2011) or freeze-thaw-induced embolism (Woodruff and Meinzer, 2011). Given the importance of NSC pools for both carbon and water balance of long-lived woody plants, maintaining a minimum pool size may be a key trait for surviving resource limitations across various temporal scales.
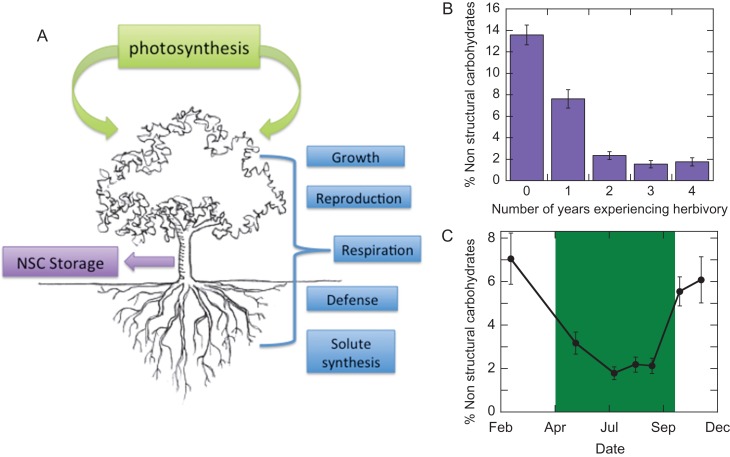
Traditionally, NSC storage was viewed as a consequence of weakening carbon sinks from reduced growth and respiration near the conclusion of the growing season when there was a surplus of carbon being acquired (Chapin et al., 1990). Recent evidence, however, suggests that NSC storage may be highly regulated and is often a competing sink for recently acquired carbon throughout the growing season (Fig. 3) (Hoch et al., 2003; Sala et al., 2012). Active regulation of carbon allocation may be coordinated by a complex genetic linkage among traits related to photosynthesis, growth, storage and other carbon movement (Smith and Stitt, 2007). Therefore, carbon allocation strategies could be highly variable within and among plant populations, depending on selective pressures from competition, resource limitation and disturbance. For example, if total carbon pools are equal between two plants (i.e. similar rates of total carbon assimilation via photosynthesis), then one plant demonstrating greater tissue synthesis and subsequent growth will do so at the expense of having less photosynthate to allocate to other sinks including NSC storage (Fig. 3). In locations where competition for sunlight is high and episodic disturbance is low (e.g. tropical rainforests), natural selection should favour individuals who maximize growth at the expense of NSC storage. Conversely, where competition is low, but disturbance or stress is high, or resource availability fluctuates, natural selection should favour individuals who allocate more of their photosynthate to NSC storage. However, plants that grow in riparian ecosystems, including Tamarix, are often exposed to competition, stress and episodic disturbance, requiring a high level of functional diversity in physiological traits. Therefore, riparian plant species will likely express a range of carbon allocation strategies, particularly in locations where there is strong gene flow among populations.
Figure 3:
(A) A conceptualized carbon budget of woody plants showing the source of carbon coming from photosynthesis and the major carbon sinks. The conceptual diagram highlights NSC storage (purple box on the left) as a competing sink with all other sinks (shown on the right) including tissue growth, respiration (mitochondrial plus photo-respiration), reproduction, defense and solute synthesis. In Tamarix plants, the various carbon sinks that compete with NSC storage can be strong at different periods of the year given its typical environmental niche, trait expression and herbivory pressure. (B) (Redrawn from Hudgeons et al., 2007): Mean percentage of NSC storage measured in the root crown of mature Tamarix trees in northern Nevada. The trees had been exposed to a range of 0–4 years of episodic herbivory by the northern Tamarix leaf beetle (Diorhabda carinulata). Error bars represent the standard error of the means. (C) (Redrawn from Hultine et al., 2015b): The seasonal pattern of NSC concentrations in the twigs of mature Tamarix trees occurring in southeastern Utah (n = 20 trees). The patterns show a reduction in NSC storage during the growing season, revealing competing sinks between growth and storage as shown in the schematic on the left of Figure 3.
The biocontrol agent, D. carinulata and other recently released Diorhabda species are exerting intense herbivory pressure on Tamarix throughout the southwestern US, resulting in a potentially significant shift in what could be optimal plant carbon allocation. Tamarix has evolved under intense pressure from herbivory: in its home range, Tamarix is attacked by >320 species of insects and mites from 88 genera (Kovalev, 1995). This plant–insect co-evolution has likely contributed to a diverse set of strategies to cope with damaging insects. Among these strategies could be one in which Tamarix plants reserve relatively large pools of NSC with which to replace damaged tissues following herbivory (Hultine et al., 2015b), although these pools can be rapidly depleted following recurrent episodic herbivory (Hudgeons et al., 2007; Fig. 3A). As mentioned above, replenishing NSC pools comes at the cost of reduced allocation to other sinks (Fig. 3B), especially if canopy-scale photosynthetic capacity is reduced as a consequence of foliage herbivory (Strauss and Agrawal, 1999; Pattison et al., 2011). Herbivore pressure by Diorhabda is, nonetheless, a new phenomenon in North America where previously there had been little selection for herbivory tolerance in Tamarix plants. This shift in selective pressures could result in a potentially significant change in optimal plant carbon allocation. However, desert riparian settings require physiological traits to best cope with a combination of competition, temperature extremes, salinity and stochastic recruitment opportunities (Table 1). These selective pressures will, at least in part, favour allocation to growth, respiration (growth and maintenance respiration), reproduction and solute synthesis (Box 1), all at the potential expense of reduced allocation to NSC storage. Thus, plants that exhibit allocation to traits associated with success in the most taxing desert riparian settings (e.g. high salinity with extreme temperatures) may be maladapted to deal with herbivory-caused reduced NSC storage compared with individuals growing in low stress sites.
Table 1:
Common environmental conditions Tamarix plants face during their life history and the carbon allocation strategies required to maximize fitness and survival under specific conditions
| Environmental condition | Carbon cost |
|---|---|
| Insect/pathogen infestation | Resistance from defensive chemistry/secondary metabolite production |
| High soil/groundwater salinity | Solute synthesis to osmotically exude salts from leaves |
| Increasing depth to groundwater | Tissue construction for rapid root growth |
| Intra–inter-specific competition for sunlight | Tissue construction for rapid canopy growth rates |
| High growing season temperature | High mitochondrial respiration rates |
| Stochastic recruitment opportunities | Continuous flower and seed production to cope with unpredictable soil moisture conditions |
| Potential growing season freezing events | High NSC concentrations for tissue growth following frost-induced dieback |
| High fire frequency | High NSC concentrations for resprouting following episodic fire |
| High episodic foliage herbivory | High NSC concentrations to construct new foliage following herbivory events |
Box 1. Mechanisms and costs of salinity tolerance in Tamarix.
Halophytes, such as Tamarix spp., are defined as plants that have a high tolerance to salt. Generally speaking, there are three distinct ways that plants tolerate high salt concentrations (Munns and Tester, 2008): (i) tolerance to osmotic stress; (ii) salt exclusion by plant roots so that salts do not accumulate in the leaves; and (iii) compartmentalization of salts to avoid toxic concentrations in the cytoplasm, especially the cytoplasm of mesophyll cells in the leaf. Compared with most riparian plants, Tamarix has a fairly high tolerance to osmotic stress (Cui et al., 2010; Ding et al., 2010) and therefore can maintain gas exchange at lower leaf water potentials than most co-occurring species such as willows (Salix spp.) or cottonwoods (Populus spp.) (Pockman and Sperry, 2000; Hultine and Bush, 2011). More importantly, Tamarix avoids long-term salt toxicity in leaf tissues by compartmentalizing and excreting salts through specialized salt glands (Storey and Thompson, 1994; Glenn et al., 2012). Compartmentalization takes place by synthesizing compatible organic solutes, such as sucrose and other compounds, at high enough concentrations in the cytosol and organelles of leaf cells to balance the osmotic pressure of ions in the cell vacuole (Flowers et al., 1977; Munns and Tester, 2008). However, compatible solute synthesis and subsequent removal of salts once they have entered the leaf come with a considerable metabolic cost resulting in a potential reduction in carbon allocation to other sinks (Fig. 3). To be more precise, about seven moles of ATP are required to accumulate one mole of Na+ as an osmoticum, whereas it takes ~52 moles of ATP to synthesize one mole of sucrose (Raven, 1985). The high metabolic cost of solute synthesis may allow plants to survive the presence of high external concentrations of salt, but at the expense of higher vulnerability to other stressors. For example, Tamarix dieback and mortality in response to episodic herbivory by D. carinulata increased along a soil salinity gradient in a Mojave Desert river watershed (Hultine et al., 2015a). Therefore, soil salinity may exert a combination of high osmotic stress (resulting in lower net photosynthesis) and increased metabolic costs (resulting in lower carbon allocation to NSC storage) that may synergistically predispose Tamarix to the negative effects of episodic foliage herbivory and other stressors.
Across the geographic range of Tamarix, allocation to NSC storage may be selected for in populations that experience freezing temperatures during the growing season. A recent common garden experiment indicated that Tamarix genotypes from higher latitudes, where freezing temperatures during the growing season are common, showed greater tolerance to herbivory by D. carinulata (i.e. expressed higher canopy regrowth following herbivory) than lower latitude genotypes (Williams et al., 2014). One explanation for the observed pattern is that high-latitude genotypes maintained higher NSC storage due to a reduction in sink strength as plant growth ceases at the conclusion of the growing season (Hoch et al., 2002). In other words, NSC pools could increase as a consequence of imbalances between carbon supply and demand as growth and respiration decline (Chapin et al., 1990; Sala et al., 2012). Alternatively, high-latitude genotypes may have been selected to actively maintain high NSC pools. The same common garden experiment revealed evidence for natural selection and subsequent adaptation to local environment conditions as high-latitude genotypes expressed earlier leaf senescence, lower biomass production and higher root-to-shoot ratios compared with genotypes from lower latitudes which exhibited increased aboveground biomass accumulation (Friedman et al., 2011; Williams et al., 2014). The latter trait is important because the largest NSC pool is presumably stored belowground (Canham et al., 1999). Given that high-latitude populations experience occasional canopy dieback associated with freezing temperatures during the growing season, natural selection may favour genotypes that allocate more biomass to belowground NSC storage presumably for rebuilding frost-damaged tissues. As a by-product of selection to cope with low temperatures, high latitude genotypes may be better adapted to tolerate episodic disturbance.
The relative strength of carbon allocation tradeoffs within and among Tamarix populations is an open question, but recent evidence suggests that these tradeoffs might be profound. Measurements of radial growth from annual tree rings showed that surviving trees within populations grew slower in years prior to the arrival of D. carinulata beetles compared with trees that ultimately succumbed to repeated herbivory events (Hultine et al., 2013). These patterns were presumably a function of surviving trees allocating a higher proportion of their carbon pool to NSC storage at the expense of slower annual growth rates (Hultine et al., 2013). If plants selected for faster growth are killed by beetles at a higher rate than slower growing plants, then population mean productivity of individuals may decline in response to episodic herbivory. This pattern of directional selection could in turn impact population fitness by altering competitive interactions with other species. This is particularly true given evidence that Tamarix already has lower growth rates relative to native riparian tree species Salix spp. and Populus spp. at germination (Sher and Marshall, 2003).
Implications for conservation and future research directions
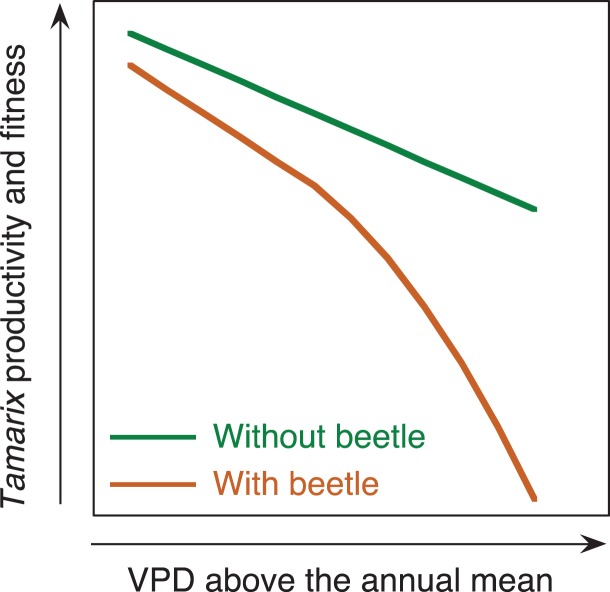
This review highlights three main points. The first is that despite its recent introduction into North America, Tamarix expresses several functional trait variations consistent with adaptation to regional climate conditions, which may be in part a result of extensive hybridization. This result is consistent with previous studies showing local adaptation occurs in invasive plant species (Liao et al., 2016, Oduor et al. 2016). The second is that Tamarix genotypes adapted to warmer climates may be maladapted to the effects of episodic herbivory due to allocating a larger proportion of their total carbon pool to growth and respiration at the expense of lower overall storage of NSCs. Finally, both of these patterns suggest that the combination of herbivory and increased aridity driven by climate change could synergistically reduce growth, fitness and overall dominance of Tamarix (Fig. 4). As the distribution of D. carinulata and related species continue to expand their range into warmer and more arid regions in North America, rates of Tamarix mortality will likely increase compared with current mortality rates in more northern or higher elevation locations. Likewise, Tamarix growing under conditions of high soil and/or groundwater salinity may be especially sensitive to episodic herbivory. Plants growing in high salinity will not only be exposed to chronic stress from lower soil water potentials, but will potentially require greater allocation of carbon to solute synthesis in order to osmotically facilitate salt exudation from leaf tissues (Box 1). To better understand how the combination of climate and episodic disturbance (i.e. herbivory) will impact Tamarix plants in the southwestern US, we suggest that future research projects take place on four inter-related thrusts: (i) the construction and maintenance of common gardens, such as the one described earlier and currently in operation in Yuma, AZ; (ii) expanded efforts to quantify plant carbon budgets over time, particularly better seasonal accounting of changes in whole-plant NSC pools across genotypes and populations; (iii) genomics and epigenetics research to link phenotypic traits such as resource allocation, tolerance to salinity and drought to specific molecular markers and gene expression, and (iv) ground and remote sensing-based monitoring of Diorhabda spp. distributions and subsequent impact on Tamarix fitness, recruitment and survival.
Figure 4:
The predicted relationship between growing season aridity (i.e. vapor pressure deficit) above the annual mean and the productivity and fitness of Tamarix genotypes with and without the presence of the northern Tamarix leaf beetle (Diorhabda carinulata).
Information acquired from current research activities, and research approaches advocated here would provide critical information for targeted restoration and conservation of valued riparian ecosystems. Data on plant genetics and phenotypic trait expression could potentially guide restoration ecologists and land managers on how to best use limited resources for riparian restoration by improving predictive ability based on phenotype/genotype screening on when and where climate change by defoliation interactions will most impact Tamarix. These projects could target a wide range of specific objectives depending on the projected impacts of foliage herbivory on Tamarix including fire prevention, native plant restoration and habitat restoration. For example, Tamarix populations that are predicted to experience the highest levels of mortality and canopy dieback could be areas that are given the highest priority for restoration efforts. Knowing these patterns may be particularly important for guiding habitat restoration of threatened and endangered avian species such as the southwestern willow flycatcher (Empidomax traillii extimus) that under some circumstances rely on Tamarix canopies for nesting (Bateman et al., 2010). It is currently unclear to what extent biological control of Tamarix will impact riparian habitat for E. trallii extimus. However, the combination of common garden studies of plant resource allocation and stress tolerance with technological advances in molecular genomics will provide critical information on how Tamarix populations will likely respond to multiple stressors going into the future.
Acknowledgements
This project was supported by a grant by the US Department of Agriculture, National Institute of Food and Agriculture (Award # 2015-67013-23138). The authors would like to thank Davis Blasini, Brian Glucksman, Dan Koepke and Bethany Zumwalde for their help collecting the preliminary data from the Yuma common garden presented in this paper, and their contribution to the ideas presented.
References
- Alexander JM, Ghezzi R, Edwards PJ (2012) Different genetic clines in response to temperature across the native and introduced ranges of a global plant invader. J Ecol 100: 771–781. [Google Scholar]
- Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Hogg EH, et al. (2010) A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For Ecol Manage 259: 660–84. [Google Scholar]
- Allred KW. (2002) Identification and taxonomy of (Tamaricaceae) in New Mexico. Des Plant 18: 26–32. [Google Scholar]
- Andregg WL, Hicke JA, Fisher RA, Allen CD, Aukema J, Bentz B, Hood S, Lichstein JW, Macalady AK, McDowell N, et al. (2015) Tree mortality from drought, insects, and their interactions in a changing climate. New Phytol 208: 674–683. [DOI] [PubMed] [Google Scholar]
- Anderson E. (1953) Introgressive hybridization. Bio Rev Camb Philos Soc 28: 280–307. [Google Scholar]
- Baker HG. (1965) Characteristics and modes of origin of weeds In: Baker HG, Stebbins GL eds. The Genetics of Colonizing Species. Academic, New York, pp 147–168. [Google Scholar]
- Baker HG. (1974) The evolution of weeds. Annu Rev Ecol Syst 5: 1–24. [Google Scholar]
- Bateman HL, Dudley TL, Bean DW, Ostoja SM, Hultine KR, Kuehn MJ (2010) A river system to watch: documenting the effects of saltcedar (Tamarix spp.) biocontrol in the Virgin River Valley. Ecol Res 28: 405–410. [Google Scholar]
- Baum B. (1978) The Genus Tamarix. Publications of the Israel Academy of Sciences and Humanities, Jerusalem. [Google Scholar]
- Bean DW, Dalin P, Dudley TL (2012) Evolution of critical day length for diapause induction enables range expansion of Diorhabda carinulata, a biological control agent against tamarisk (Tamarix spp.). Evol Appl 5: 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom AJ, Chapin FS, Mooney HA (1985) Resource limitation in plants—an economic analogy. Annu Rev Ecol Syst 16: 363–392. [Google Scholar]
- Bucci SJ, Scholz FG, Goldstein G, Meinzer FC, Sternberg LDL (2003) Dynamic changes in hydraulic conductivity in petioles of two savanna tree species: factors and mechanisms contributing to the refilling of embolized vessels. Plant Cell Environ 26: 1633–1645. [Google Scholar]
- Canham CD, Kobe KR, Latty EF, Chazdon RL (1999) Interspecific and intraspecific variation in tree seedling survival: effects of allocation to roots versus carbohydrate reserves. Oecologia 121: 1–11. [DOI] [PubMed] [Google Scholar]
- Carnicer J, Coll M, Ninyerola M, Pons X, Sánchez G, Peñuelas J (2011) Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proc Natl Acad Sci USA 108: 1474–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin FS III, Shulze ED, Mooney HA (1990) The ecology and economics of storage in plants. Annu Rev Ecol Syst 21: 423–447. [Google Scholar]
- Clausen J, Keck DD, Hiesey W (1941) Regional differentiation in plant species. Am Soc Nat 75: 231–250. [Google Scholar]
- Cui B, Yang Q, Zhang K, Zhao X, You Z (2010) Responses of saltcedar (Tamarix chinensis to water table depth and soil salinity in the Yellow River Delta, China. Plant Ecol 209: 279–290. [Google Scholar]
- DeLoach CJ, Lewis PA, Herr JC, Carruthers RI, Tracy JL, Johnson J (2003) Host specificity of the leaf beetle, Diorhabda elongata deserticola (Coleoptera: Chrysomelidae) from Asia, a biological control agent for saltcedars (Tamarix: Tamaricaceae) in the Western United States. Biol Control 27: 117–147. [Google Scholar]
- Ding X, Tian C, Zhang S, Song J, Zhang F, Mi G, Feng G (2010) Effects of NO3¯-N on the growth and salinity tolerance of Tamarix laxa (Willd.). Plant Soil 331: 57–67. [Google Scholar]
- Dlugosch KM, Parker IM (2008) Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Mol Ecol 17: 431–449. [DOI] [PubMed] [Google Scholar]
- Dudley TL, Bean DW (2012) Tamarisk biocontrol, endangered species risk and resolution of conflict through riparian restoration. BioControl 57: 331–47. [Google Scholar]
- Dudley TL, Bean DW, Pattison RR, Cares A (2012) Selectivity of a biological control agent, Diorhabda carinulata Desbrochers, 1870 (Coleoptera: Chrysomelidae) for host species within the genus Tamarix Linneaus, 1753. Pan-Pacific Ento 88: 319–341. [Google Scholar]
- Flowers TJ, Troke PF, Yeo AR (1977) The mechanism of salt tolerance in halophytes. Annu Rev Plant Physiol 28: 89–121. [Google Scholar]
- Friedman JM, Auble GT, Shafroth PB, Scott ML, Merigliano MF, Freehling MD, Griffin ER (2005) Dominance of non-native riparian trees in western USA. Biol Invasions 7: 747–751. [Google Scholar]
- Friedman JM, Roelle JE, Gaskin JF, Pepper AE, Manhart JR (2008) Latitudinal variation in cold hardiness in introduced Tamarix and native Populus. Evol Appl 1: 598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM, Roelle JE, Cade BS (2011) Genetic and environmental influences on leaf phenology and cold hardiness of native and introduced riparian trees. Int J Biometeorol 55: 775–787. [DOI] [PubMed] [Google Scholar]
- Gaskin JF. (2003) Molecular systematics and the control of invasive plants: a case study of Tamarix (Tamaricaceae). Ann Mo Bot Gard 90: 109–118. [Google Scholar]
- Gaskin JF, Kazmer DJ (2006) Comparison of ornamental and wild saltcedar (Tamarix spp.) along Eastern Montana, USA riverways using chloroplast and nuclear DNA sequence markers. Wetlands 26: 939–950. [Google Scholar]
- Gaskin JF, Kazmer DJ (2009) Introgression between invasive saltcedars (Tamarix chinensis and T. ramosissima) in the USA. Biol Invasions 11: 1121–1130. [Google Scholar]
- Gaskin JF, Schaal BA (2002) Hybrid Tamarix widespread in US invasion and undetected in native Asian range. Proc Natl Acad Sci 99: 11256–11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin JF, Schaal BA (2003) Molecular phylogenetic investigation of U.S. invasive Tamarix. Syst Bot 28: 86–95. [Google Scholar]
- Gaskin JF, Shafroth PB (2005) Hybridization of Tamarix ramosissima and T. chinensis (saltcedars) with T. aphylla (Athel) (Tamaricaceae) in the southwestern USA determined from DNA sequence data. Madroño 52: 1–10. [Google Scholar]
- Gaskin JF, Pepper AE, Manhart JM (2006) Isolation and characterization of ten polymorphic microsatellites in saltcedars (Tamarix chinensis and T. ramosissima). Mol Ecol Notes 6: 1146–1149. [Google Scholar]
- Gaskin JF, Birken AS, Cooper DJ (2012) Levels of novel hybridization in the saltcedar invasion compared over seven decades. Biol Invasions 14: 693–699. [Google Scholar]
- Glenn EP, Nelson SG, Ambrose B, Martinez R, Soliz D, Pabendinskas V, Hultine K (2012) Comparison of salinity tolerance of three Atriplex spp. in well-watered and drying soils. Environ Exp Bot 83: 62–72. [Google Scholar]
- Grady KC, Ferrier SM, Whitham TG, Kolb TE, Hart SC, Allan GJ. 2011. Genetic variation in productivity of foundation riparian species at the edge of their distribution: implications for restoration and assisted migration in a warming climate. Glob Change Biol 17: 3724–3735. [Google Scholar]
- Gray LK, Hamann A, John S, Rweyongeza D, Barnhardt L, Thomas BR (2016) Climate change risk management in tree improvement selection and movement of genotypes. Tree Gen Genom doi:10.1007/s11295-016-0983-1. [Google Scholar]
- Hoch G, Popp M, Körner (2002) Altitudinal increases in mobile carbon pools in Pinus cembra suggests sink limitation on growth at the Swiss treeline. Oikos 98: 361–374. [Google Scholar]
- Hoch G, Richter A, Körner C (2003) Non-structural carbon compounds in temperate forest trees. Plant Cell Environ 26: 1067–1081. [Google Scholar]
- Horton JS. (1977) The development and perpetuation of the permanent tamarisk type in the phreatophyte zone of the Southwest. In Johnson RR, Jones DA, eds, Importance, preservation and management of riparian habitat: a symposium. U. S. Department of Agriculture, Forest Service General Technical Report RM-43, Ft. Collins, Colorado, pp 124–127.
- Howe GT, Hackett WP, Furnier GR, Klevorn RE (1995) Photoperiodic responses of a northern and southern ecotype of black cottonwood. Physiol Plant 93: 695–708. [Google Scholar]
- Hudgeons JL, Knutson AE, Heinz KM, DeLoach CJ, Dudley TL, Pattison RR, Kiniry JR (2007) Defoliation by introduced Diorhabda elongata leaf beetles (Coleoptera Chrysomelidae) reduces carbohydrate reserves and regrowth of Tamarix (Tamaricaceae). Biol Conv 43: 213–221. [Google Scholar]
- Hultine KR, Bush SE (2011) Ecohydrological consequences of non-native riparian vegetation in the southwestern U.S.: a review from an ecophysiological perspective. Water Res Res 47W07542, doi: 10.1029/2010WR010317. [Google Scholar]
- Hultine KR, Dudley TL (2013) Tamarix from organism to landscape In Sher A, Quigley MF, eds, Tamarix: A Case Study of Ecological Change in the American West. Oxford University Press, New York, pp 149–167. [Google Scholar]
- Hultine KR, Dudley TL, Leavitt SW (2013) Herbivory-induced mortality increases with radial growth in an invasive riparian phreatophyte. Ann Bot 111: 1197–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultine KR, Dudley TL, Koepke DF, Bean DW, Glenn EP, Lambert AM (2015. a) Patterns of herbivory-induced mortality of a dominant non-native tree/shrub (Tamarix spp.) in a southwestern US watershed. Biol Invasions 17: 1729–1742. [Google Scholar]
- Hultine KR, Bean DW, Dudley TL, Gehring CA (2015. b) Species introductions and their cascading impacts on biotic interactions in desert riparian ecosystems. Integr Comp Biol 55: 587–601. [DOI] [PubMed] [Google Scholar]
- IPCC , 2013: Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovern mental Panel on Climate Change. In Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM eds. Chapter 12 Long Term Climate Change: Projections, Commitments and Irreversibly, Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 1535p. [Google Scholar]
- Kawecki TJ, Ebert D (2004) Conceptual issues in local adaptation. Ecol Lett 7: 1225–1241. [Google Scholar]
- Kennard D, Louden N, Gemoets D, Ortega S, González E, Bean D, Cunningham P, Johnson T, Rosen K, Stalke A (2016) Tamarix dieback and vegetation patterns following release of the northern tamarisk beetle (Diorhabda carinulata) in western Colorado. Bio Con 101: 114–122. [Google Scholar]
- Kirkpatrick M, Barton NH (1997). Evolution of a species’ range. Am Nat 150: 1–23. [DOI] [PubMed] [Google Scholar]
- Knutson AE, DeLoach CJ, Tracy JL, Randal CW (2012) Field evaluation of Diorhabda elongata and D. carinata (Coleoptera: Chrysomelidae) for biological control of saltcedars (Tamarix spp.) in Northwest Texas. Southwest Entomol 37: 91–102. [Google Scholar]
- Kolbe JJ, Glor RE, Schettino LRG, Lara AC, Larson A, Losos JB (2004) Genetic variation increases during biological invasion by a Cuban lizard. Nature 431: 177–181. [DOI] [PubMed] [Google Scholar]
- Kovalev OV. (1995) Co-evolution of tamarisk (Tamaricaceae) and pest arthroods (Insecta: Arachnida: Acarina), with special reference to biological control prospects. Pensoft, Sofia: 110p. [Google Scholar]
- Kurz WA, Dymond CC, Stinson G, Rampley GJ, Neilson ET, Carroll AL, Ebata T, Safranyik L (2008) Mountain pine beetle and forest carbon feedback to climate change. Nature 452: 987–990. [DOI] [PubMed] [Google Scholar]
- Liao HX, D'Antonio CM, Chen BM, Huang QQ, Peng SL (2016) How much do phenotypic plasticity and local genetic variation contribute to phenotypic divergences along environmental gradients in widespread invasive plants? A meta-analysis. Oikos 125: 905–917. [Google Scholar]
- Levanic T, Matjaz C, McDowell NG (2011) Associations between growth, wood anatomy, carbon isotope discrimination and mortality in a Quercus robur forest. Tree Physiol 31: 298–308. [DOI] [PubMed] [Google Scholar]
- Macnair MR. (1991) Why the evolution of the resistance to anthropogenic toxins normally involves major gene changes—the limits to natural-selection. Genetica 84: 213–219. [Google Scholar]
- Manion PD. (1991) Tree Disease Concepts, Ed 2 Prentice-Hall, Upper Saddle River, NJ, USA, 416p. [Google Scholar]
- Mayonde SG, Cron GV, Gaskin JF, Byrne MJ (2016) Tamarix (Tamaricaceae) hybrids: the dominant invasive genotype in southern Africa. Biol Invasions doi:10.1007/s10530-016-1249-4. [Google Scholar]
- McDowell NG, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG, et al. (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought. New Phytol 178: 719–739. [DOI] [PubMed] [Google Scholar]
- Moore J-S, Hendry AP (2009) Can gene flow have negative demographic consequences? Mixed evidence from stream threespine stickleback. Philos Trans R Soc Lond B Biol Sci 364: 1533–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59: 651–681. [DOI] [PubMed] [Google Scholar]
- Nagler PL, Glenn EP, Jarnevich CS, Shafroth PB (2011) Distribution and abundance of Saltcedar and Russian Olive in the Western United States. Crit Rev Plant Sci 30: 508–523. [Google Scholar]
- Nardini A, Lo Gullo MA, Salleo S (2011) Refilling embolized xylem conduits: is it a matter of phloem unloading. Plant Sci 180: 604–611. [DOI] [PubMed] [Google Scholar]
- Oduor AMO, Leimu R, van Kleunen M (2016) Invasive plant species are locally adapted just as frequently and at least as strongly as native plant species. J Ecol 104: 957–968. [Google Scholar]
- Pattison RR, D'Antonio CM, Dudley TL, Allander KK, Rice B (2011) Early impacts of biological control on canopy cover and water use of the invasive saltcedar tree (Tamarix spp.) in western Nevada, USA. Oecologia 165: 605–616. [DOI] [PubMed] [Google Scholar]
- Plaut JA, Yepez EA, Hill J, Pangle R, Sperry JS, :Pockman WT, McDowell NG (2012) Hydraulic limits preceding mortality in a piñon-juniper woodland under experimental drought. Plant Cell Environ. 35: 1601–1617. [DOI] [PubMed] [Google Scholar]
- Pockman WT, Sperry JS (2000) Vulnerability to xylem cavitation and the distribution of Sonoran desert vegetation. Am J Bot 87: 1287–1299. [PubMed] [Google Scholar]
- Powers RF, Reynolds PE (1999) Ten-year responses of ponderosa pine plantations to repeated vegetation and nutrient control along an elevation gradient. Can J For Res 29: 1027–1038. [Google Scholar]
- Rice KJ, Black RA, Radamaker G, Evans RD (1992) Photosynthesis, growth, and biomass allocation in habitat ecotypes of cheatgrass (Bromus tectorum). Funct Ecol 6: 32–40. [Google Scholar]
- Raven JA. (1985) Regulation of pH and generation of osmolarity in vascular plants: a cost-benefit analysis in relation to efficiency of use of energy, nitrogen and water. New Phytol 101: 25–77. [DOI] [PubMed] [Google Scholar]
- Robinson TW. (1965) Introduction, spread, and area extent of saltcedar (Tamarix) in the western states. US Geo Survey Profes Paper 491-A.
- Sala A, Hoch G (2009) Height-related growth declines in ponderosa pine are not due to carbon limitation. Plant Cell Environ 32: 22–30. [DOI] [PubMed] [Google Scholar]
- Sala A, Woodruff DR, Meinzer F (2012) Carbon dynamics in trees: feast or famine. Tree Physiol 32: 764–775. [DOI] [PubMed] [Google Scholar]
- Savolainen O, Pyhäjärvi T, Knürr (2007) Gene flow and local adaptation in trees. Ecol Evol Syst 38: 595–619. [Google Scholar]
- Seager R, Ting M, Held I, Kushnir Y, Lu J, Vecchi G, Huang HP, Harnik N, Leetmaa A, Lau NC, et al. (2007) Model projections of an imminent transition to a more arid climate in Southwestern North America. Science 316: 1181–1184. [DOI] [PubMed] [Google Scholar]
- Sevanto S, McDowell NG, Dickman TL, Pangle R, Pockman WT (2014) How do trees die? A test of the hydraulic failure and carbon starvation hypothesis. Plant Cell Environ. 37: 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton JP, McKay JK, Sala A (2002) Plasticity and genetic diversity may allow saltcedar to invade cold climates in North America. Ecol Appl 12: 1652–1660. [Google Scholar]
- Shafroth PB, Auble GT, Stromberg JC, Patten DT (1998) Establishment of woody riparian vegetation in relation to annual patterns of streamflow, Bill Williams River, Arizona. Wetlands 18: 577–590. [Google Scholar]
- Shafroth PB, Cleverly JR, Dudley TL, Taylor JP, Van Riper C, Weeks EP, Stuart JN (2005) Control of Tamarix in the Western United States: implications for water salvage, wildlife use, and riparian restoration. Environ Manage 35: 231–246. [DOI] [PubMed] [Google Scholar]
- Sher A. (2013) Introduction to the paradox plant In Sher A, Quigley MF, eds, Tamarix: A Case Study of Ecological Change in the American West. Oxford University Press, New York, pp 1–20. [Google Scholar]
- Sher AA, Marshall DL (2003) Seedling competition between native Populus deltoides (Salicaceae) and exotic Tamarix ramosissima (Tamaricaceae) across water regimes and substrate types. Am J Bot 90: 413–422. [DOI] [PubMed] [Google Scholar]
- Smith AM, Stitt M (2007) Coordination of carbon supply and plant growth. Plant Cell Environ 30: 1126–1149. [DOI] [PubMed] [Google Scholar]
- Storey R, Thompson WW (1994) An X-ray microanalysis study of salt-glands and intracellular calcium crystals of Tamarix. Ann Bot 73: 307–313. [Google Scholar]
- Strauss SY, Agrawal AA (1999) The ecology and evolution of plant tolerance to herbivory. Trends Ecol Evol 14: 179–185. [DOI] [PubMed] [Google Scholar]
- van der Werf GR, Randerson JT, Giglio L, Collatz GJ, Mu M, Kasibhatla PS, Morton DC, DeFries RS, Jin Y, van Leewen TT (2010) Global fire emissions and the contribution of deforestation, savanna, forest, agricultural, and peat fires (1997–2009). Atmos Chem Phys 10: 11707–11735. [Google Scholar]
- Williams GC. (1966) Natural selection, the costs of reproduction, and a refinement of Lack's principle. Am Nat 100: 687–690. [Google Scholar]
- Williams WI, Friedman JM, Gaskin JF, Norton AP (2014) Hybridization of an invasive shrub affects tolerance and resistance to defoliation by a biological control agent. Evol Appl 7: 381–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff DR, Meinzer FC (2011) Water stress, shoot growth and storage and non-structural carbohydrates along a tree height gradient in a tall conifer. Plant Cell Environ 34: 1920–1930. [DOI] [PubMed] [Google Scholar]
- Yanchuk AD, Murphy JC, Wallin KF (2008) Evaluation of genetic variation of attack and resistance in lodgepole pine in the early stages of a mountain pine beetle outbreak. Tree Genet Genom 4: 171–180. [Google Scholar]
- Zavaleta E. (2000) The economic value of controlling an invasive shrub. Ambio 29: 462–467. [Google Scholar]
- Zhang J, Marshall JD, Jaquish BC (1993) Genetic differentiation in carbon isotope discrimination and gas exchange in Pseudotsuga menziesii. Oecologia 93: 80–87. [DOI] [PubMed] [Google Scholar]