Abstract
Geraniaceae have emerged as a model system for investigating the causes and consequences of variation in plastid and mitochondrial genomes. Incredible structural variation in plastid genomes (plastomes) and highly accelerated evolutionary rates have been reported in selected lineages and functional groups of genes in both plastomes and mitochondrial genomes (mitogenomes), and these phenomena have been implicated in cytonuclear incompatibility. Previous organelle genome studies have included limited sampling of Geranium, the largest genus in the family with over 400 species. This study reports on rates and patterns of nucleotide substitutions in plastomes and mitogenomes of 17 species of Geranium and representatives of other Geraniaceae. As detected across other angiosperms, substitution rates in the plastome are 3.5 times higher than the mitogenome in most Geranium. However, in the branch leading to Geranium brycei/Geranium incanum mitochondrial genes experienced significantly higher dN and dS than plastid genes, a pattern that has only been detected in one other angiosperm. Furthermore, rate accelerations differ in the two organelle genomes with plastomes having increased dN and mitogenomes with increased dS. In the Geranium phaeum/Geranium reflexum clade, duplicate copies of clpP and rpoA genes that experienced asymmetric rate divergence were detected in the single copy region of the plastome. In the case of rpoA, the branch leading to G. phaeum/G. reflexum experienced positive selection or relaxation of purifying selection. Finally, the evolution of acetyl-CoA carboxylase is unusual in Geraniaceae because it is only the second angiosperm family where both prokaryotic and eukaryotic ACCases functionally coexist in the plastid.
Keywords: accD, clpP, rpoA, gene duplication, positive selection, accelerated substitution rate, cytonuclear incompatibility
Introduction
Plant cells harbor two endosymbiotic organelles (plastids and mitochondria) that have retained their own genomes (Lang et al. 1999; Keeling 2010). In the early stages of endosymbiosis the two showed parallel evolutionary trajectories that have shaped their genomes, including genome reduction, massive gene loss, DNA transfer to the host nuclear genome and functional replacement of organelle genes (Timmis et al. 2004). The majority of organelle proteins are nuclear-encoded, synthesized in the cytosol and then transported into the organelles (Timmis et al. 2004; Kleine et al. 2009). Many of these nuclear genes are involved in the maintenance of organelle genome stability (Day and Madesis 2007; Sloan and Taylor 2012), and a number are targeted to both plastids and mitochondria (Maréchal and Brisson 2010). Although many proteins involved in DNA replication, repair, and recombination (DNA-RRR) are common to both plastids and mitochondria (Carrie and Small 2013), their genomes exhibit variability in architecture and rates of nucleotide substitution.
Among angiosperms, the contrast between the two organelle genomes is particularly sharp when considering structural organization, which appears to be much more complicated in mitochondria compared with plastids (Mower et al. 2012; Ruhlman and Jansen 2014). For example, plant mitochondrial genomes (mitogenomes) contain an abundance of noncoding sequence, including repetitive and foreign DNA, and have a dynamic, multipartite organization due to active recombination between repeat regions. In contrast, most plastid genomes (plastomes) typically have a quadripartite structure with an inverted repeat (IR) separated by large and small single copy (LSC and SSC) regions. However, several lineages, such as Campanulaceae, Ericaceae, Fabaceae, Geraniaceae, and Oleaceae, have experienced extreme and rapid divergence in plastome architecture (Chumley et al. 2006; Lee et al. 2007; Haberle et al. 2008; Cai et al. 2008; Blazier et al. 2011; Guisinger et al. 2011; Fajardo et al. 2013; Martínez-Alberola et al. 2013; Weng et al. 2014; Ruhlman et al. 2017).
Plastomes and mitogenomes also exhibit nucleotide substitution rate variation. Plastid substitution rates are generally higher than those in the mitochondria of seed plants (Wolfe et al. 1987; Drouin et al. 2008; Richardson et al. 2013). Examples of mitochondrial-specific rate accelerations have been documented, including gene-specific heterogeneity in synonymous substitution rates within the genome in a number of angiosperm lineages: Geraniaceae, Ajuga, Plantago, Silene, and Viscum (Cho et al. 2004; Parkinson et al. 2005; Mower et al. 2007; Sloan et al. 2012a; Zhu et al. 2014; Skippington et al. 2015). However, only two studies in Silene and Ajuga (Sloan et al. 2012b; Zhu et al. 2014) have compared substitution rates of plastid and mitochondrial gene sequences across the entire genomes to assess the evolutionary parallels between the organelle genomes.
The functional transfer of plastid and mitochondrial genes to the nucleus is an ongoing evolutionary process (Timmis et al. 2004; Kleine et al. 2009). In angiosperms, the majority of recent, functional relocations has involved the transfer of mitochondrial genes to the nucleus (Adams and Palmer 2003; Liu et al. 2009; Park et al. 2014, 2015a), with multiple independent transfers of only four plastid genes documented (infA, rpl22, rpl32, and accD) (Millen et al. 2001; Ueda et al. 2007; Magee et al. 2010; Sabir et al. 2014; Park et al. 2015b). Although the role of selection pressure in intracellular gene transfer (IGT) remains uncertain, rates of organelle mutation accumulation could influence selection for IGT to the nucleus (Brandvain and Wade 2009).
Substitution of a nuclear-encoded protein to complement pseudogenization or loss of an organelle-encoded protein has also been documented. Most gene substitutions involve nuclear gene duplication, with one copy supplying the cytosol and the other supplying the organelle(s). For example, the product of the nuclear rpl23 gene in Spinacia oleracea and Geranium maderense substitutes for the missing plastid rpl23 gene product (Bubunenko et al. 1994; Weng et al. 2016). A different kind of substitution has been documented for accD in Brassicaceae (Schulte et al. 1997) and Poaceae (Konishi et al. 1996; Gornicki et al. 1997). With the loss of plastid-encoded accD, the eukaryotic monomeric ACC gene product substituted the function of the multisubunit plastid ACCase.
Gene substitutions have occurred through different mechanisms. A protein already targeted to an organelle could be duplicated and the copy could undergo transit peptide evolution. For example, a duplicate copy of nuclear-encoded Rps16 that substitutes for the mitochondrial-encoded protein in Medicago and Populus was recruited through adaptation of its transit peptide to allow import into the plastid as well (Ueda et al. 2008). Likewise, transit peptide recruitment has facilitated replacement of proteins encoded in the mitochondria with nuclear-encoded proteins of plastid origin, which now serve both compartments. Examples include Rpl10 in Brassicaceae and monocots (Kubo and Arimura 2010) and Rps13 in Arabidopsis (Mollier et al. 2002).
Geraniaceae organelle genomes offer an excellent system to study the evolutionary dynamics of structural organization and rates of nucleotide substitution. Sequencing of organelle genomes from this family has provided unprecedented examples of genomic change and highly accelerated nucleotide substitution rates, and improper DNA-RRR machinery has been suggested to cause this syndrome (Parkinson et al. 2005; Guisinger et al. 2008, 2011; Blazier et al. 2011, 2016a; Weng et al. 2014). Geranium plastomes exhibit extreme reconfiguration including reduction of the IR, operon disruption, extensive gene duplication and a high frequency of repetitive DNA (Guisinger et al. 2011; Zhang et al. 2016). Exploration of the evolutionary phenomena in Geranium mitogenomes revealed extensive horizontal gene transfer (HGT), IGT, and loss of introns and RNA editing sites (Park et al. 2015a). In particular, two Geranium species, Geranium brycei and Geranium incanum, contain considerable foreign organellar DNA from diverse eudicots, including many parasitic plants.
Little is known, however, about nucleotide substitution rates within Geranium organelle genomes. To assess rates of nucleotide substitution of protein coding genes and the relationship between Geranium plastome and mitogenome evolution, we analyzed 98 plastid and mitochondrial genes of 17 Geranium species and representatives of other genera of Geraniaceae. We examined whether substitution rates were elevated and variable within the two organelle genomes and assessed the rate correlation between them. In addition, we characterized accD and clpP in the plastids of Geraniaceae and correlate structural variation with substitution rates in these genes. We document the evolutionary fate of the plastid-encoded accD and discuss the complex evolutionary history of Acetyl-Co-A carboxlase in Geraniaceae.
Materials and Methods
Organelle Gene Sequence Analysis
To compare rates of sequence evolution in plastid and mitochondrial genes of Geranium, protein-coding genes from 17 species spanning all three currently recognized subgenera (Erodioidea, Geranium, and Robertium; Aedo et al. 1998) were selected along with the three outgroup taxa California macrophylla, Erodium texanum and Monsonia emarginata from Geraniaceae (supplementary table S1, Supplementary Material online). DNA isolation and Illumina sequencing methods were described in Park et al. (2015a). Plastid protein-coding genes for the 17 Geranium species were obtained by de novo assembly of paired-end Illumina reads with Velvet v. 1.2.08 (Zerbino and Birney 2008) using multiple k-mer sizes from 69 to 91 at the Texas Advanced Computing Center (TACC). Plastid genes were identified in each draft genome by performing BlastN searches using BLAST+ v.2.2.31 (Camacho et al. 2009) against published protein-coding genes from G. palmatum (NC_014573) and related Geraniaceae as query sequences. For mitochondrial protein-coding genes, previously published gene sequences of 17 species of Geranium and the outgroups C. macrophylla, E. texanum and M. emarginata were used (Park et al. 2015a). Genbank accession numbers for both mitochondrial and plastid sequences of all species are in supplementary table S1, Supplementary Material online.
Estimation of Substitution Rate Variation
Ninety-eight organelle protein-coding genes were shared by all 20 taxa sampled (supplementary table S2, Supplementary Material online). Individual gene alignments were generated based on “back-translate” with MAFFT v. 7.017 (Katoh et al. 2002) in Geneious R6 v. 6.1.8 (Biomatters Ltd., Auckland, New Zealand, http://www.geneious.com) and all shared organelle protein-coding genes were concatenated into a single alignment. The N-terminal portion of the clpP was extremely divergent so only the conserved domain of the gene was used. The 5′ end of matK was also removed due to unalignable sequences. In Geranium phaeum and Geranium reflexum, which contain two distinct copies of clpP and rpoA, the two copies were arbitrarily named copies 1 and 2 and only the first copy (G. phaeum1 and G. reflexum1) was used for rate analyses. Phylogenetic relationships from the concatenated sequence were estimated using maximum likelihood methods in RAxML v. 7.2.8 (Stamatakis 2006) with the “GTRGAMMA” model under the rapid bootstrap algorithm (1,000 replicates) on TACC and the resulting ML tree used as the constraint tree.
To estimate rates of nucleotide substitution, nonsynonymous (dN) and synonymous (dS) rates were calculated in PAML v. 4.8 (Yang 2007) using the codeml option with codon frequencies estimated with the F3 × 4 model. Gapped regions were removed by using “cleandata = 1” option. Rate estimations were performed on the following data sets: 1) each concatenated plastid and mitochondrial data set, 2) concatenated gene sets for the functional groups ATP synthase (atp), cytochrome b6f (pet), cytochrome c oxidase (cox), cytochrome c maturation (ccm), NADH-plastoquinone oxidoreductase (ndh), NADH-ubiquinone oxidoreductase (nad), photosystem I (psa), photosystem II (psb), RNA polymerase (rpo), and small (rps) and large (rpl) ribosomal subunits, and 3) all individual genes from both organelles. For accD and clpP, extensive comparisons of dN and dS were performed to investigate the relationship between the rates of nucleotide substitution and structural divergence of across selected angiosperms (supplementary table S3, Supplementary Material online). Five plastid genes (atpB, ndhF, psaA, psbA, and rbcL) were chosen to generate phylogenetic trees, which were used as a constraint tree for rate comparisons of clpP and accD genes (supplementary figs. S1 and S2, Supplementary Material online). Only the conserved domains of clpP and accD were used because other regions of the genes were too divergent to generate reliable nucleotide alignments.
To test for evidence of selection acting on duplicated genes, dN/dS was calculated. Likelihood ratio tests (LRTs) were performed to compare the goodness fit of two models, H0 (dN/dS is fixed across entire tree) and H1 (dN/dS allowed to be different in a given clade). Statistical analyses were conducted by using R v. 3.2.0 (R Development Core Team 2015) and the Bonferroni correction for multiple comparisons was applied.
Prediction of Biological Features and Structures in Genes and Proteins
The effective number of codons (ENC) and GC content at synonymous third codon positions (GC3S) for each organelle gene were calculated using CodonW (http://codonw.sourceforge.net). The NCBI Conserved Domain Database (CDD v. 3.14) was used for conserved domain annotation (Marchler-Bauer et al. 2015). Gene conversion was examined using GENECONV in the Recombination Detection Program (RDP) v. 4.56b (Martin et al. 2015). Previously assembled transcriptomes (Ruhlman et al. 2015; Zhang et al. 2015) for three Geranium species and the other genera from Geraniales were used to identify IGT to the nucleus or substitution of a nuclear gene for a plastid gene. Transferred or substituted genes were identified using BLAST+ v.2.2.31 (Camacho et al. 2009) by performing “TBlastN” of the ACC sequences from Arabidopsis thaliana (ACC1, ACC2, accA, accB, and accC, NC_003070; accD, NC_000932) against the transcriptomes. Organelle transit peptides were predicted using TargetP v.1.1 (Emanuelsson et al. 2007). Phylogenetic analysis was performed on data set of ACC homologs (supplementary table S4, Supplementary Material online). Divergence time was estimated by Bayesian MCMC analysis using BEAST v.2.1.3 (Bouckaert et al. 2014) following the method described in Park et al. (2015a).
Results
Nucleotide Substitution Rates in Geranium Organelle Genes
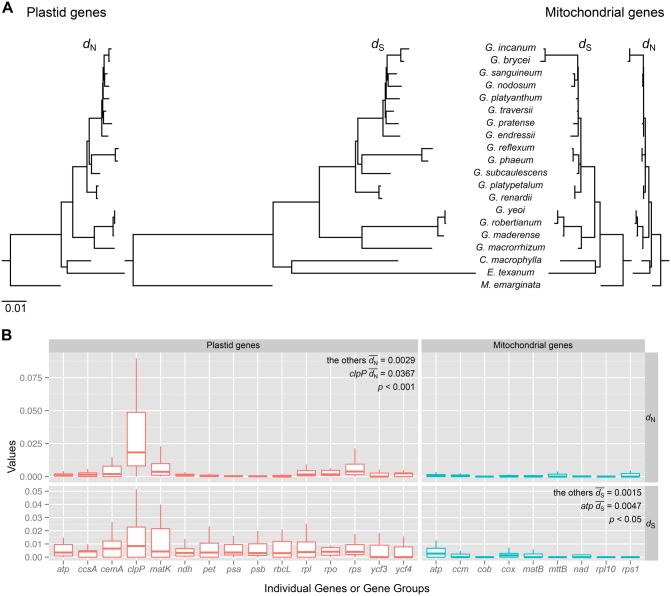
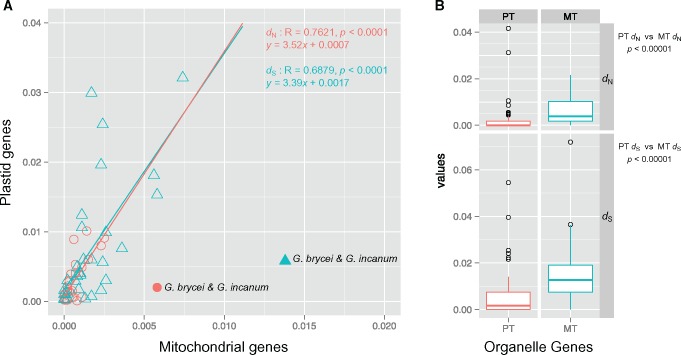
All 17 Geranium species and representative species of the related Geraniaceae genera California, Erodium, and Monsonia shared 72 plastid-encoded and 26 mitochondrial-encoded genes (supplementary table S2, Supplementary Material online). To examine rate variation in organelle genes among Geranium species, nonsynonymous (dN) and synonymous (dS) substitution rates were estimated using a constraint tree generated by phylogenetic analyses of a concatenated data set of all 98 genes (supplementary fig. S3A, Supplementary Material online). Estimates of nucleotide substitution rates in Geranium showed that plastid genes generally evolved faster than mitochondrial genes in both dN and dS (figs. 1 and 2). Linear regression of plastid and mitochondrial substitution rates for all branches, except for the branch leading to G. brycei and G. incanum, showed that plastid rates are 3.5 times faster for dN and 3.4 times faster for dS than mitochondrial rates (fig. 2A). A significant correlation between the organelles was found for both dN and dS (P < 0.0001, fig. 2A). Examination of rate variation in individual organelle genes or gene groups revealed both gene-specific and lineage-specific accelerations in substitution rates within Geranium (fig. 1B [see supplementary fig. S4, Supplementary Material online, for outlier information], supplementary figs. S5–S7, Supplementary Material online). For example, the average value of dN for the plastid-encoded clpP gene was ∼3 times higher than other plastid-encoded genes (fig. 1B), and this difference was significant (pairwise Wilcoxon rank-sum test, P < 0.001 after Bonferroni correction; supplementary table S5, Supplementary Material online). A significant difference of dS for mitochondrial-encoded atp gene group was detected compared with most other mitochondrial-encoded genes or gene groups (supplementary table S6, Supplementary Material online). The rate of synonymous substitutions in atp6 and atp9 were highest among mitochondrial genes across Geranium species (supplementary fig. S5, Supplementary Material online). Accelerated dS was detected for several other mitochondrial genes in three lineages, A2, A4, and B (shaded in gray in supplementary fig. S7, Supplementary Material online). Lineage-specific accelerations of dN of the plastid genes cemA, clpP, matK and genes encoding ribosomal proteins and RNA polymerase subunits were observed within clades A, A1, A2, and B (supplementary fig. S6, Supplementary Material online).
Fig. 1.
—Organelle gene divergence among 17 species of Geranium. (A) Plastid and mitochondrial phylograms of nonsynonymous (dN) or synonymous (dS) substitution rates based on 72 plastid and 26 mitochondrial genes, respectively. All trees are drawn to the same scale and scale bar indicates the number of substitutions per site. C., California; E., Erodium; G., Geranium; M., Monsonia. (B) Box plots of the values of dN and dS for Geranium plastid (red) and mitochondrial (blue) individual genes or functional groups of genes (see supplementary table S2, Supplementary Material online). The box represents values between quartiles, solid lines extend to minimum and maximum values, horizontal lines in boxes show median values but outliers were eliminated (see supplementary fig. S4 for outlier information, Supplementary Material online). Significance of fit was evaluated by pairwise Wilcoxon rank sum tests in the R package.
Fig. 2.
—Plastid and mitochondrial sequence divergence among Geranium species. (A) Correlation of nonsynonymous (dN, red circles) and synonymous (dS, blue triangles) substitution rates between each shared branch in the phylogram shown in supplementary fig. S3A, Supplementary Material online. Linear regression analyses included all branches except for the branch leading to G. brycei and G. incanum (closed circle and triangle). (B) Boxplot distribution of the dN and dS values for all individual organelle genes from G. brycei and G. incanum. The box represents values between quartiles, solid lines extend to minimum and maximum values, outliers are shown as circles and horizontal lines in boxes show median values. Significance of fit was evaluated by Pearson’s correlation and pairwise Wilcoxon rank sum tests in the R package. PT, plastid; MT, mitochondrion.
Comparison of the effective number of codons (ENC) and GC content at synonymous third codon positions (GC3S) showed that most Geranium plastid genes fall below Wright’s curve (Wright 1990), reflecting low GC content at synonymous third codon positions (supplementary fig. S8A, Supplementary Material online). Mitochondrial genes were consistent with the plastid results except for matR, which has GC3S values between 53.2% and 55.4% (supplementary fig. S8B, Supplementary Material online). The accelerated mitochondrial atp9 gene was below Wright’s curve (supplementary fig. S8B, Supplementary Material online), and showed a significant negative correlation between GC3S and dS (Pearson’s correlation, R = −0.7993, P < 0.001; supplementary fig. S9A, Supplementary Material online) and a minor but not significant increase in ENC relative to other genes (supplementary fig. S9B, Supplementary Material online).
Elevated and Variable Mitochondrial Substitution Rates in a Specific Geranium Lineage
Substitution rates of organelle genes in the G. brycei/G. incanum clade showed the opposite pattern from other Geranium species in which the plastid genes had much higher rates than mitochondrial genes (figs. 1A and 2). The dN and dS of mitochondrial genes from G. brycei and G. incanum were 2.9 times and 2.4 times higher than plastid genes, respectively, and this difference was significant (Wilcoxon rank-sum test, P < 0.00001; fig. 2B). LRTs indicated that dN/dS values for mitochondrial-encoded ccmFn, matR, and nad2 genes were significantly different in the G. brycei/G. incanum clade compared with other Geranium mitochondrial genes (supplementary table S7, Supplementary Material online).
To address whether accelerated nucleotide substitution rates in G. brycei and G. incanum were affected by HGT (i.e., these two species contain intact native and foreign copies of atp8, cox2, nad2, and rps1; Park et al. 2015a), native copies were compared with their orthologs from other Geranium species. Significant differences in rate variation were identified in six of the eight comparisons; the two exceptions were dS of atp8 and dN of cox2 (Wilcoxon rank-sum test, P < 0.05; supplementary fig. S10, Supplementary Material online).
Elevated Substitution Rates Following Duplication of Plastid-Encoded clpP and rpoA Genes
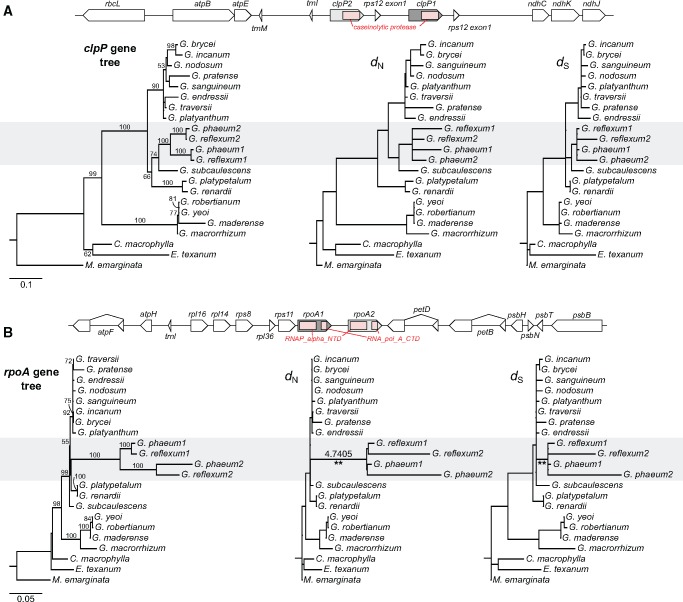
Both G. phaeum and G. reflexum contained two divergent copies of clpP (75.2% and 75.8% nucleotide identity between paralogs) and rpoA (88.5% and 87.8% nucleotide identity between paralogs) genes in the plastome single copy (SC) region, and all copies include complete conserved domains (fig. 3). No gene conversion between the paralogs of clpP and rpoA genes was detected by GENECONV (Martin et al. 2015). Phylogenetic analyses of these copies with other Geranium orthologs indicated that the extra copies derived from duplication events that occurred in the common ancestor of G. phaeum and G. reflexum (fig. 3). To examine sequence evolution of the duplicated genes, pairwise comparisons of dN and dS among their homologs were computed using a constraint tree (supplementary fig. S3B, Supplementary Material online). Branch lengths of dN for clpP for G. phaeum1 and G. reflexum1 were 2.0 times and 2.4 times longer than their paralogs G. phaeum2 and G. reflexum2, whereas dS branch lengths for G. phaeum2 and G. reflexum2 were 7.5 times and 7.4 times longer than their paralogs, respectively (fig. 3A). Fourteen branches with dN/dS > 1 were detected (shown in red font in supplementary table S8, Supplementary Material online) but LRTs showed that none of them were significantly different (P = 1.00 after Bonferroni correction, supplementary table S8, Supplementary Material online). dN branches for G. phaeum2 and G. reflexum2 rpoA were 15.4 times and 9.6 times longer than their paralogs G. phaeum1 and G. reflexum1 and dS branches were 21.5 times and 7.0 times longer, respectively (fig. 3B). Six branches with dN/dS >1 were detected (shown in red font in supplementary table S9, Supplementary Material online). LRTs showed that only one branch, leading to G. phaeum and G. reflexum, had a significantly different dN/dS for rpoA (P < 0.001 after Bonferroni correction, supplementary table S9, supplementary fig. S3b, Supplementary Material online), indicating positive selection (dN/dS = 4.7) in the ancestor of these two species. Lineage-specific rate accelerations of the other three plastid-encoded polymerase (PEP) genes rpoB, rpoC1, and rpoC2 were observed on the branch leading to G. phaeum and G. reflexum compared with their sister species Geranium subcaulescens (supplementary fig. S11, Supplementary Material online), although among the four PEP genes only rpoB had a significantly different dN/dS in LRTs (P < 0.05 after Bonferroni correction; supplementary table S10, Supplementary Material online).
Fig. 3.
—Duplication of plastid-encoded clpP and rpoA genes in G. phaeum and G. reflexum. (A) Schematic diagram of the genomic regions surrounding the plastid clpP homologs from G. phaeum and G. reflexum. Pink box indicates conserved domain (caseinolytic protease). Maximum likelihood (ML) gene tree (left) based on clpP nucleotide sequences. ML phylograms used the constraint tree in supplementary fig. S3B, Supplementary Material online to show nonsynonymous (dN) or synonymous (dS) substitution rates of clpP (middle, right, respectively). (B) Schematic diagram of the genomic regions surrounding the rpoA homologs from G. phaeum and G. reflexum plastomes. Pink box indicates two conserved domains (RNA_alpha_NTD and RNA_pol_A_CTD). ML gene tree (left) based on rpoA nucleotide sequences. ML phylograms used the constraint tree in supplementary fig. S3B, Supplementary Material online to show dN and dS of rpoA (middle, right, respectively). Branches with significantly higher dN/dS (4.7405) detected by likelihood ratio test (LRT) are marked with two asterisks (P < 0.001 after Bonferroni correction; supplementary table S9, Supplementary Material online). Clade A2 within Geranium (supplementary fig. S3A, Supplementary Material online) that has duplication events is highlighted in gray. The numbers “1” and “2” after each species in the phylograms represent paralogs for clpP and rpoA. Bootstrap support values >50% are shown on the branches.
Structural Evolution of Plastid-Encoded clpP Gene
Geranium and Monsonia plastomes contain clpP-like open reading frames (ORFs) that are intronless but retain conserved domains (supplementary fig. S12A, Supplementary Material online). The first 71 nucleotides of the ORFs is a highly divergent region that represents the former first exon (43–55% nucleotide sequence identity compared with Nicotiana tabacum, supplementary fig. S13, Supplementary Material online). The C-terminal portions of the ORFs included the conserved domain and the remaining sequence of the Geranium and Monsonia clpP-like ORFs was highly variable, ranging from 252 to 488 bp in length (supplementary fig. S12A, Supplementary Material online). The conserved C to U editing site of the clpP gene, which was characterized by Chateigner-Boutin et al. (2008), was also detected in the 3′ end of the sequence (supplementary fig. S14, Supplementary Material online). To investigate a correlation between structural evolution of the plastid-encoded clpP gene and substitution rates, dN and dS of clpP for representative angiosperms and Geraniales were calculated using sequences from the conserved domain. Multiple lineage-specific accelerations of dN and dS were detected within the groups that experienced the intron loss in clpP (supplementary fig. S12B, Supplementary Material online). The dN and dS values were more strongly correlated in the groups that contain structural changes in clpP (Pearson’s correlation, R = 0.8985, P < 0.0001) than in other angiosperms (R = 0.7704, P < 0.0001), and Wilcoxon rank-sum test showed that dN is significantly different between these groups (dN, P < 0.001; dS, P = 0.0708; supplementary fig. S15A, Supplementary Material online). LRTs showed that the branches leading to clades A2 (branch 18), A3 (branch 24), B (branch 32), and California (branch 34) had significantly different dN/dS, and that the branches within Geraniaceae including Geranium also had significantly different dN/dS (indicated in red font in supplementary table S11, Supplementary Material online).
Interruption of Plastid-Encoded accD
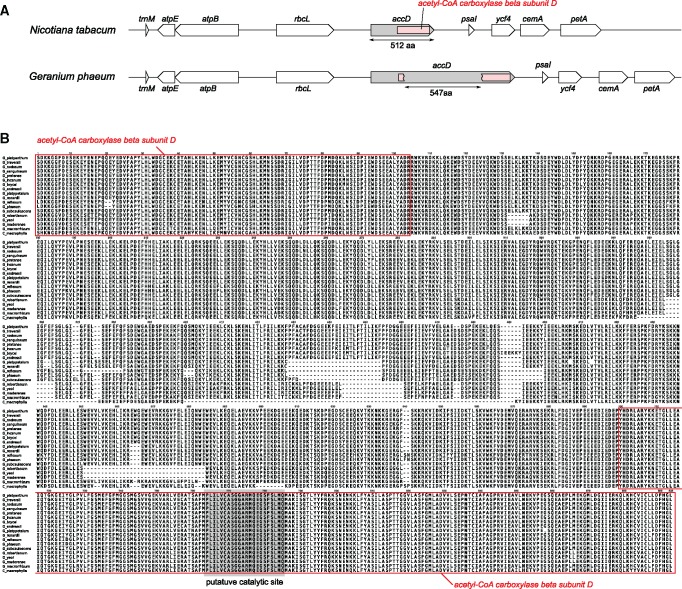
Plastomes of all Geranium species examined and C. macrophylla have an expanded acetyl-CoA carboxylase subunit β (accD) ORF compared with N. tabacum (1,539 bp), ranging in size from 2,748 bp in C. macrophylla to 3,576 bp in G. brycei. The conserved domain of accD is split into two portions by a large insertion of 547 amino acids (fig. 4). Interruption of the conserved domain was also detected in accD-like ORFs from three other genera of Geraniales (i.e., Pelargonium alternans, NC_023261; Pelargonium cotyledonis, KM459516; and Viviania marifolia, NC_023259).
Fig. 4.
—Characterization of the plastid-encoded acetyl-CoA carboxylase beta subunit (accD) genes of Geranium. (A) Schematic diagram of the genomic region surrounding accD in the G. phaeum plastome compared with Nicotiana tabacum. Boxes inside accD gene (gray) indicate the conserved domain (acetyl-CoA carboxylase beta subunit; pink). (B) Amino acid sequence alignment of plastid-encoded acetyl-CoA carboxylase beta subunit conserved domain including the insertion regions from 17 Geranium species and California macrophylla. Red boxes indicate the conserved domain of acetyl-CoA carboxylase beta subunit and shaded gray box indicates well-known putative catalytic site (Lee et al. 2004).
To investigate the effect of the insertion on nucleotide substitution rates of accD, dN, and dS were calculated for Geraniaceae and representative angiosperms using only the conserved domain sequences. Higher values of dN and dS were identified in accD genes on branches leading to Geraniaceae and Viviania compared with other angiosperms (supplementary fig. S16, Supplementary Material online). Both dN and dS values were more strongly correlated in the divergent groups with the interrupted accD conserved domain (Pearson’s correlation, R = 0.9699, P = 0.0301) than in angiosperms with intact domains (R = 0.6598, P < 0.0001), and Wilcoxon rank-sum test showed that branch values of dN and dS were significantly different between the two groups (dN, P < 0.001; dS, P < 0.001; supplementary fig. S15B, Supplementary Material online).
Coordination of Acetyl-CoA Carboxylase Gene Function between Plastid and Nuclear Genomes
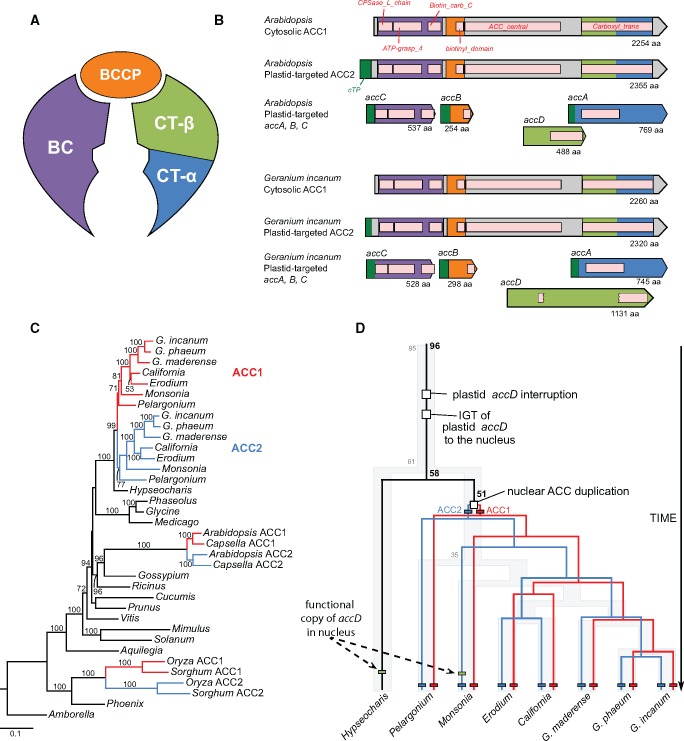
Examination of transcriptomes (Ruhlman et al. 2015; Zhang et al. 2015) provided evidence that all Geraniaceae except Hypseocharis contained two transcripts for the nuclear, eukaryotic acetyl-CoA carboxylase (ACC) gene (ACC1 and ACC2; fig. 5). One of these copies (arbitrarily referred to as ACC2) has an extended sequence at the 5′ end predicted by TargetP to be a plastid transit peptide in the product (supplementary table S12, Supplementary Material online). Three nuclear-encoded transcripts (accA, accB, and accC) for plastid-targeted proteins similar to Arabidopsis homologs were also detected across Geraniaceae (fig. 5B, supplementary table S12, Supplementary Material online). In contrast, an accD gene transfer from the plastid to the nucleus was identified in the Hypseocharis and Monsonia transcriptomes and the predicted ORF contained a transit peptide sequence (supplementary fig. S17, supplementary table S12, Supplementary Material online). The target peptides were 75 and 76 amino acids long in Hypseocharis and Monsonia, respectively, and their alignment length was 78 amino acids long with a sequence identity of 19.2% (supplementary fig. S17, Supplementary Material online). The nuclear-encoded accD of these two species contains a large insertion that interrupted the conserved domains (supplementary fig. S17A, Supplementary Material online), similar to the plastid-encoded accD in Geraniaceae (fig. 4).
Fig. 5.
—Evolutionary implications of acetyl-CoA carboxylase variation in Geraniaceae. (A) Schematic diagram of Acetyl-coenzyme A carboxylase (ACCase) composed of four subunits. BC = Biotin carboxylase (purple), BCCP = Biotin carboxyl carrier protein (orange), CT = Carboxyl transferase (alpha: blue, beta: green). (B) Architecture of ACCase from Geranium incanum compared with Arabidopsis thaliana. Colors indicate subunits of ACCase corresponding to the schematic diagram in (A). Pink boxes indicate the conserved domains of ACCase. Dark green boxes in N-terminus indicate a transit peptide. (C) Maximum likelihood (ML) gene tree based on nuclear-encoded ACC homologs among angiosperms. The numbers “1” and “2” after each species in the phylogram represents paralogs for ACC (red and blue). Bootstrap support values >50% are shown on the branches. (D) Summary of gene duplication and intracellular gene transfer (IGT) events with divergence times. See supplementary fig. S19, Supplementary Material online for more detailed divergence time estimates for Geraniaceae. The evolutionary events are marked with squares and are shown in colored boxes. Numbers at nodes indicate divergence time estimates in Ma. Background tree with numbers (gray) adapted from Park et al. (2015a). Color branches correspond to each ortholog (red, ACC1; blue, ACC2).
Phylogenetic analyses of nuclear-encoded ACC homologs from Geraniaceae showed that each of the orthologs grouped together (fig. 5C), suggesting that the duplication event occurred in the common ancestor of Geraniaceae excluding Hypseocharis (fig. 5D). Pairwise analysis of dN and dS for ACC homologs showed that the ACC1 ortholog had elevated dS and lower dN values (supplementary fig. S18, Supplementary Material online) and Wilcoxon rank-sum test showed that dN is significantly different between ACC orthologs (P = 0.01017, supplementary fig. S18B, Supplementary Material online). On the basis of the organismal divergence time in Geraniaceae (Park et al. 2015a), the interruption of the conserved domains of plastid accD occurred between 61 Ma and 95 Ma (fig. 5D). Divergence time estimates suggest that the nuclear ACC gene duplication occurred 51 Ma in Geraniaceae excluding Hypseocharis (fig. 5D;supplementary fig. S19, Supplementary Material online for more detailed divergence time estimates).
Discussion
Organelle sequencing across the angiosperm family Geraniaceae revealed accelerated substitution rates and rapid structural evolution in plastomes (Guisinger et al. 2008, 2011; Weng et al. 2014, 2017; Blazier et al. 2016b; Ruhlman et al. 2017) as well as highly accelerated substitution rates in mitogenomes (Parkinson et al. 2005, Bakker et al. 2006, Weng et al. 2012). However, because only one to three species of the largest genus Geranium with >400 species (Aedo et al. 1998) were included in previous studies, little is known about the evolution of their organelle genomes. In this study, we analyzed all shared protein coding genes from both plastomes and mitogenomes of 17 Geranium species and closely related species in the family to compare patterns of evolutionary rates in both organelles. We identified several unusual phenomena, including gene-specific and lineage-specific rate accelerations with one lineage showing significantly higher rates in mitochondrial genes than plastid genes, different rates of nucleotide substitutions in duplicate copies of plastid genes, a correlation between accelerated rates and structural variation in the plastid-encoded clpP gene and a complex evolutionary history of gene interruption, transfer and duplication of both the eukaryotic and prokaryotic acetyl-CoA carboxylase genes. Our discussion will focus on these unusual phenomena, which highlight the dynamic evolutionary history of Geranium organellar genomes.
Substitution Rate Variation in Geranium Organelle Genomes
Extensive variation in organelle substitution rates was detected in Geranium. Our study identified gene-specific patterns of rate variation in organelle protein coding genes (figs. 1B, supplementary figs. S6 and S7, Supplementary Material online). Among plastid genes, several had accelerated dN, including cemA, clpP, matK, and genes encoding large and small subunit ribosomal proteins and PEP subunits. These are the same plastid genes/functional groups that had significantly accelerated dN in a family-wide comparison (Guisinger et al. 2008). The most extreme case was clpP, which had significantly higher dN with a nearly 13-fold increase. In the case of mitochondrial-encoded genes, rate accelerations were restricted to synonymous sites with members of the atp functional group having a significantly higher dS with a 3-fold increase. This pattern of accelerated dN in plastid genes and dS in mitochondrial genes in Geranium is similar to that previously reported in Pelargonium, another large genus of Geraniaceae (Weng et al. 2012).
Lineage-specific accelerated rates of nucleotide substitutions occur in both genomes, which are especially evident from the longer branches in clades A2 and A4 that include G. incanum/G. brycei and G. phaeum/G. reflexum (fig. 1A, supplementary figs. S6 and S7, Supplementary Material online). The rate accelerations in mitochondrial and plastid genes occur in different clades indicating that different lineages are affected by rate variation in the two genomes. Previous studies have shown that substitution rate is generally three times slower in mitogenomes than in plastomes (Wolfe et al. 1987; Drouin et al. 2008). Some lineages (Geraniaceae, Ajuga, Plantago, and Silene) have experienced a substantial rate increase in the mitogenome (Cho et al. 2004; Parkinson et al. 2005; Mower et al. 2007; Sloan et al. 2008; Zhu et al. 2014). Geranium brycei and G. incanum exhibit accelerated rates in mitochondrial genes compared with their plastid counterparts (fig. 2B). This pattern contrasts with a previous study that showed that in most angiosperms nonsynonymous and synonymous substitution rates are 1.8 and 4.9 times faster in the plastome than the mitogenome, respectively (Zhu et al. 2014). Rate comparisons in Ajuga by Zhu et al. (2014) showed a similar pattern of higher rates of nucleotide substitutions in the mitogenome than the plastome. They suggested that localized hypermutation or mRNA retroprocessing was likely responsible for this phenomenon. A previous study in Geranium (Park et al. 2015a) showed that all examined Geranium mitochondrial genes had similar editing sites, suggesting that the accelerated rates in G. brycei and G. incanum genes are not caused by RNA editing frequency. The mitogenomes of these two species have acquired a number of foreign organelle genes (atp8, cox2, nad2, and rps1) via HGT (Park et al. 2015a). It is possible that the substitution rates correlate with gene conversion between the native and foreign copies, however, gene conversion was only found in the cox2 gene of G. brycei (Park et al. 2015a). The mitochondrial rate accelerations in G. brycei and G. incanum do not appear to be a gene-specific phenomenon because many genes show higher rates in these species (supplementary figs. S5 and S7, Supplementary Material online). Furthermore, accelerated rates of these same genes have occurred in several other Geranium lineages. One possible cause for this pattern in Geranium is dysfunction of the DNA-RRR machinery, which has been suggested previously in groups with accelerated mitochondrial rates (Parkinson et al. 2005; Mower et al. 2007; Sloan et al. 2012a). The correlation between nucleotide substitution rates and structural evolution in Geranium, particularly where mitochondrial-targeted DNA-RRR genes are involved, should be explored to test this hypothesis.
Rate Variation among Duplicated Plastid Genes
Most plastid gene duplications are caused by IR expansion (Jansen and Ruhlman 2012), and duplicate copies are homogenized by gene conversion during homologous recombination between the repeats. However, partial or complete gene duplications have been documented in SC regions in a few lineages including Geraniaceae (Guisinger et al. 2011; Ruhlman et al. 2017), Fabaceae (Cai et al. 2008; Sabir et al. 2014; Sveinsson and Cronk 2014), Campanulaceae (Haberle et al. 2008), and Caryophyllaceae (Erixon and Oxelman 2008). Geranium phaeum and G. reflexum plastomes each contain two divergent copies of clpP and rpoA genes in the LSC region (fig. 3). The phylogenetic placement of the duplicate copies indicates that clpP and rpoA were duplicated in the common ancestor of two species (fig. 3). On the basis of previously published divergence times (Park et al. 2015a), the duplication events occurred between 2.0 and 8.7 Ma.
Elevated substitution rates and positive selection or relaxed functional constraints are often associated with gene duplication (Ohta 1994). Duplicate copies of clpP and rpoA in G. phaeum and G. reflexum have experienced asymmetric rate divergence (fig. 3). Analysis of dN/dS for clpP did not detect positive selection on the branch leading to G. phaeum and G. reflexum (supplementary table S8, Supplementary Material online). In the case of rpoA, however, the branch leading to G. phaeum/G. reflexum had a dN/dS significantly >1 after Bonferroni-correction, indicating positive selection or relaxation of purifying selection acting on the gene in the ancestor of these two species (fig. 3B, supplementary table S9, Supplementary Material online). In Campanulastrum americanum (Campanulaceae) positive selection was also detected in clpP and some populations had a partial duplication of the first exon (Barnard-Kubow et al. 2014). The authors suggested that accelerated evolution in the plastome may contribute to cytonuclear incompatibility. This hypothesis was supported in a recent study of rapidly evolving plastid-nuclear protein complexes in Silene (Rockenbach et al. 2016).
Duplications of genes in the SC regions have been reported for several genes in unrelated groups of angiosperms and in some cases this involves the same two genes, clpP and rpoA, that are duplicated in Geranium. In two genera of the tribe Sileneae (Silene and Lychnis, Caryophyllaceae), high substitution rates were accompanied by gene duplications, loss of introns and positive selection in clpP (Erixon and Oxelman 2008). A similar pattern was observed in the G. phaeum/G. reflexum clade in which the tandemly duplicated clpP genes lack both introns. Another example of gene duplication in SC regions was recently reported for rpl20 in M. emarginata in Geraniaceae (Ruhlman et al. 2017). In this case, there are five copies included in a larger repeat in this IR-lacking species that have very high nucleotide sequence identity (96%) and there is GC biased gene conversion between the copies. Furthermore, replication dependent recombination between the rpl20 repeats produces alternative plastome arrangements. This species also has duplicated, intronless copies of clpP.
Duplication of rpoA has also been documented in Pelargonium but in this case duplicate copies reside in the IR (Blazier et al. 2016a; Weng et al. 2017). Up to six copies of rpoA are present in each IR in some species and although rates of nucleotide substitutions are very high and levels of amino acid sequence identity is as low as 19% compared with the outgroup Eucalyptus, this gene is experiencing purifying selection and functional domains remain intact, suggesting that the rpoA is functional in Pelargonium. In this case, the multiple copies are homogenized via gene conversion.
Evolution of the ATP-Dependent Clp Protease Proteolytic Subunit
In plant cells, the ATP-dependent Clp protease proteolytic subunit gene (clpP) that contains two introns is encoded in plastids and is involved in protein metabolism (Peltier et al. 2004; Stanne et al. 2009). Several angiosperm lineages have experienced loss or structural evolution (i.e., lack of first intron or both introns) of the plastid-encoded clpP (Erixon and Oxelman 2008; Martinez-Alberola et al. 2013; Sloan et al. 2014; Barnard-Kubow et al. 2014; Wang et al. 2016; Ruhlman et al. 2017). We identified an extremely divergent, intronless clpP gene that was previously annotated as a pseudogene or loss in Geranium and Monsonia plastomes (Guisinger et al. 2011; Weng et al. 2014). Intron loss may be explained by direct genomic deletion, exonization of introns, retroprocessing and HGT of an intronless version of a gene followed by gene conversion with native copies (reviewed in Hepburn et al. 2012). The most parsimonious interpretation for clpP intron loss in Geraniaceae involves the loss of the first intron in the common ancestor of Geranium, California, Erodium, and Monsonia followed by loss of the second intron independently in Geranium and Monsonia. The mechanism of the first intron loss is indiscernible from the present data, although two possible mechanisms are exonization of the intron or intron loss followed by an insertion. However, the second intron is likely to have been lost in Geranium and Monsonia through retroprocessing, which involves genetic integration of an intronless cDNA intermediate produced by reverse transcription of a spliced mRNA. This explanation is supported by the fact that Geranium and Monsonia clpP genes show the parallel loss of introns and a RNA editing site (fig. 3A, supplementary fig. S14, Supplementary Material online).
Extensive analyses of the plastid-encoded clpP gene across angiosperms revealed that structural changes in clpP genes are correlated with elevated substitution rates in several lineages (supplementary fig. S12, Supplementary Material online). In Geraniaceae lineages, high substitution rates were accompanied by positive or relaxed purifying selection. Our results also showed that increased nonsynonymous substitution rates in clpP were recurrent in the family. A similar situation was observed in some Silene lineages (Erixon and Oxelman 2008; Sloan et al. 2014) where clpP genes had elevated dN and dS associated with the loss of the introns. Gene-specific rate accelerations could be explained by localized hypermutation (Sloan et al. 2009; Magee et al. 2010) or mutagenic retroprocessing (Parkinson et al. 2005; Zhu et al. 2014). Consistent with the mechanism of the intron losses, mutagenic retroprocessing is favored as a potential explanation for clpP in Geranium and Monsonia.
Evolution of Acetyl-CoA Carboxylase
Acetyl-CoA carboxylase (ACCase) is crucial to fatty acid metabolism in most living organisms (Wakil et al. 1983; Kode et al. 2005) and two types of ACCase (i.e., eukaryotic and prokaryotic) are known (Wakil et al. 1983). The eukaryotic ACCase is a single multifunctional polypeptide chain encoded in the nucleus that operates in the cytoplasm. The prokaryotic ACCase comprises four subunits: The acetyl-CoA carboxylase subunit α (accA), the biotin carboxyl carrier protein subunit (accB), the biotin carboxylase subunit (accC), all nuclear-encoded, and the acetyl-CoA carboxylase subunit β (accD), which is encoded in the plastid (fig. 5A and B). Plant cells contain both types of ACCase, the eukaryotic type and the prokaryotic type, and the latter assembles in the plastid subsequent to the import of the three nuclear-encoded subunits. The plastid-encoded accD gene has been lost multiple times across angiosperms (Ruhlman and Jansen 2014), however, because the plastid ACCase is essential a functional replacement must occur. Several studies have shown that the missing plastid-encoded accD gene has been transferred to the nucleus (Rousseau-Gueutin et al. 2013; Sabir et al. 2014; Li et al. 2016), or alternatively that the prokaryotic ACCase has been replaced by the nuclear-encoded eukaryotic type (Konishi et al. 1996; Gornicki et al. 1997). Substitution of the duplicated eukaryotic ACCase for the plastid ACCase has been documented in Brassica (Schulte et al. 1997) and Poaceae (Konishi et al. 1996; Gornicki et al. 1997). Co-existence of the plastid-targeted eukaryotic ACCase and the plastid-encoded, prokaryotic ACCase has been documented in Arabidopsis (Babiychuk et al. 2011) and likely occurs more widely in Brassicaceae (Rousseau-Gueutin et al. 2013). According to Babiychuk et al. (2011) the prokaryotic ACCase, including the subunit encoded by accD, provides most of the ACCase activity.
The evolutionary history of ACCase in Geraniaceae is complex (fig. 5D). All species examined have copies of the three nuclear encoded prokaryotic subunits (A, B, C; supplementary table S12, Supplementary Material online) and the plastid-encoded accD with a large insertion that interrupts the conserved domain. The acetyl-CoA carboxylase subunit β, encoded by plastid accD, is likely functional in Geraniaceae as it retains the putative catalytic site at the C-terminus (Rousseau-Gueutin et al. 2013). Large insertions in accD have been documented in other lineages including cupressophyte conifer clade (Hirao et al. 2008; Yi et al. 2013) and the legume Medicago truncatula (Gurdon and Maliga 2014). Like Geraniaceae, in both of these cases accD preserves an intact reading frame suggesting functionality. The conserved domain of accD likely was interrupted in the common ancestor of Geraniaceae. Two examined Geraniaceae, Hypeocharis and Monsonia, have a plastid-to-nucleus functional transfer of the formerly plastid-encoded accD gene, and this likely also occurred in the ancestor of the family because the nuclear copies have the interrupted conserved domain (supplementary fig. S17, Supplementary Material online). Although transfer of DNA from the plastid to the nuclear genome is frequent (Noutsos et al. 2005), functionalization of transferred sequences is far less common. The low level of amino acid identity (19.2%) indicates that the transit peptides have diverse origins within the nuclear genomes of each species. The Geraniaceae is only the second angiosperm family in which a plastid-targeted eukaryotic ACCase and the plastid-encoded, prokaryotic ACCase are known to co-exist.
Organelle genomes of Geranium exhibit both lineage-specific and gene-specific rate accelerations where mitogenomes have increased rates in synonymous substitutions whereas nonsynonymous substitutions are accelerated in plastomes. The two most unusual phenomena in Geranium organelle genomes concern substitution rates in the mitogenome and the evolutionary history of acetyl-CoA carboxylase. Geranium provides only the second example among angiosperms in which rates are significantly higher in the mitogenome than the plastome. Evolution of ACCase is complex in the Geraniaceae because of the interruption and transfer of the prokaryotic, plastid encoded accD subunit and the duplication of the eukaryotic subunit with one copy now targeted to the plastid, making it only the second example among angiosperms of the co-existence a plastid-targeted eukaryotic ACCase and the plastid-encoded, prokaryotic ACCase. Finally, our results support the suggestion that accelerated evolution in the plastome in angiosperm lineages like Geraniaceae may contribute to cytonuclear incompatibility (Barnard-Kubow et al. 2014; Zhang et al. 2015; Rockenbach et al. 2016; Ruhlman et al. 2017).
Supplementary Material
Supplementary data are available at Genome Biology and Evolution online.
Supplementary Material
Acknowledgments
Support was provided by the National Science Foundation (IOS-1027259 to R.J.K. and T.A.R.) and from the President of King Abdulaziz University, Prof Dr Abdulrahman O. Alyoubi (to J.S.M.S., H.H.H., and R.K.J.). The authors thank Robin Parer at www.Geraniaceae.com for providing living material of Geraniaceae species. We also thank the Genome Sequencing and Analysis Facility at the University of Texas at Austin for performing the Illumina sequencing, the Texas Advanced Computing Center (TACC) at UT Austin for access to supercomputers, the Plant Resources Center (TEX-LL) at UT Austin for housing voucher specimens and two anonymous reviewers for valuable suggestions on an earlier version of the manuscript.
Literature Cited
- Adams KL, Palmer JD.. 2003. Evolution of mitochondrial gene content: gene loss and transfer to the nucleus. Mol Phylogen Evol. 29:380–395. [DOI] [PubMed] [Google Scholar]
- Aedo C, Garmenia FM, Pando F.. 1998. World checklist of Geranium L. (Geraniaceae). Anal Jard Bot Madrid. 56:211–252. [Google Scholar]
- Babiychuk E, et al. 2011. Plastid gene expression and plant development require a plastidic protein of the mitochondrial transcription termination factor family. Proc Natl Acad Sci U S A. 108:6674–6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker FT, Breman F, Merckx V.. 2006. DNA sequence evolution in fast evolving mitochondrial DNA nad1 exons in Geraniaceae and Plantaginaceae. Taxon 55:887–896. [Google Scholar]
- Barnard-Kubow KB, Sloan DB, Galloway LF.. 2014. Correlation between sequence divergence and polymorphism reveals similar evolutionary mechanisms acting across multiple timescales in a rapidly evolving plastid genome. BMC Evol Biol. 14:268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazier JC, Guisinger MM, Jansen RK.. 2011. Recent loss of plastid-encoded ndh genes within Erodium (Geraniaceae). Plant Mol Biol. 76:263–272. [DOI] [PubMed] [Google Scholar]
- Blazier JC, et al. 2016a. Divergence of RNA polymerase α subunits in angiosperm plastid genomes is mediated by genomic rearrangement. Sci Rep. 6:24595.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazier JC, et al. 2016b. Variable presence of the inverted repeat and plastome stability in Erodium. Ann Bot. 117:1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouckaert R, et al. 2014. BEAST 2: a software platform for Bayesian evolutionary analysis. PLoS Comput Biol. 10:e1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandvain Y, Wade MJ.. 2009. The functional transfer of genes from the mitochondria to the nucleus: the effects of selection, mutation, population size and rate of self-fertilization. Genetics 182:1129–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubunenko MG, Schmidt J, Subramanian AR.. 1994. Protein substitution in chloroplast ribosome evolution: a eukaryotic cytosolic protein has replaced its organelle homologue (L23) in spinach. J Mol Biol. 240:28–41. [DOI] [PubMed] [Google Scholar]
- Cai Z, et al. 2008. Extensive reorganization of the plastid genome of Trifolium subterraneum (Fabaceae) is associated with numerous repeated sequences and novel DNA insertions. J Mol Evol. 67:696–704. [DOI] [PubMed] [Google Scholar]
- Camacho C, et al. 2009. BLAST+: architecture and applications. BMC Bioinform. 10:421.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrie C, Small I.. 2013. A reevaluation of dual-targeting of proteins to mitochondria and chloroplasts. Biochim Biophys Acta. 1833:253–259. [DOI] [PubMed] [Google Scholar]
- Chateigner-Boutin AL, et al. 2008. CLB19, a pentatricopeptide repeat protein required for editing of rpoA and clpP chloroplast transcripts. Plant J. 56:590–602. [DOI] [PubMed] [Google Scholar]
- Cho Y, Mower JP, Qiu YL, Palmer JD.. 2004. Mitochondrial substitution rates are extraordinarily elevated and variable in a genus of flowering plants. Proc Natl Acad Sci U S A. 101:17741–17746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumley TW, et al. 2006. The complete chloroplast genome sequence of Pelargonium × hortorum: organization and evolution of the largest and most highly rearranged chloroplast genome of land plants. Mol Biol Evol. 23:2175–2190. [DOI] [PubMed] [Google Scholar]
- Day A, Madesis P.. 2007. DNA replication, recombination, and repair in plastids In: Bock R, editor. Cell and molecular biology of plastids. Berlin (Germany: ): Springer; p. 65–119. [Google Scholar]
- Drouin G, Daoud H, Xia J.. 2008. Relative rates of synonymous substitutions in the mitochondrial, chloroplast and nuclear genomes of seed plants. Mol Phylogenet Evol. 49:827–831. [DOI] [PubMed] [Google Scholar]
- Emanuelsson O, Brunak S, von Heijne G, Nielsen H.. 2007. Locating proteins in the cell using TargetP, SignalP, and related tools. Nat Protoc. 2:953–971. [DOI] [PubMed] [Google Scholar]
- Erixon P, Oxelman B.. 2008. Whole-gene positive selection, elevated synonymous substitution rates, duplication, and indel evolution of the chloroplast clpP1 gene. PLoS ONE. 3:e1386.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo D, et al. 2013. Complete plastid genome sequence of Vaccinium macrocarpon: structure, gene content, and rearrangements revealed by next generation sequencing. Tree Genet Genomes. 9:489–498. [Google Scholar]
- Gornicki P, et al. 1997. Plastid-localized acetyl-CoA carboxylase of bread wheat is encoded by a single gene on each of the three ancestral chromosome sets. Proc Natl Acad Sci U S A. 94:14179–14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisinger MM, Kuehl JV, Boore JL, Jansen RK.. 2008. Genome-wide analyses of Geraniaceae plastid DNA reveal unprecedented patterns of increased nucleotide substitutions. Proc Natl Acad Sci U S A. 105:18424–18429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisinger MM, Kuehl JV, Boore JL, Jansen RK.. 2011. Extreme reconfiguration of plastid genomes in the angiosperm family Geraniaceae: rearrangements, repeats, and codon usage. Mol Biol Evol. 28:583–600. [DOI] [PubMed] [Google Scholar]
- Gurdon C, Maliga P.. 2014. Two distinct plastid genome configurations and unprecedented intraspecies length variation in the accD coding region in Medicago truncatula. DNA Res. 21:417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberle RC, Fourcade HM, Boore JL, Jansen RK.. 2008. Extensive rearrangements in the chloroplast genome of Trachelium caeruleum are associated with repeats and tRNA genes. J Mol Evol. 66:350–361. [DOI] [PubMed] [Google Scholar]
- Hepburn NJ, Schmidt DW, Mower JP.. 2012. Loss of two introns from the Magnolia tripetala mitochondrial cox2 gene implicates horizontal gene transfer and gene conversion as a novel mechanism of intron loss. Mol Biol Evol. 29:3111–3120. [DOI] [PubMed] [Google Scholar]
- Hirao T, Watanabe A, Kurita M, Kondo T, Takata K.. 2008. Complete nucleotide sequence of the Cryptomeria japonica D. Don. chloroplast genome and comparative chloroplast genomics: diversified genomic structure of coniferous species. BMC Plant Biol. 8:70.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen RK, Ruhlman TA.. 2012. Plastid genomes of seed plants In: Bock R, Knoop V, editors. Genomics of chloroplasts and mitochondria. Netherlands: Springer; pp. 103–126 [Google Scholar]
- Katoh K, Misawa K, Kuma K, Miyata T.. 2002. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 30:3059–3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling PJ. 2010. The endosymbiotic origin, diversification and fate of plastids. Philos Trans R Soc Lond B Biol Sci. 365:729–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine T, Maier UG, Leister D.. 2009. DNA transfer from organelles to the nucleus: the idiosyncratic genetics of endosymbiosis. Annu Rev Plant Biol. 60:115–138. [DOI] [PubMed] [Google Scholar]
- Kode V, Mudd EA, Iamtham S, Day A.. 2005. The tobacco plastid accD gene is essential and is required for leaf development. Plant J. 44:237–244. [DOI] [PubMed] [Google Scholar]
- Konishi T, Shinohara K, Yamada K, Sasaki Y.. 1996. Acetyl-CoA carboxylase in higher plants: most plants other than Gramineae have both the prokaryotic and the eukaryotic forms of this enzyme. Plant Cell Physiol. 37:117–122. [DOI] [PubMed] [Google Scholar]
- Kubo N, Arimura S.. 2010. Discovery of the rpl10 gene in diverse plant mitochondrial genomes and its probable replacement by the nuclear gene for chloroplast RPL10 in two lineages of angiosperms. DNA Res. 17:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang BF, Gray MW, Burger G.. 1999. Mitochondrial genome evolution and the origin of eukaryotes. Annu Rev Genet. 33:351–397. [DOI] [PubMed] [Google Scholar]
- Lee HL, Jansen RK, Chumley TW, Kim KJ.. 2007. Gene relocations within chloroplast genomes of Jasminum and Menodora (Oleaceae) are due to multiple, overlapping inversions. Mol Biol Evol. 24:1161–1180. [DOI] [PubMed] [Google Scholar]
- Lee SS, et al. 2004. Characterization of the plastid-encoded carboxyltransferase subunit (accD) gene of potato. Mol Cells. 17:422–429. [PubMed] [Google Scholar]
- Li J, et al. 2016. Evolution of short inverted repeat in cupressophytes, transfer of accD to nucleus in Sciadopitys verticillata and phylogenetic position of Sciadopityaceae. Sci Rep. 6:20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SL, Zhuang Y, Zhang P, Adams KL.. 2009. Comparative analysis of structural diversity and sequence evolution in plant mitochondrial genes transferred to the nucleus. Mol Biol Evol. 26:875–891. [DOI] [PubMed] [Google Scholar]
- Magee AM, et al. 2010. Localized hypermutation and associated gene losses in legume chloroplast genomes. Genome Res. 20:1700–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler-Bauer A, et al. 2015. CDD: NCBI's conserved domain database. Nucleic Acids Res. 43:D222–D226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DP, Murrell B, Golden M, Khoosal A, Muhire B.. 2015. RDP4: detection and analysis of recombination patterns in virus genomes. Virus Evol. 1:vev003.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Alberola F, et al. 2013. Balanced gene losses, duplications and intensive rearrangements led to an unusual regularly sized genome in Arbutus unedo chloroplasts. PLoS ONE. 8:e79685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal A, Brisson N.. 2010. Recombination and the maintenance of plant organelle genome stability. New Phytol. 186:299–317. [DOI] [PubMed] [Google Scholar]
- Millen RS, et al. 2001. Many parallel losses of infA from chloroplast DNA during angiosperm evolution with multiple independent transfers to the nucleus. Plant Cell. 13:645–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollier P, Hoffmann B, Debast C, Small I.. 2002. The gene encoding Arabidopsis thaliana mitochondrial ribosomal protein S13 is a recent duplication of the gene encoding plastid S13. Curr Genet. 40:405–409. [DOI] [PubMed] [Google Scholar]
- Mower JP, Sloan DB, Alverson AJ.. 2012. Plant mitochondrial genome diversity: the genomics revolution In: Wendel JH, editor. Plant genome diversity volume 1: Plant genomes, their residents, and their evolutionary dynamics. New York: Springer; p. 123–144. [Google Scholar]
- Mower JP, Touzet P, Gummow JS, Delph LF, Palmer JD.. 2007. Extensive variation in synonymous substitution rates in mitochondrial genes of seed plants. BMC Evol Biol. 7:135.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noutsos C, Richly E, Leister D.. 2005. Generation and evolutionary fate of insertions of organelle DNA in the nuclear genomes of flowering plants. Genome Res. 15:616–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T. 1994. Further examples of evolution by gene duplication revealed through DNA sequence comparisons. Genetics 138:1331–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, et al. 2015a. Dynamic evolution of Geranium mitochondrial genomes through multiple horizontal and intracellular gene transfers. New Phytol. 208:570–583. [DOI] [PubMed] [Google Scholar]
- Park S, Jansen RK, Park SJ.. 2015b. Complete plastome sequence of Thalictrum coreanum (Ranunculaceae) and transfer of the rpl32 gene to the nucleus in the ancestor of the subfamily Thalictroideae. BMC Plant Biol. 15:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, et al. 2014. Complete sequences of organelle genomes from the medicinal plant Rhazya stricta (Apocynaceae) and contrasting patterns of mitochondrial genome evolution across asterids. BMC Genomics. 15:405.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson CL, et al. 2005. Multiple major increases and decreases in mitochondrial substitution rates in the plant family Geraniaceae. BMC Evol Biol. 5:73.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier JB, et al. 2004. Clp protease complexes from photosynthetic and non-photosynthetic plastids and mitochondria of plants, their predicted three-dimensional structures, and functional implications. J Biol Chem. 279:4768–4781. [DOI] [PubMed] [Google Scholar]
- Richardson AO, Rice DW, Young GJ, Alverson AJ, Palmer JD.. 2013. The "fossilized" mitochondrial genome of Liriodendron tulipera: Ancestral gene content and order, ancestral editing sites and extraordinarily low mutation rate. BMC Biol. 11:29.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. 2015. R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. Available from: https://www.R-project.org/.
- Rockenbach K, et al. 2016. Positive selection in rapidly evolving plastid-nuclear enzyme complexes. Genetics 204:1507–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau-Gueutin M, et al. 2013. Potential functional replacement of the plastidic acetyl-CoA carboxylase subunit (accD) gene by recent transfers to the nucleus in some angiosperm lineages. Plant Physiol. 161:1918–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhlman TA, et al. 2015. NDH expression marks major transitions in plant evolution and reveals coordinate intracellular gene loss. BMC Plant Biol. 15:100.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhlman TA, Jansen RK.. 2014. The plastid genomes of flowering plants In: Maliga P, editor. Chloroplast biotechnology: methods and protocols. Vol. 1132. New York: Springer Science and Business Media, LLC; p. 3–38. [DOI] [PubMed] [Google Scholar]
- Ruhlman TA, Zhang J, Blazier JC, Sabir JSM, Jansen RK.. 2017. Recombination-dependent replication and gene conversion homogenize repeat sequences and diversify plastid genome structure. Am J Bot. 104:1–14. [DOI] [PubMed] [Google Scholar]
- Sabir J, et al. 2014. Evolutionary and biotechnology implications of plastid genome variation in the inverted-repeat-lacking clade of legumes. Plant Biotechnol J. 12:743–754. [DOI] [PubMed] [Google Scholar]
- Schulte W, Töpfer R, Stracke R, Schell J, Martini N.. 1997. Multi-functional acetyl-CoA carboxylase from Brassica napus is encoded by a multi-gene family: indication for plastidic localization of at least one isoform. Proc Natl Acad Sci U S A. 94:3465–3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skippington E, Barkman TJ, Rice DW, Palmer JD.. 2015. Miniaturized mitogenome of the parasitic plant Viscum scurruloideum is extremely divergent and dynamic and has lost all nad genes. Proc Natl Acad Sci U S A. 112:E3515–E3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DB, et al. 2012a. Rapid evolution of enormous, multichromosomal genomes in flowering plant mitochondria with exceptionally high mutation rates. PLoS Biol. 10:e1001241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DB, Alverson AJ, Wu M, Palmer JD, Taylor DR.. 2012b. Recent acceleration of plastid sequence and structural evolution coincides with extreme mitochondrial divergence in the angiosperm genus Silene. Genome Biol Evol. 4:294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DB, Barr CM, Olson MS, Keller SR, Taylor DR.. 2008. Evolutionary rate variation at multiple levels of biological organization in plant mitochondrial DNA. Mol Biol Evol. 25:243–246. [DOI] [PubMed] [Google Scholar]
- Sloan DB, Oxelman B, Rautenberg A, Taylor DR.. 2009. Phylogenetic analysis of mitochondrial substitution rate variation in the angiosperm tribe Sileneae. BMC Evol Biol. 9:260.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan DB, Taylor DR.. 2012. Evolutionary rate variation in organelle genomes: the role of mutational processes In: Bullerwell CE, editor. Organelle genetics. Heidelberg (Germany: ): Springer-Verlag; p. 123–146. [Google Scholar]
- Sloan DB, et al. 2014. A recurring syndrome of accelerated plastid genome evolution in the angiosperm tribe Sileneae (Caryophyllaceae). Mol Phylogenet Evol. 72:82–89. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. 2006. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690. [DOI] [PubMed] [Google Scholar]
- Stanne TM, Sjögren LL, Koussevitzky S, Clarke AK.. 2009. Identification of new protein substrates for the chloroplast ATP-dependent Clp protease supports its constitutive role in Arabidopsis. Biochem J. 417:257–268. [DOI] [PubMed] [Google Scholar]
- Sveinsson S, Cronk Q.. 2014. Evolutionary origin of highly repetitive plastid genomes within the clover genus (Trifolium). BMC Evol Biol. 14:228.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis JN, Ayliffe MA, Huang CY, Martin W.. 2004. Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nat Rev Genet. 5:123–135. [DOI] [PubMed] [Google Scholar]
- Ueda M, et al. 2007. Loss of the rpl32 gene from the chloroplast genome and subsequent acquisition of a preexisting transit peptide within the nuclear gene in Populus. Gene 402:51–56. [DOI] [PubMed] [Google Scholar]
- Ueda M, et al. 2008. Substitution of the gene for chloroplast RPS16 was assisted by generation of a dual targeting signal. Mol Biol Evol. 25:1566–1575. [DOI] [PubMed] [Google Scholar]
- Wakil SJ, Stoops JK, Joshi VC.. 1983. Fatty acid synthesis and its regulation. Annu Rev Biochem. 52:537–579. [DOI] [PubMed] [Google Scholar]
- Wang C-W, Chen S-Y, Zhang X-Z.. 2016. Chloroplast genome evolution in Actinidiaceae: clpP loss, heterogeneous divergence and phylogenomic practice. PLoS ONE. 11:e0162324.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng M-L, Ruhlman TA, Gibby M, Jansen RK.. 2012. Phylogeny, rate variation, and genome size evolution of Pelargonium (Geraniaceae). Mol Phylog Evol. 64:654–670. [DOI] [PubMed] [Google Scholar]
- Weng ML, Blazier JC, Govindu M, Jansen RK.. 2014. Reconstruction of the ancestral plastid genome in Geraniaceae reveals a correlation between genome rearrangements, repeats, and nucleotide substitution rates. Mol Biol Evol. 31:645–659. [DOI] [PubMed] [Google Scholar]
- Weng ML, Ruhlman TA, Jansen RK.. 2016. Plastid-nuclear interaction and accelerated coevolution in plastid ribosomal genes in Geraniaceae. Genome Biol Evol. 8:1824–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng ML, Ruhlman TA, Jansen RK.. 2017. Expansion of inverted repeat does not decrease substitution rates in Pelargonium plastid genomes. New Phytol. 214:842–851. [DOI] [PubMed] [Google Scholar]
- Wolfe KH, Li WH, Sharp PM.. 1987. Rates of nucleotide substitution vary greatly among plant mitochondrial, chloroplast, and nuclear DNAs. Proc Natl Acad Sci U S A. 84:9054–9058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright F. 1990. The effective number of codons used in a gene. Gene 87:23–29. [DOI] [PubMed] [Google Scholar]
- Yang Z. 2007. PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol. 24:1586–1591. [DOI] [PubMed] [Google Scholar]
- Yi X, Gao L, Wamg B, Su Y-J, Wang T.. 2013. The complete chloroplast genome sequence of Cephalotaxus oliveri (Cephalotaxaceae): evolutionary comparison of Cephalotaxus chloroplast DNAs and insights into the loss of inverted repeat copies in gymnosperms. Genome Biol Evol. 5:688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbino DR, Birney E.. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18:821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ruhlman TA, Sabir J, Blazier JC, Jansen RK.. 2015. Coordinated rates of evolution between interacting plastid and nuclear genes in Geraniaceae. Plant Cell. 27:563–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, et al. 2016. Coevolution between nuclear-encoded DNA replication, recombination, and repair genes and plastid genome complexity. Genome Biol Evol. 8:622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A, Guo W, Jain K, Mower JP.. 2014. Unprecedented heterogeneity in the synonymous substitution rate within a plant genome. Mol Biol Evol. 31:1228–1236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.