Abstract
Background
Recent years have seen increasing use of rituximab (RTX) for various types of primary and secondary glomerulopathies. However, there are no studies that specifically address the risk of infection related to this agent in patients with these conditions.
Methods
We reviewed the outcomes of all patients who received RTX therapy for glomerular disease between June 2000 and October 2011 in eight French nephrology departments. Each case was analysed for survival, cause of death if a non-survivor and/or the presence of infectious complications, including severe or opportunistic infection occurring within the 12 months following RTX infusion.
Results
Among 98 patients treated with RTX, 25 presented with at least one infection. We report an infection rate of 21.6 per 100 patient-years. Five patients died within 12 months following an RTX infusion, of whom four also presented with an infection. The median interval between the last RTX infusion and the first infectious episode was 2.1 months (interquartile range 0.5–5.1). Most infections were bacterial (79%) and pneumonia was the most frequent infection reported (27%). The presence of diabetes mellitus (P = 0.006), the cumulative RTX dose (P = 0.01) and the concomitant use of azathioprine (P = 0.03) were identified as independent risk factors. Renal failure was significantly associated with an increased infection risk by bivariate analysis (P = 0.03) and was almost significant by multivariate analysis (P = 0.05). Nephrotic syndrome did not further increase the risk of infection and/or death.
Conclusion
The risk of infection after RTX-based immunosuppression among patients with glomerulopathy must be considered and patients should receive close monitoring and appropriate infection prophylaxis, especially in those with diabetes and high-dose RTX regimens.
Keywords: glomerulonephritis, immunosuppression, rituximab, sepsis, safety
Introduction
Autoimmune glomerulonephritis can occur either as a primary disorder or as part of a systemic disease, such as systemic lupus erythematosus (SLE) or anti-neutrophil cytoplasmic antibodies (ANCA)-associated vasculitis (AAV). A variety of immunosuppressive treatments have been used in these diseases in order to control autoimmune response and to prevent progression to end-stage renal disease (ESRD). For many years, standard therapy has relied predominantly on corticosteroids, cytotoxic agents and antiproliferative drugs. More recently, studies in experimental models as well as advances in human pathology have highlighted the pivotal role of B cells in autoimmune disease that in turn has led to modern biotherapies that directly target B cell populations [1].
Rituximab (RTX) is a murine/human chimeric anti-CD20 monoclonal antibody that depletes B cells and was initially approved for the treatment of non-Hodgkin's lymphoma. Subsequent studies using RTX as an immunosuppressive agent have resulted in extending its indications to include treatment of some non-hematologic diseases. Although RTX has been approved by regulatory agencies only for the treatment of lymphoproliferative disease, rheumatoid arthritis and AAV [2–6], this drug has been shown to be useful in a large spectrum of autoimmune conditions. Off-label use of RTX has been extended to treatment of several kidney diseases, whether antibody-mediated or not, including membranous nephropathy [7–9], cryoglobulinemic membranoproliferative glomerulonephritis (MPGN) [10], lupus nephritis [11–13], minimal change disease [14, 15] and focal segmental glomerulosclerosis (FSGS) [16, 17]. RTX is also used in renal transplantation as part of induction treatment [18], desensitization of immunized patients [19, 20] and treatment of acute humoral rejection [21, 22].
As is the case for all other immunosuppressive agents, RTX increases the risk of infectious complications. Estimates of the infectious complication rate after RTX administration range from 1.9 to 5.6 per 100 patient-years for patients with rheumatoid arthritis [23–26], from 6.6 to 16.6 per 100 patient-years for patients with SLE [11, 27, 28] and 14.1 per 100 patient-years for patients with cryoglobulinemia [10]. After kidney transplantation, patients receiving RTX in addition to their standard immunosuppressive regimen have a significantly higher infectious complication rate, estimated at approximately 50% [29, 30]. Previous studies have revealed that the infection risk depends not only on the indication for RTX treatment, but also on the burden of the overall immunosuppressive therapy. Given the increasing use of RTX in nephrology, this study was designed to describe the infectious complications after RTX in this specific population, to consider the potential risk factors for infection after RTX and to determine the outcomes of these patients.
Materials and methods
Patients
We included in this retrospective study all adult patients who received RTX between June 2000 and October 2011 in eight French nephrology departments for the treatment of an underlying renal disease. RTX was administrated according to the local protocol. We excluded renal transplant recipients and patients who had received concomitant treatment with high-dose cytotoxic drugs [i.e. a cumulative dose of cyclophosphamide (CYC) > 3 g] given for hematological malignancy.
Outcome
We reviewed all patient files from the time of first RTX infusion through the last available information. We collected data regarding patient baseline demographic characteristics, indications for RTX, RTX treatment regimens and severe infectious episodes. Taking into account the long duration of action of RTX, we retrieved events that occurred within the 12-month period following an infusion of RTX (initial treatment or re-treatment). Other immunosuppressive treatments received prior to RTX or within the 12 months following an RTX infusion were also retrieved. Severe infection was defined as an infection that required hospitalization and/or intravenous antibiotics and/or resulted in death. We also specifically retrieved opportunistic infections defined according to the Centers for Disease Control and Prevention classification system for HIV infection and the revised case definition for AIDS [31]. Laboratory results were collected at baseline (before RTX infusion) and at 3, 6 and 12 months after treatment initiation and then every 12 months until the last follow-up. Nephrotic syndrome was defined as a serum albumin concentration <30 g/L associated with proteinuria >3 g/24 h. Renal function was evaluated using the estimated glomerular filtration rate (eGFR) calculated according to the Modification of Diet in Renal Disease (MDRD) four-variable formula. Leucopenia was graded in accordance with the Common Terminology Criteria for Adverse Events v3.0: grade 1, total white blood (WBC) count ≥3000–<4000/mm3; grade 2, ≥2000–<3000/mm3; grade 3, ≥1000–<2000/mm3; grade 4 <1000/mm3.
Statistical analysis
Baseline clinical and laboratory data are expressed as percentages, means (± SD) or medians [interquartile range (IQR)], as appropriate. Bivariate analysis was used to identify factors associated with occurrence of the combined end point (infectious complication and/or death during the 12-month period following RTX) by using the chi-square test or the Fisher's exact test for categorical predictors and logistic regression for continuous variables. We conducted a multivariate logistic regression analysis to study associations between ‘death/infection’ (dependent variable) and predictor variables. Parameters correlated with outcome in bivariate analysis with a P-value <0.2 were considered for entry in the multiple logistic regression model. Parameters with missing data >10% (gamma globulin level at 3 months) were excluded from multivariate analysis. Age, creatinine and RTX dose were kept as continuous variables in the logistic regression (hypothesis of linear modelling not rejected). We then assessed the absence of interaction between concomitant immunosuppressive treatment [plasmapheresis, steroids, azathioprine (AZA), calcineurin inhibitor (CNI)] and the cumulative dose of RTX. The model was fitted by selection of variables using the Wald test. Finally, the quality of adjustment of the model was tested with the Hosmer–Lemeshow statistic. Odds ratios are expressed with 95% confidence intervals (CI). Kaplan–Meier analysis was used to compare long-term survival rates across different subgroups of patients according to their initial eGFR. Statistical analyses were performed using JMP8 8.2.0 software (SAS, Cary, NC, USA) and STATA version 10.0 for Macintosh (StataCorp, College Station, TX, USA).
Results
Patient characteristics
The study included 98 patients who received RTX between June 2000 and October 2011. The main indications for RTX therapy were MPGN (mainly cryoglobulinemia-associated nephropathy), membranous nephropathy, lupus nephritis and AAV (Table 1).
Table 1.
Main characteristics at inclusion
| Main demographic characteristics at inclusion | |
|---|---|
| Age, mean ± SD (years) | 49.2 ± 19.4 |
| No. of female/No. of male | 45/53 |
| Body mass index, mean ± SD (kg/m2) | 25.5 ± 4.4 |
| Diabetes mellitus, n (%) | 18 (18.4%) |
| Serum creatinine at inclusion, median (IQR)(µmol/L) | 127.5 (80.5–224.3) |
| eGFR according to MDRD, median (IQR)(mL/min/1.73 m2) | 47.7 (23.8–87.5) |
| Patients with renal failure, n (%) | 61 (62.2%) |
| Patients with nephrotic syndrome, n (%) | 44 (44.9%) |
| Previous immunosuppressive agent, n (%) | 71 (72.4%) |
| Steroids, n (%) | 69 (70.4%) |
| CYC, n (%) | 36 (36.7%) |
| Total dose CYC, mean ± SD (g) | 7 ± 4.05 |
| Interval since CYC/RTX, median (IQR) (months) | 9.4 (1.6–43.3) |
| MMF, n (%) | 32 (32.6%) |
| Interval since MMF/RTX, median (IQR) (months) | 0.0 (0.0–12.4) |
| Calcineurine inhibitor, n (%) | 16 (16.3%) |
| Interval since CNI/RTX, median (IQR) (months) | 2.8 (0.0–8.4) |
| Azathioprine, n (%) | 8 (8.1%) |
| Interval since AZA/RTX, median (IQR) (months) | 15.1 (0.2–18.8) |
| Rituximab protocol administration | |
| 375 mg/m2/week ×4 infusions, n (%) | 58 (59.1%) |
| 1 g/2 weeks ×2 infusions, n (%) | 19 (19.4%) |
| Other regimen, n (%) | 21 (21.4%) |
| Reinjection after initial protocol, n (%) | 37 (37.7%) |
| Cumulate dose, median (IQR) (mg) | 2800 (2000–3562) |
| Immunosuppressive therapy prescribed within the 12 months following RTXs, n (%) | 75 (76.5%) |
| Steroids, n (%) | 68 (69.4%) |
| Plasmapheresis, n (%) | 19 (19.4%) |
| Patients with other immunosuppressive therapy, n (%), including | 41 (41.8%) |
| MMF, n (%) | 26 (26.5%) |
| CNI, n (%) | 12 (12.4%) |
| AZA, n (%) | 6 (6.1%) |
| CYC, n (%) | 5 (5.1%) |
| Chloraminophen, n (%) | 2 (2%) |
| Lenalidomide, n (%) | 1 (1%) |
We analysed the patient demographic characteristics at the time of the first RTX infusion (Table 1). Of note, 44.9% of patients were nephrotic at RTX initiation. The median serum creatinine value and eGFR were 127.5 µmol/L (IQR 80.5–224.3) and 47.7 mL/min/1.73 m2 (IQR 23.8–87.5), respectively. Sixty-one patients (62.2%) had renal dysfunction as defined by an eGFR <60 mL/min/1.73 m2. Dialysis was needed for six patients. The median gamma globulin level was 5.9 g/L (IQR 4–8.5; normal range 6.4–13) at the time of first RTX infusion.
The median duration of follow-up in the whole cohort, from the time of the first RTX infusion until the study was shortened 12 months after the last observed infusion, was 12.7 months (IQR 12.0–17.8).
Treatment characteristics
In most cases, RTX was used in conjunction with other immunosuppressive drugs. The details concerning the immunosuppressive regimens given before and after RTX administration are summarized in Table 1.
Prior to RTX treatment, 69 patients (70.4%) had received corticosteroids. Sixty-two patients (63.3%) had previously received other immunosuppressive agents, either alone or in combination. Previous immunosuppression included CYC in 36 patients (36.7%), mycophenolate mofetil (MMF) in 32 (32.7%), CNI in 16 (16.3%) and AZA in 8 (8.3%).
Other therapies were frequently administered during or after RTX infusion. These immunosuppressive regimens most often included steroids (69.4%). Plasmapheresis was used in 19 patients (19.4%) and purine inhibitors (MMF or AZA) were administered to 28 patients (28.6%), including 4 patients who received both AZA and MMF during follow-up. CNIs were used in 12 patients (12.2%) (Table 1).
Most patients received four weekly RTX infusions of 375 mg/m2 (n = 58) as their initial protocol. The median cumulative RTX dose was 2800 mg (IQR 2000–3562). Of note, 37 patients received one or more RTX reinfusions several months after the initial protocol. Therefore, the total number of infusions varied greatly among the study population, ranging from 1 to 12 infusions, with a median of 4.
In regard to the use of preventive treatment for infection, 35 patients received trimethoprim-sulfamethoxazole prophylaxis for Pneumocystis jiroveci infection, including 18 who had received CYC prior to RTX. Fifteen received primary prophylaxis with intravenous immunoglobulin because of severe hypogammaglobulinemia and one patient had been given tuberculosis prophylaxis including rifampicin and isoniazid.
Patient survival
Five patients died within the year following RTX injection, yielding a mortality rate of 3.7 per 100 patient-years. Three patients died due to an infection, one patient died of neoplasia (pancreatic adenocarcinoma discovered 4 months after the first RTX infusion) and one suffered sudden death. The specific fatal infections were pneumonia, candida septicaemia and cellulitis with septic shock.
Death occurred after a median interval of 4.0 months (IQR 1.6–7.2) after the last RTX administration. Of note, the patients who died were significantly older than those who survived the year following RTX infusion (mean age 80.7 ± 3.0 years versus 47.5 ± 18.4; P = 0.0003).
Infectious complications
Overall, 33 infectious episodes were noted in 25 patients, resulting in an infection rate of 21.6 per 100 patient-years. Nineteen patients experienced only one infection and six patients had at least two infectious episodes. The median interval between the first RTX infusion and the first infectious episode was 4.6 months (IQR 1.6–9.4). The median interval between the last RTX infusion and the first subsequent infectious episode was 2.1 months (IQR 0.5–5.1). For the whole group, infection-free survival at 12 months was 79.5% after the initiation of RTX and 75.1% after the last RTX infusion.
The spectrum of infectious complications in the 25 patients is depicted in Figure 1. Most infections were of bacterial origin (79%). Pneumonia (n = 9), acute pyelonephritis (n = 5) and cellulitis (n = 4) were the most frequent types of bacterial infection. We observed five viral infections: one case of febrile cytomegalovirus (CMV) viremia without any organ involvement and four cases of non-complicated herpes simplex virus (HSV) or varicella-zoster virus (VZV) infections. Fungal infections included one case each of pulmonary aspergillosis and invasive candidiasis. No case of pneumocystosis was noted, even though only 35 patients received routine trimethoprim-sulfamethoxazole-based prophylaxis.
Fig. 1.
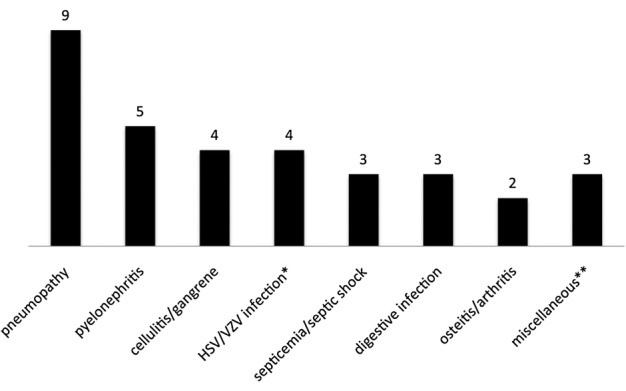
Infections observed within 12 months after rituximab therapy. HSV, herpes simplex virus; VZV, varicella zoster virus. *HSV/VZV infections were HSV stomatitis, HSV genital infection and two zoster recurrences. **Miscellaneous infections were pulmonary aspergillosis, invasive candidiasis and cytomegalovirus viremia.
White blood cell counts at 3 and 6 months were available for 89 and 88 patients of our cohort, respectively. Among them, 8 patients were leucopenic (defined as WBC <4000/mm3) at month 3 and 12 patients at month 6. In most cases, leucopenia was mild to moderate (grade 1 or 2). Only one patient each had grade 3 or grade 4 leucopenia, both occurring at 6 months. Of note, none of the patients with severe leucopenia presented with an infectious complication during this period. Among the 9 patients who received CYC in the 3-month period before RTX administration, none were leucopenic at RTX initiation or at 3 months after RTX initiation.
Follow-up beyond the initial 12-month period
Because of the long duration of action of RTX, we extended our observations to include all severe infections after RTX administration regardless of the time interval after the infusion. The median duration of this follow-up was 24 months (IQR 12–36) after the first RTX infusion and 11.7 months (IQR 7.5–23.5) after the last one. After extending the follow-up period beyond the initial 12 months following RTX infusion, we retrieved eight new infectious episodes in six patients, including three patients who had not experienced infectious complications during the year immediately following the last RTX administration. Importantly, those with infections identified after the extension of the observation period included two patients who presented with mycobacterial infection at 24 and 28 months, respectively, after the last RTX infusion. In one case the patient experienced tuberculosis lymphadenitis, while in the other the clinical localization was a subcutaneous cellulitis secondary to mycobacterium avium infection. Of note, these two patients did not receive any additional immunosuppressive treatment beyond RTX except for some steroids in one case, with concomitant steroid dosage that was <5 mg/day of prednisone at the time of infection. Notably in both cases, the CD19 population remained low (<20/mm3) 2 years after the last RTX course. For the four other patients, their infections included three episodes of pneumonia, two episodes of septic shock (including one episode leading to death) and one case of cellulitis (Table 2). Most patients (five of six) who presented an infection beyond the initial 12 months following RTX had been treated for MPGN.
Table 2.
Description of late infections that occurred >12 months after last RTX injection
| Patient | Nephropathy | eGFR at RTX initiation (mL/min/1.73 m2) | Cumulative RTX dose received | Associated immunosuppressive treatment | Infection | Time since the last RTX (months) | Gamma globulin levela | CD19 counta |
|---|---|---|---|---|---|---|---|---|
| 1 | Lupus nephritis | 39.2 | 3400 | Steroids, plasmapheresis, AZA, MMF | Pneumonia | 13 | NA | 3 |
| Septic shock S. aureus | 24 | 13.8 | NA | |||||
| Septic shock P.aeruginosa leading to death | 25 | NA | NA | |||||
| 2 | MPGN | 29.1 | 4440 | Plasmapheresis, steroids | Pneumonia | 14 | NA | NA |
| 3 | MPGN | 46.2 | 4000 | Plasmapheresis, steroids | Pneumonia | 14 | 8.5 | 14 |
| 4 | MPGN | 41.2 | 2000 | Steroids | Mycobacterium avium cellulitis | 24 | 5.3 | 0 |
| 5 | MPGN | 24.9 | 2000 | Steroids | Cellulitis | 25 | NA | NA |
| 6 | MPGN | 22.2 | 3700 | None | Tuberculous lymphadenitis | 27.3 | 4.3 | 19 |
aLevels at the time of the infection.
NA, not available.
Risk factors for death and/or infection
By bivariate analysis (Table 3), the presence of diabetes mellitus, either pre-existing or related to the immunosuppressive treatment, was significantly associated with occurrence of the combined end point (infection or death). Initial renal dysfunction determined by eGFR (MDRD) was also associated with a higher risk of subsequent infection. The presence of nephrotic syndrome, initial serum albumin level or proteinuria was not predictive of subsequent infection. Patient age tended towards a significantly increased risk of infection; sex and body mass index at RTX initiation were not associated with a higher risk of infection. Even though we found no statistical association of any specific glomerular disease with the outcome by bivariate analysis, patients with MPGN tended to have a higher infection rate when compared with other nephropathies (Table 3).
Table 3.
Comparison of main clinical characteristics by bivariate analysis between patients for the principal combined outcome death and/or infectious complication
| No infection/death (n = 72) |
Infection and/or death (n = 26) |
P-value | |
|---|---|---|---|
| Age, mean ± SD (years) | 47.3 ± 19.2 | 54.6 ± 19.4 | 0.11 |
| BMI, mean ± SD (kg/m2) | 25.8 ± 4.4 | 24.7 ± 3.5 | 0.38 |
| Diabetes mellitus, n (%) | 8 (11.1%) | 10 (38.4%) | 0.002 |
| Nephrotic syndrome, n (%) | 31 (43.1%) | 13 (50%) | 0.72 |
| Serum creatinine at first RTX infusion (D0), median (IQR) (μmol/L) | 118.5 (72.5–186) | 163.5 (115.5–279.8) | 0.03 |
| eGFR at D0, median (IQR) (mL/min/1.73 m2) | 53.2 (31.0–91.8) | 32.2 (18.6–52.9) | 0.03 |
| Serum albumin at D0, mean ± SD (g/L) | 28.6 ± 7.4 | 28.1 ± 7.9 | 0.77 |
| Proteinuria at D0, median (IQR) (g/day) | 3.0 (1.3–6.2) | 3.0 (1.1–6.6) | 0.8 |
| Previous immunosuppressive treatment, n (%) | 54 (75.0%) | 17 (65.4%) | 0.27 |
| Type of nephropathy, n | 0.7 | ||
| MPGN | 15 | 10 | |
| Membranous nephropathy | 16 | 4 | |
| Lupus nephritis | 15 | 4 | |
| AAV | 10 | 3 | |
| Minimal change disease | 7 | 2 | |
| FSGS | 4 | 2 | |
| Other | 5 | 1 | |
| RTX administration | |||
| Cumulative dose, median (IQR) (mg) | 2720 (2000–3425) | 3100 (2055–4200) | 0.02 |
| Cotrimoxazole prophylaxis, n (%) | 23 (32%) | 12 (46%) | 0.24 |
| Concomitant immunosuppressive treatment | |||
| Concomitant immunosuppression, n (%) | 53 (73.6%) | 22 (84.6%) | 0.11 |
| Plasmapheresis, n (%) | 11 (15.3%) | 8 (30.8%) | 0.06 |
| Steroids, n (%) | 47 (65.2%) | 21 (80.8%) | 0.18 |
| MMF, n (%) | 21 (29.1%) | 5 (19.2%) | 0.32 |
| CNI, n (%) | 11 (15.3%) | 1 (3.8%) | 0.12 |
| AZA, n (%) | 2 (2.8%) | 4 (15.4%) | 0.02 |
| Variables with missing data | No infection/death | Infection and/or death | |
| Gamma globulin level at M3, median (IQR) (g/L) (n = 58a) |
6.2 (4.8–8.6) (n = 40a) |
4.1 (2.8–6.8) (n = 18a) |
0.10 |
| Hypogammaglobulinemiab at M3, n (%) (n = 58a) |
19 (47.5%) (n = 40a) |
13 (72.2%) (n = 18a) |
0.06 |
| Leucopenia <3000/mm3 at M6, n (%) (n = 88a) |
7 (10.6%) (n = 66a) |
1 (4.5%) (n = 22a) |
0.39 |
| Lymphocyte count at M6, median (IQR) (n = 81a) |
1240 (810–1650) (n = 61a) |
1371 (740–2070) (n = 20a) |
0.35 |
| Count of CD19+ cells at M12, median (IQR) (/mm3) (n = 68a) |
28.0 (3.5–88) (n = 52a) |
1.5 (0–31) (n = 16a) |
0.59 |
aNumber of patients with data available for the considered variable.
bHypogammaglobulinemia defined as gamma globulin level <6 g/L.
The burden of immunosuppression contributed significantly to the risk of infection in our cohort. The total dose of RTX was significantly higher in patients who reached the combined end point. Our study did not reveal an increased risk for those patients receiving RTX and either long-term corticosteroid therapy or another immunosuppressive agent simultaneously (Table 3).
Data concerning gamma globulin level and CD19+ cell counts were available for 58 and 68 patients of our cohort, respectively. Neither the total gamma globulin level nor B cell depletion were statistically associated with the risk of reaching the composite end point.
Multiple logistic regression analysis was performed including all parameters that were associated (P < 0.2) with the infection risk in bivariate tests (age, diabetes, RTX dose, initial serum creatinine, AZA use, CNI use, plasmapheresis use, steroid use), except gamma globulin due to missing data. These analyses showed that diabetes, AZA use and total RTX dose remained statistically associated with the risk of reaching the composite end point in our population (Table 4).
Table 4.
Multivariate analysis for the combined primary outcome death and/or infectious complication using a multiple logistic regression analysis, which included all parameters that were associated with the infectious risk by bivariate tests with P-value <0.2
| P-value | Odds ratio (95% CI) | |
|---|---|---|
| Age | 0.93 | 1.0020 (0.9621–1.0432) |
| RTX dose | 0.01 | 1.0005 (1.0001–1.0009) |
| Diabetes mellitus | 0.006 | 6.8057 (1.7212–26.9095) |
| Initial serum creatinine | 0.05 | 1.0047 (0.9999–1.0094) |
| Plasmapheresis | 0.92 | 1.0687 (0.2670–4.2768) |
| Steroids | 0.59 | 1.4266 (0.3820–5.3276) |
| CNI | 0.58 | 0.4864 (0.0349–6.7317) |
| AZA | 0.03 | 10.4347 (1.3132–82.9109) |
A Kaplan–Meier survival analysis for the combined end point of death and/or infection was performed after grouping the patients according to their initial eGFR. A threshold of 45 mL/min/1.73 m2, corresponding to the median value of initial eGFR in our cohort, clearly divided the patients into two groups. The higher eGFR group had better event-free survival, as illustrated in Figure 2. Of note, the RTX cumulative dose was higher in the group with a higher eGFR, with a median RTX cumulative dose of 2960 mg (IQR 2350–3860) for patients with an eGFR >45 mL/min/1.73 m2 versus 2400 mg (IQR 2000–3400) for patients with an eGFR <45 mL/min/1.73 m2 (P = 0.09). The immunosuppressive regimen according to the eGFR is described in the Supplementary data, Table S1. Although plasmapheresis was associated with an eGFR <45 mL/min/1.73 m2 and MMF or CNI with an eGFR >45 mL/min/1.73 m2, none of these variables were associated with the primary end point by bivariate or multivariate analysis (Tables 3 and 4).
Fig. 2.
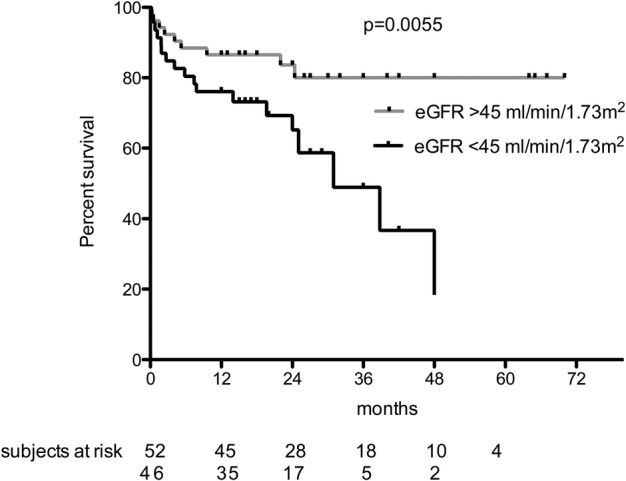
Kaplan–Meier survival analysis without death and/or infectious complication by grouping the patients according to their initial eGFR.
Discussion
In the present study, we showed that the overall incidence of severe infectious complications after RTX treatment in a population with glomerular disease was especially high and reached 21.6 per 100 patient-years. Compared with other published study populations without overt renal disease, this incidence is significantly higher than has been reported in SLE (6.6 per 100 patient-years to 9.5%) [27, 28] or rheumatoid arthritis (1.9–5.6 per 100 patient-years) [23–26]. Higher infection rates have been observed for autoimmune diseases with renal involvement, as in the examples of lupus nephritis (16.1 per 100 patient-years) [11], cryoglobulinemic vasculitis (14.1 per 100 patient-years) [10] and AAV (18–36%) [3, 5, 32].
Our results identify the severity of renal dysfunction as a risk factor for severe infectious events by bivariate analysis and it remains borderline significant by multivariate analysis (P = 0.05). The eGFR cut-off value of 45 mL/min/1.73 m2 used in the Kaplan–Meier analysis is the median eGFR value in our cohort, matching with the stage 3a–3b threshold according to the Kidney Disease Outcomes Quality Initiative chronic kidney disease (CKD) classification. These data are consistent with results of previous studies in RTX-treated patients showing that worse renal function may be associated with a higher risk of infection [10, 30]. According to the literature, RTX pharmacokinetics are not modified by kidney failure [33]. However, CKD is known to cause defects in innate and adaptive immunity for both T cell– and B cell–mediated responses [34, 35]. This effect of CKD could be additive to the risk of infection induced by RTX-induced B cell depletion. Of note, neither the specific glomerular disease nor the presence of nephrotic syndrome was associated with a significantly increased risk of infection and/or death. Another risk factor we show for the development of infection is the presence of prior or new-onset diabetes mellitus. This is in accordance with the findings from Kamar et al. [29] in a renal transplant recipient population and Heusele et al. [36] in a population of patients with autoimmune diseases.
One possible explanation for the higher incidence of infectious complications in our cohort compared with other studies might be the combination of RTX with other immunosuppressive therapies. Of note, 69.4% of our patients were on corticosteroid therapy in association with RTX treatment and 41.8% received another immunosuppressive drug during follow-up. In contrast, a remarkably safe profile of RTX monotherapy has been shown in membranous nephropathy [8, 9] or idiopathic nephrotic syndrome [37]. We found an association for an increased infection risk with AZA use that remained significant in multivariate analysis. Nevertheless, the number of patients was very small, with only six patients taking some AZA in combination with RTX and most of them (four of six patients) receiving other immunosuppressive treatments, such as MMF. We did not find any significant associations for administration of other immunosuppressive treatments used before or after RTX administration, except for AZA. However, we did not retrieve the cumulative dose of each immunosuppressive treatment. This point could be a limitation of our study, considering that in some studies steroid dosage has been identified as a risk factor for severe infection whereas steroid use per se was not associated with a higher risk of infection [26].
Another potential explanation for the high risk of infection seen in our population could be hypogammaglobulinemia. Hypogammaglobulinemia is frequently seen in nephrotic syndrome, even before the introduction of immunosuppression, but it is also a known side effect of RTX. Even though the link between hypoglobulinemia and infection risk after RTX [38] is not found consistently, several studies have identified it as a risk factor for infectious complication [10, 26, 36, 39]. In our study with limited data on gamma globulin levels, the association between the gamma globulin level and the outcome did not reach significance. Indeed, some data were missing and neither immunoglobulin G levels nor complement values were available to refine our analysis. Moreover, most of the 25 patients in our cohort with MPGN were cryoglobulinemic, a condition that can lead to functional hypogammaglobulinemia even if the gamma globulin level is preserved. Also, 15 patients in our study received prophylactic intravenous immunoglobulin infusion for hypogammaglobulinemia, possibly masking the relationship between gamma globulin level and infection risk due to a preventive effect as suggested by Roberts et al. [40]. Of note, anti-infectious prophylaxis with polyvalent immunoglobulin is actually recommended only for hypogammaglobulinemic patients suffering from recurrent infectious complications. Therefore, prospective studies are needed before suggesting their systematic use as primary prevention for patients with RTX-induced hypogammaglobulinemia.
Finally, we observed that patients who died or presented with an infectious complication in this cohort had received a higher cumulative dose of RTX than those who did not reach these end points. Despite a very low odds ratio (OR) [1.0005 (95% CI 1.0001–1.0009)], the RTX dose remained significant by multivariate analysis (P = 0.01) and thus could have a clinical impact for the highest cumulative doses (up to 8000 mg in our cohort). Although it seems that a single course of RTX is possibly sufficient in specific indications (e.g. membranous nephropathy [9]), other diseases seem to require repeated infusions in order to avoid relapse (e.g. AAV [41]). Preliminary data, such as those published in the context of membranous nephropathy, suggest that when RTX is given as a single-course treatment without any other immunosuppressive agents, infectious side effects are extremely rare [9]. Due to the large variety of protocols for RTX maintenance therapy in our cohort, we cannot address a specific risk according to the reinfusion protocol. Further studies are needed to quantify the precise risk for follow-up doses of RTX. Moreover, some patients develop a very prolonged CD19 depletion after RTX infusion [42, 43]. In our cohort, we did not define any significant risks due to cytopenia or prolonged CD19 depletion. However, our observation of two late mycobacterial infections emphasizes the infection risk in those patients with prolonged effects of RTX. Interestingly, most of the patients who presented with a late infectious complication had MPGN. The follow-up over 12 months in our cohort is too heterogeneous to draw any specific conclusions. However this observation could point out a long-term risk for these patients that has not been shown before, as most studies identified infections only during the 6- to 12-month period following RTX treatment.
A very large majority of the reported infections were bacterial and these were mostly pneumonia. Our study does not include data regarding vaccination, but the frequency of bacterial pneumonia diagnosis suggests that Streptococcus pneumonia and Haemophilus influenzae vaccinations should be proposed for every renal patient prior to RTX use. Of interest, Heusele et al. [36] found a lower incidence of infection after RTX treatment in patients with a history of prior pneumococcal vaccination.
On the other hand, a number of our patients presented with rare opportunistic infections, such as invasive candidiasis, aspergillosis or CMV infection. This observation underlines the potential role of B cells not only in classical antibody-mediated protection against encapsulated bacteria, but also in defence against other opportunistic agents thought to be controlled by innate or T cell cytotoxic immune response. Our study is not sufficiently powered to support specific guidelines, however, antifungal prophylaxis might be considered as a recommended treatment in AAV cases treated with RTX [44]. Of note, we did not observe any hepatitis B reactivations in our series, even though many case reports and an alert from the US Food and Drug Administration have acknowledged the potential risk in RTX-treated patients [45, 46]. No case of pneumocystosis was observed in this study, although only a small proportion of our patients (35%) received a cotrimoxazole-based prophylaxis. Rare cases of pneumocystosis have been reported in some series of patients following RTX therapy [47, 48]. No patient presented with progressive multifocal leukoencephalopathy (PML), even though rare cases have been previously described after RTX infusion for SLE, rheumatoid arthritis or immune thrombocytopenia [49, 50].
Our study has several limitations, such as its retrospective design, missing data for some biologic parameters such as WBC or immunoglobulin level and the heterogeneity of underlying diseases and treatment protocols. Importantly, the absence of a control cohort in our study strongly limits analysis of the role pf RTX in the assessment of infection risk. Nevertheless, our findings underline the need for more prospective, controlled trials to investigate the efficacy and tolerance of RTX in this specific population. This study, conducted in multiple reference nephrology centres, reflects the ‘pragmatic’ use of RTX and has defined some points that should be explored in future studies.
In summary, the risk of infection after RTX therapy must be considered among patients with nephropathy, especially in those with diabetes and renal failure. Concomitant use of AZA may increase this risk and further studies are needed to clarify these results. Clinicians should be attentive to prophylaxis (pneumococcal and haemophilus vaccination; hepatitis B virus immunization; pneumocystis prophylaxis, especially in AAV; intravenous immunoglobulin in symptomatic hypogammaglobulinemia) as highlighted in recent guidelines for AAV [44, 51].
Supplementary data
Supplementary data are available online at http://ndt.oxfordjournals.org.
Conflict of interest statement
A. Karras declared lecture fees from Roche.
Supplementary Material
References
- 1. Mei HE, Schmidt S, Dörner T. Rationale of anti-CD19 immunotherapy: an option to target autoreactive plasma cells in autoimmunity. Arthritis Res Ther 2012; 14Suppl 5: S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stone JH, Merkel PA, Spiera R et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363: 221–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jones RB, Tervaert JWC, Hauser T et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010; 363: 211–220 [DOI] [PubMed] [Google Scholar]
- 4. Roccatello D, Sciascia S, Rossi D et al. Long-term effects of rituximab added to cyclophosphamide in refractory patients with vasculitis. Am J Nephrol 2011; 34: 175–180 [DOI] [PubMed] [Google Scholar]
- 5. Guillevin L, Pagnoux C, Karras A et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med 2014; 371: 1771–1780 [DOI] [PubMed] [Google Scholar]
- 6. Jones RB, Furuta S, Tervaert JWC et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis: 2-year results of a randomised trial. Ann Rheum Dis 2015; 74: 1178–1182 [DOI] [PubMed] [Google Scholar]
- 7. Bomback AS, Derebail VK, McGregor JG et al. Rituximab therapy for membranous nephropathy: a systematic review. Clin J Am Soc Nephrol 2009; 4: 734–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fervenza FC, Abraham RS, Erickson SB et al. Rituximab therapy in idiopathic membranous nephropathy: a 2-year study. Clin J Am Soc Nephrol 2010; 5: 2188–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruggenenti P, Cravedi P, Chianca A et al. Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol 2012; 23: 1416–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Terrier B, Launay D, Kaplanski G et al. Safety and efficacy of rituximab in nonviral cryoglobulinemia vasculitis: data from the French Autoimmunity and Rituximab registry. Arthritis Care Res 2010; 62: 1787–1795 [DOI] [PubMed] [Google Scholar]
- 11. Rovin BH, Furie R, Latinis K et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum 2012; 64: 1215–1226 [DOI] [PubMed] [Google Scholar]
- 12. Condon MB, Ashby D, Pepper RJ et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis 2013; 72: 1280–1286 [DOI] [PubMed] [Google Scholar]
- 13. Catapano F, Chaudhry AN, Jones RB et al. Long-term efficacy and safety of rituximab in refractory and relapsing systemic lupus erythematosus. Nephrol Dial Transplant 2010; 25: 3586–3592 [DOI] [PubMed] [Google Scholar]
- 14. Munyentwali H, Bouachi K, Audard V et al. Rituximab is an efficient and safe treatment in adults with steroid-dependent minimal change disease. Kidney Int 2013; 83: 511–516 [DOI] [PubMed] [Google Scholar]
- 15. Iijima K, Sako M, Nozu K et al. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet 2014; 384: 1273–1281 [DOI] [PubMed] [Google Scholar]
- 16. Suri M, Tran K, Sharma AP et al. Remission of steroid-resistant nephrotic syndrome due to focal and segmental glomerulosclerosis using rituximab. Int Urol Nephrol 2008; 40: 807–810 [DOI] [PubMed] [Google Scholar]
- 17. Kronbichler A, Kerschbaum J, Fernandez-Fresnedo G et al. Rituximab treatment for relapsing minimal change disease and focal segmental glomerulosclerosis: a systematic review. Am J Nephrol 2014; 39: 322–330. [DOI] [PubMed] [Google Scholar]
- 18. van den Hoogen MWF, Kamburova EG, Baas MC et al. Rituximab as induction therapy after renal transplantation: a randomized, double-blind, placebo-controlled study of efficacy and safety. Am J Transplant 2015; 15: 407–416 [DOI] [PubMed] [Google Scholar]
- 19. Vo AA, Lukovsky M, Toyoda M et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med 2008; 359: 242–251 [DOI] [PubMed] [Google Scholar]
- 20. Jackson AM, Kraus ES, Orandi BJ et al. A closer look at rituximab induction on HLA antibody rebound following HLA-incompatible kidney transplantation. Kidney Int 2015; 87: 409–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Faguer S, Kamar N, Guilbeaud-Frugier C et al. Rituximab therapy for acute humoral rejection after kidney transplantation. Transplantation 2007; 83: 1277–1280 [DOI] [PubMed] [Google Scholar]
- 22. Kaposztas Z, Podder H, Mauiyyedi S et al. Impact of rituximab therapy for treatment of acute humoral rejection. Clin Transplant 2009; 23: 63–73 [DOI] [PubMed] [Google Scholar]
- 23. Edwards JCW, Szczepanski L, Szechinski J et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 2004; 350: 2572–2581 [DOI] [PubMed] [Google Scholar]
- 24. Cohen SB, Emery P, Greenwald MW et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum 2006; 54: 2793–2806 [DOI] [PubMed] [Google Scholar]
- 25. Emery P, Fleischmann R, Filipowicz-Sosnowska A et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum 2006; 54: 1390–1400 [DOI] [PubMed] [Google Scholar]
- 26. Gottenberg JE, Ravaud P, Bardin T et al. Risk factors for severe infections in patients with rheumatoid arthritis treated with rituximab in the autoimmunity and rituximab registry. Arthritis Rheum 2010; 62: 2625–2632 [DOI] [PubMed] [Google Scholar]
- 27. Terrier B, Amoura Z, Ravaud P et al. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French Autoimmunity and Rituximab registry. Arthritis Rheum 2010; 62: 2458–2466 [DOI] [PubMed] [Google Scholar]
- 28. Merrill JT, Neuwelt CM, Wallace DJ et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase ii/iii systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 2010; 62: 222–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kamar N, Milioto O, Puissant-Lubrano B et al. Incidence and predictive factors for infectious disease after rituximab therapy in kidney-transplant patients. Am J Transplant 2010; 10: 89–98 [DOI] [PubMed] [Google Scholar]
- 30. Scemla A, Loupy A, Candon S et al. Incidence of infectious complications in highly sensitized renal transplant recipients treated by rituximab: a case-controlled study. Transplantation 2010; 90: 1180–1184 [DOI] [PubMed] [Google Scholar]
- 31. Whalen C, Horsburgh CR, Hom D et al. Site of disease and opportunistic infection predict survival in HIV-associated tuberculosis. AIDS 1997; 11: 455–460 [DOI] [PubMed] [Google Scholar]
- 32. Guillevin L. Infections in vasculitis. Best Pract Res Clin Rheumatol 2013; 27: 19–31 [DOI] [PubMed] [Google Scholar]
- 33. Jillella AP, Dainer PM, Kallab AM et al. Treatment of a patient with end-stage renal disease with rituximab: pharmacokinetic evaluation suggests rituximab is not eliminated by hemodialysis. Am J Hematol 2002; 71: 219–222 [DOI] [PubMed] [Google Scholar]
- 34. Kato S, Chmielewski M, Honda H et al. Aspects of immune dysfunction in end-stage renal disease. Clin J Am Soc Nephrol 2008; 3: 1526–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cohen G, Haag-Weber M, Hörl WH. Immune dysfunction in uremia. Kidney Int Suppl 1997; 62: S79–S82 [PubMed] [Google Scholar]
- 36. Heusele M, Clerson P, Guery B et al. Risk factors for severe bacterial infections in patients with systemic autoimmune diseases receiving rituximab. Clin Rheumatol 2014; 33: 799–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ruggenenti P, Ruggiero B, Cravedi P et al. Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol 2014; 25: 850–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van Vollenhoven RF, Emery P, Bingham CO et al. Longterm safety of patients receiving rituximab in rheumatoid arthritis clinical trials. J Rheumatol 2010; 37: 558–567 [DOI] [PubMed] [Google Scholar]
- 39. Besada E, Koldingsnes W, Nossent JC. Long-term efficacy and safety of pre-emptive maintenance therapy with rituximab in granulomatosis with polyangiitis: results from a single centre. Rheumatology 2013; 52: 2041–2047 [DOI] [PubMed] [Google Scholar]
- 40. Roberts DM, Jones RB, Smith RM et al. Immunoglobulin G replacement for the treatment of infective complications of rituximab-associated hypogammaglobulinemia in autoimmune disease: a case series. J Autoimmun 2015; 57: 24–29 [DOI] [PubMed] [Google Scholar]
- 41. Smith RM, Jones RB, Jayne DRW. Progress in treatment of ANCA-associated vasculitis. Arthritis Res Ther 2012; 14: 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lu TY-T, Jónsdóttir T, Jónsdóttir T et al. Prolonged B-cell depletion following rituximab therapy in systemic lupus erythematosus: a report of two cases. Ann Rheum Dis 2008; 67: 1493–1494 [DOI] [PubMed] [Google Scholar]
- 43. Genberg H, Hansson A, Wernerson A et al. Pharmacodynamics of rituximab in kidney allotransplantation. Am J Transplant 2006; 6: 2418–2428 [DOI] [PubMed] [Google Scholar]
- 44. Ntatsaki E, Carruthers D, Chakravarty K et al. BSR and BHPR guideline for the management of adults with ANCA-associated vasculitis. Rheumatology 2014; 53: 2306–2309 [DOI] [PubMed] [Google Scholar]
- 45. Mitka M. FDA: increased HBV reactivation risk with ofatumumab or rituximab. JAMA 2013; 310: 1664. [DOI] [PubMed] [Google Scholar]
- 46. Droz N, Gilardin L, Cacoub P et al. Kinetic profiles and management of hepatitis B virus reactivation in patients with immune-mediated inflammatory diseases. Arthritis Care Res 2013; 65: 1504–1514 [DOI] [PubMed] [Google Scholar]
- 47. Martin-Garrido I, Carmona EM, Specks U et al. Pneumocystis pneumonia in patients treated with rituximab. Chest 2013; 144: 258–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Besada E, Nossent JC. Should Pneumocystis jiroveci prophylaxis be recommended with rituximab treatment in ANCA-associated vasculitis? Clin Rheumatol 2013; 32: 1677–1681 [DOI] [PubMed] [Google Scholar]
- 49. Carson KR, Evens AM, Richey EA et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 2009; 113: 4834–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kamar N, Mengelle C, Rostaing L. Incidence of JC-virus replication after rituximab therapy in solid-organ transplant patients. Am J Transplant 2009; 9: 244–245 [DOI] [PubMed] [Google Scholar]
- 51. Charles P, Bienvenu B, Bonnotte B et al. Rituximab: recommendations of the French Vasculitis Study Group (FVSG) for induction and maintenance treatments of adult, antineutrophil cytoplasm antibody-associated necrotizing vasculitides. Presse Med 2013; 42: 1317–1330 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


