Abstract
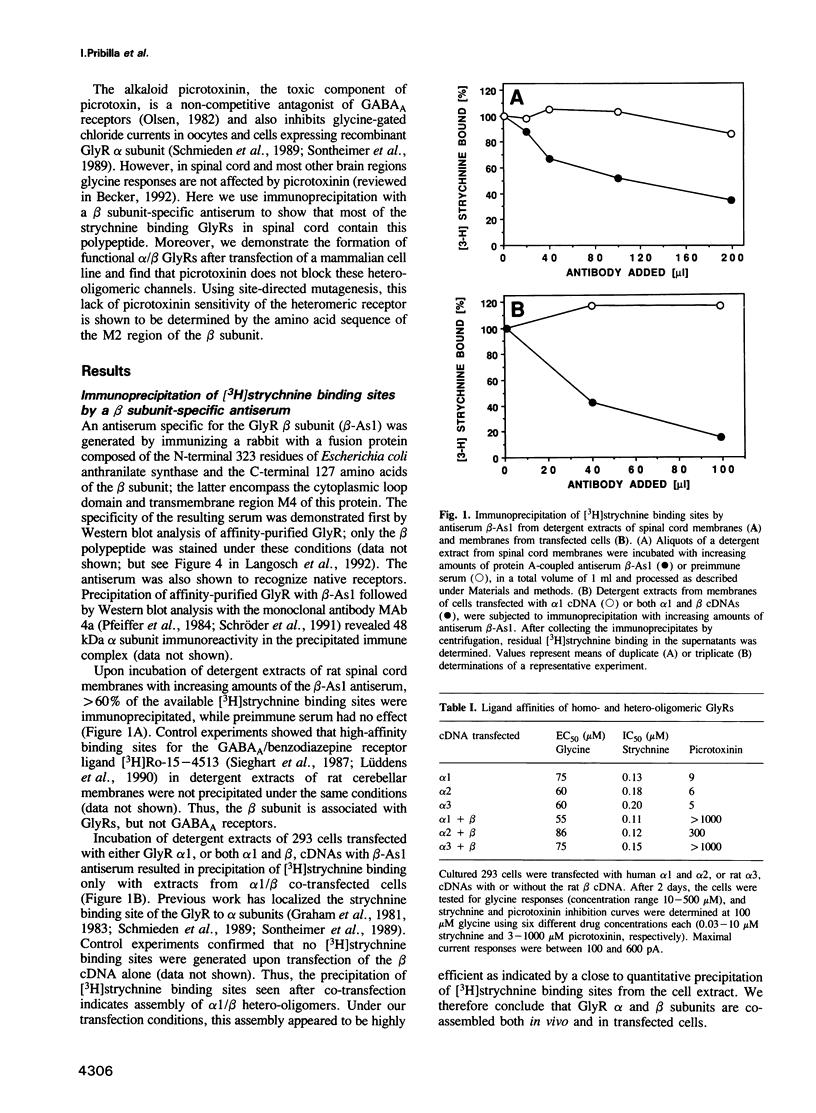
Purified preparations of the inhibitory glycine receptor (GlyR) contain alpha and beta subunits, which share homologous primary structures and a common transmembrane topology with other members of the ligand-gated ion channel superfamily. Here, a beta subunit-specific antiserum was shown to precipitate the [3H]strychnine binding sites localized on alpha subunits from membrane extracts of both rat spinal cord and mammalian cells co-transfected with alpha and beta cDNAs. Further, inhibition of alpha homo-oligomeric GlyRs by picrotoxinin, a non-competitive blocker of ion flow, was reduced 50- to 200-fold for alpha/beta hetero-oligomeric receptors generated by cotransfection. Site-directed mutagenesis identified residues within the second predicted transmembrane segment (M2) of the beta subunit as major determinants of picrotoxinin resistance. These data implicate the M2 segment in blocker binding to and lining of the GlyR chloride channel.
Full text
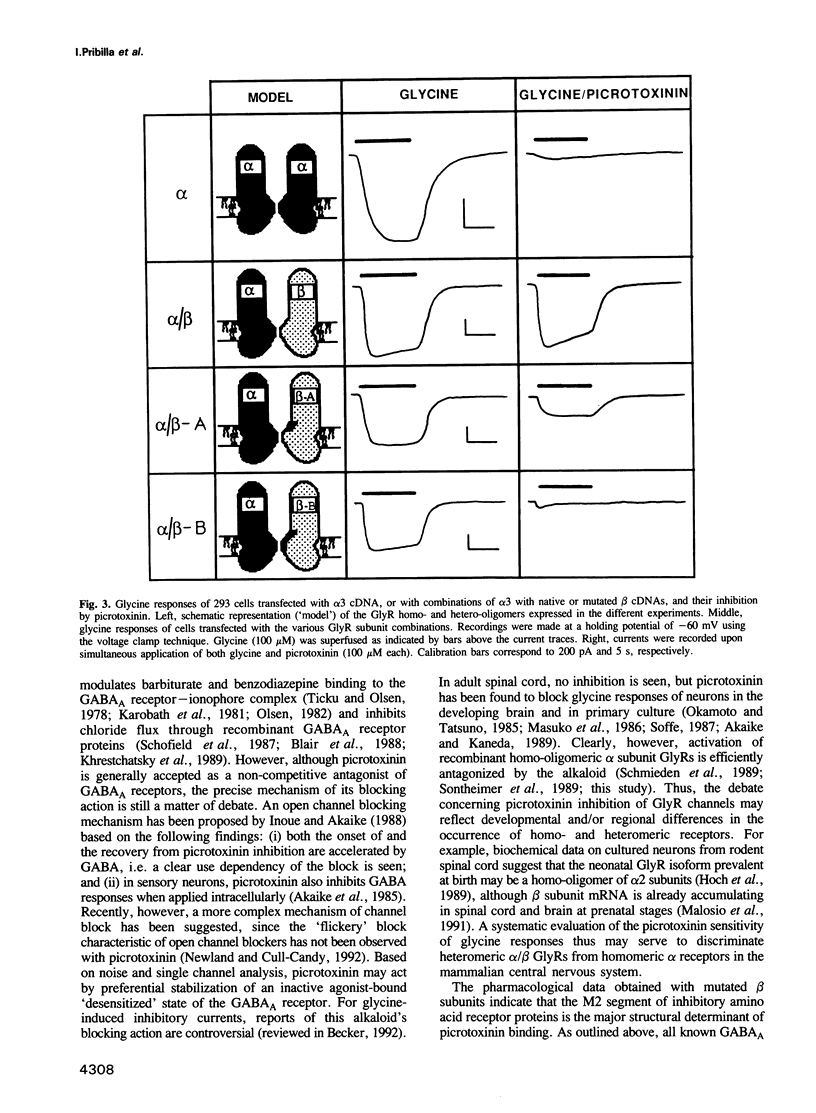
PDF

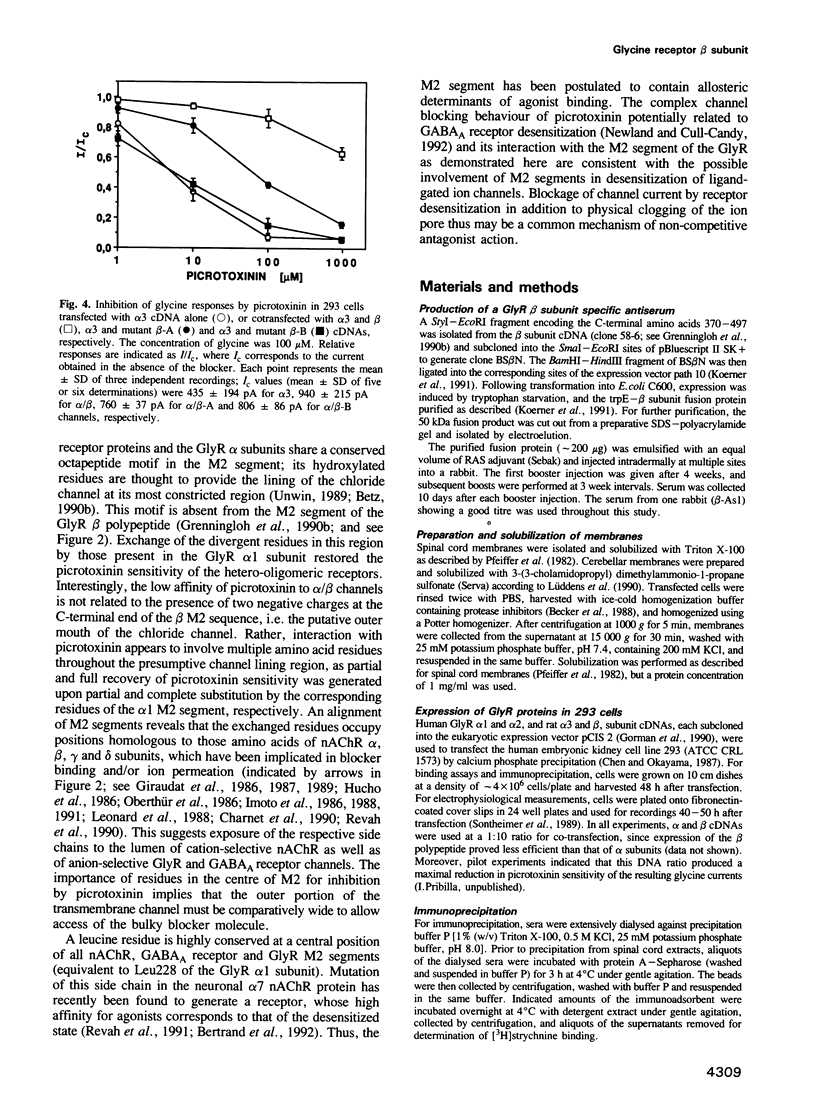


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Hattori K., Oomura Y., Carpenter D. O. Bicuculline and picrotoxin block gamma-aminobutyric acid-gated Cl- conductance by different mechanisms. Experientia. 1985 Jan 15;41(1):70–71. doi: 10.1007/BF02005880. [DOI] [PubMed] [Google Scholar]
- Akaike N., Kaneda M. Glycine-gated chloride current in acutely isolated rat hypothalamic neurons. J Neurophysiol. 1989 Dec;62(6):1400–1409. doi: 10.1152/jn.1989.62.6.1400. [DOI] [PubMed] [Google Scholar]
- Barker J. L., McBurney R. N., Mathers D. A. Convulsant-induced depression of amino acid responses in cultured mouse spinal neurones studied under voltage clamp. Br J Pharmacol. 1983 Dec;80(4):619–629. doi: 10.1111/j.1476-5381.1983.tb10051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C. M., Hermans-Borgmeyer I., Schmitt B., Betz H. The glycine receptor deficiency of the mutant mouse spastic: evidence for normal glycine receptor structure and localization. J Neurosci. 1986 May;6(5):1358–1364. doi: 10.1523/JNEUROSCI.06-05-01358.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C. M., Hoch W., Betz H. Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO J. 1988 Dec 1;7(12):3717–3726. doi: 10.1002/j.1460-2075.1988.tb03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand D., Devillers-Thiéry A., Revah F., Galzi J. L., Hussy N., Mulle C., Bertrand S., Ballivet M., Changeux J. P. Unconventional pharmacology of a neuronal nicotinic receptor mutated in the channel domain. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1261–1265. doi: 10.1073/pnas.89.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz H. Glycine receptors: heterogeneous and widespread in the mammalian brain. Trends Neurosci. 1991 Oct;14(10):458–461. doi: 10.1016/0166-2236(91)90045-v. [DOI] [PubMed] [Google Scholar]
- Betz H. Homology and analogy in transmembrane channel design: lessons from synaptic membrane proteins. Biochemistry. 1990 Apr 17;29(15):3591–3599. doi: 10.1021/bi00467a001. [DOI] [PubMed] [Google Scholar]
- Betz H. Ligand-gated ion channels in the brain: the amino acid receptor superfamily. Neuron. 1990 Oct;5(4):383–392. doi: 10.1016/0896-6273(90)90077-s. [DOI] [PubMed] [Google Scholar]
- Blair L. A., Levitan E. S., Marshall J., Dionne V. E., Barnard E. A. Single subunits of the GABAA receptor form ion channels with properties of the native receptor. Science. 1988 Oct 28;242(4878):577–579. doi: 10.1126/science.2845583. [DOI] [PubMed] [Google Scholar]
- Bormann J., Hamill O. P., Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987 Apr;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson A., Unwin P. N. Quaternary structure of the acetylcholine receptor. Nature. 1985 Jun 6;315(6019):474–477. doi: 10.1038/315474a0. [DOI] [PubMed] [Google Scholar]
- Charnet P., Labarca C., Leonard R. J., Vogelaar N. J., Czyzyk L., Gouin A., Davidson N., Lester H. A. An open-channel blocker interacts with adjacent turns of alpha-helices in the nicotinic acetylcholine receptor. Neuron. 1990 Jan;4(1):87–95. doi: 10.1016/0896-6273(90)90445-l. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E., Couturier S., Ballivet M. Pentameric structure and subunit stoichiometry of a neuronal nicotinic acetylcholine receptor. Nature. 1991 Mar 21;350(6315):235–238. doi: 10.1038/350235a0. [DOI] [PubMed] [Google Scholar]
- Giraudat J., Dennis M., Heidmann T., Chang J. Y., Changeux J. P. Structure of the high-affinity binding site for noncompetitive blockers of the acetylcholine receptor: serine-262 of the delta subunit is labeled by [3H]chlorpromazine. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2719–2723. doi: 10.1073/pnas.83.8.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudat J., Dennis M., Heidmann T., Haumont P. Y., Lederer F., Changeux J. P. Structure of the high-affinity binding site for noncompetitive blockers of the acetylcholine receptor: [3H]chlorpromazine labels homologous residues in the beta and delta chains. Biochemistry. 1987 May 5;26(9):2410–2418. doi: 10.1021/bi00383a003. [DOI] [PubMed] [Google Scholar]
- Giraudat J., Gali J., Revah F., Changeux J., Haumont P., Lederer F. The noncompetitive blocker [(3)H]chlorpromazine labels segment M2 but not segment M1 of the nicotinic acetylcholine receptor alpha-subunit. FEBS Lett. 1989 Aug 14;253(1-2):190–198. doi: 10.1016/0014-5793(89)80957-3. [DOI] [PubMed] [Google Scholar]
- Graham D., Pfeiffer F., Betz H. Photoaffinity-labelling of the glycine receptor of rat spinal cord. Eur J Biochem. 1983 Apr 5;131(3):519–525. doi: 10.1111/j.1432-1033.1983.tb07292.x. [DOI] [PubMed] [Google Scholar]
- Graham D., Pfeiffer F., Betz H. UV light-induced cross-linking of strychnine to the glycine receptor of rat spinal cord membranes. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1330–1335. doi: 10.1016/s0006-291x(81)80157-x. [DOI] [PubMed] [Google Scholar]
- Graham D., Pfeiffer F., Simler R., Betz H. Purification and characterization of the glycine receptor of pig spinal cord. Biochemistry. 1985 Feb 12;24(4):990–994. doi: 10.1021/bi00325a027. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Pribilla I., Prior P., Multhaup G., Beyreuther K., Taleb O., Betz H. Cloning and expression of the 58 kd beta subunit of the inhibitory glycine receptor. Neuron. 1990 Jun;4(6):963–970. doi: 10.1016/0896-6273(90)90149-a. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Rienitz A., Schmitt B., Methfessel C., Zensen M., Beyreuther K., Gundelfinger E. D., Betz H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987 Jul 16;328(6127):215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- Grenningloh G., Schmieden V., Schofield P. R., Seeburg P. H., Siddique T., Mohandas T. K., Becker C. M., Betz H. Alpha subunit variants of the human glycine receptor: primary structures, functional expression and chromosomal localization of the corresponding genes. EMBO J. 1990 Mar;9(3):771–776. doi: 10.1002/j.1460-2075.1990.tb08172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch W., Betz H., Becker C. M. Primary cultures of mouse spinal cord express the neonatal isoform of the inhibitory glycine receptor. Neuron. 1989 Sep;3(3):339–348. doi: 10.1016/0896-6273(89)90258-4. [DOI] [PubMed] [Google Scholar]
- Hucho F., Oberthür W., Lottspeich F. The ion channel of the nicotinic acetylcholine receptor is formed by the homologous helices M II of the receptor subunits. FEBS Lett. 1986 Sep 1;205(1):137–142. doi: 10.1016/0014-5793(86)80881-x. [DOI] [PubMed] [Google Scholar]
- Hume R. I., Dingledine R., Heinemann S. F. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science. 1991 Aug 30;253(5023):1028–1031. doi: 10.1126/science.1653450. [DOI] [PubMed] [Google Scholar]
- Imoto K., Busch C., Sakmann B., Mishina M., Konno T., Nakai J., Bujo H., Mori Y., Fukuda K., Numa S. Rings of negatively charged amino acids determine the acetylcholine receptor channel conductance. Nature. 1988 Oct 13;335(6191):645–648. doi: 10.1038/335645a0. [DOI] [PubMed] [Google Scholar]
- Imoto K., Konno T., Nakai J., Wang F., Mishina M., Numa S. A ring of uncharged polar amino acids as a component of channel constriction in the nicotinic acetylcholine receptor. FEBS Lett. 1991 Sep 9;289(2):193–200. doi: 10.1016/0014-5793(91)81068-j. [DOI] [PubMed] [Google Scholar]
- Imoto K., Methfessel C., Sakmann B., Mishina M., Mori Y., Konno T., Fukuda K., Kurasaki M., Bujo H., Fujita Y. Location of a delta-subunit region determining ion transport through the acetylcholine receptor channel. Nature. 1986 Dec 18;324(6098):670–674. doi: 10.1038/324670a0. [DOI] [PubMed] [Google Scholar]
- Inoue M., Akaike N. Blockade of gamma-aminobutyric acid-gated chloride current in frog sensory neurons by picrotoxin. Neurosci Res. 1988 Jun;5(5):380–394. doi: 10.1016/0168-0102(88)90024-7. [DOI] [PubMed] [Google Scholar]
- Karlin A., Holtzman E., Yodh N., Lobel P., Wall J., Hainfeld J. The arrangement of the subunits of the acetylcholine receptor of Torpedo californica. J Biol Chem. 1983 Jun 10;258(11):6678–6681. [PubMed] [Google Scholar]
- Karobath M., Drexler G., Supavilai P. Modulation by picrotoxin and IPTBO of 3H-flunitrazepam binding to the GABA/benzodiazepine receptor complex of rat cerebellum. Life Sci. 1981 Jan 19;28(3):307–313. doi: 10.1016/0024-3205(81)90738-4. [DOI] [PubMed] [Google Scholar]
- Khrestchatisky M., MacLennan A. J., Chiang M. Y., Xu W. T., Jackson M. B., Brecha N., Sternini C., Olsen R. W., Tobin A. J. A novel alpha subunit in rat brain GABAA receptors. Neuron. 1989 Dec;3(6):745–753. doi: 10.1016/0896-6273(89)90243-2. [DOI] [PubMed] [Google Scholar]
- Koerner T. J., Hill J. E., Myers A. M., Tzagoloff A. High-expression vectors with multiple cloning sites for construction of trpE fusion genes: pATH vectors. Methods Enzymol. 1991;194:477–490. doi: 10.1016/0076-6879(91)94036-c. [DOI] [PubMed] [Google Scholar]
- Kuhse J., Kuryatov A., Maulet Y., Malosio M. L., Schmieden V., Betz H. Alternative splicing generates two isoforms of the alpha 2 subunit of the inhibitory glycine receptor. FEBS Lett. 1991 May 20;283(1):73–77. doi: 10.1016/0014-5793(91)80557-j. [DOI] [PubMed] [Google Scholar]
- Kuhse J., Schmieden V., Betz H. A single amino acid exchange alters the pharmacology of neonatal rat glycine receptor subunit. Neuron. 1990 Dec;5(6):867–873. doi: 10.1016/0896-6273(90)90346-h. [DOI] [PubMed] [Google Scholar]
- Kuhse J., Schmieden V., Betz H. Identification and functional expression of a novel ligand binding subunit of the inhibitory glycine receptor. J Biol Chem. 1990 Dec 25;265(36):22317–22320. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langosch D., Becker C. M., Betz H. The inhibitory glycine receptor: a ligand-gated chloride channel of the central nervous system. Eur J Biochem. 1990 Nov 26;194(1):1–8. doi: 10.1111/j.1432-1033.1990.tb19419.x. [DOI] [PubMed] [Google Scholar]
- Langosch D., Hartung K., Grell E., Bamberg E., Betz H. Ion channel formation by synthetic transmembrane segments of the inhibitory glycine receptor--a model study. Biochim Biophys Acta. 1991 Mar 18;1063(1):36–44. doi: 10.1016/0005-2736(91)90350-h. [DOI] [PubMed] [Google Scholar]
- Langosch D., Hoch W., Betz H. The 93 kDa protein gephyrin and tubulin associated with the inhibitory glycine receptor are phosphorylated by an endogenous protein kinase. FEBS Lett. 1992 Feb 24;298(2-3):113–117. doi: 10.1016/0014-5793(92)80034-e. [DOI] [PubMed] [Google Scholar]
- Langosch D., Thomas L., Betz H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7394–7398. doi: 10.1073/pnas.85.19.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson E., Howlett B., Jagendorf A. Artificial reductant enhancement of the Lowry method for protein determination. Anal Biochem. 1986 Jun;155(2):243–248. doi: 10.1016/0003-2697(86)90432-x. [DOI] [PubMed] [Google Scholar]
- Leonard R. J., Labarca C. G., Charnet P., Davidson N., Lester H. A. Evidence that the M2 membrane-spanning region lines the ion channel pore of the nicotinic receptor. Science. 1988 Dec 16;242(4885):1578–1581. doi: 10.1126/science.2462281. [DOI] [PubMed] [Google Scholar]
- Lüddens H., Pritchett D. B., Köhler M., Killisch I., Keinänen K., Monyer H., Sprengel R., Seeburg P. H. Cerebellar GABAA receptor selective for a behavioural alcohol antagonist. Nature. 1990 Aug 16;346(6285):648–651. doi: 10.1038/346648a0. [DOI] [PubMed] [Google Scholar]
- Lüddens H., Wisden W. Function and pharmacology of multiple GABAA receptor subunits. Trends Pharmacol Sci. 1991 Feb;12(2):49–51. doi: 10.1016/0165-6147(91)90495-e. [DOI] [PubMed] [Google Scholar]
- Malosio M. L., Marquèze-Pouey B., Kuhse J., Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J. 1991 Sep;10(9):2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuko S., Nakajima Y., Nakajima S., Yamaguchi K. Noradrenergic neurons from the locus ceruleus in dissociated cell culture: culture methods, morphology, and electrophysiology. J Neurosci. 1986 Nov;6(11):3229–3241. doi: 10.1523/JNEUROSCI.06-11-03229.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori-Okamoto J., Tatsuno J. Development of sensitivity to GABA and glycine in cultured cerebellar neurons. Brain Res. 1985 Jun;352(2):249–258. doi: 10.1016/0165-3806(85)90112-9. [DOI] [PubMed] [Google Scholar]
- Newland C. F., Cull-Candy S. G. On the mechanism of action of picrotoxin on GABA receptor channels in dissociated sympathetic neurones of the rat. J Physiol. 1992 Feb;447:191–213. doi: 10.1113/jphysiol.1992.sp018998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Furutani Y., Hirose T., Asai M., Inayama S., Miyata T., Numa S. Primary structure of alpha-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature. 1982 Oct 28;299(5886):793–797. doi: 10.1038/299793a0. [DOI] [PubMed] [Google Scholar]
- Oberthür W., Muhn P., Baumann H., Lottspeich F., Wittmann-Liebold B., Hucho F. The reaction site of a non-competitive antagonist in the delta-subunit of the nicotinic acetylcholine receptor. EMBO J. 1986 Aug;5(8):1815–1819. doi: 10.1002/j.1460-2075.1986.tb04431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oiki S., Danho W., Madison V., Montal M. M2 delta, a candidate for the structure lining the ionic channel of the nicotinic cholinergic receptor. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8703–8707. doi: 10.1073/pnas.85.22.8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. W. GABA-benzodiazepine-barbiturate receptor interactions. J Neurochem. 1981 Jul;37(1):1–13. doi: 10.1111/j.1471-4159.1981.tb05284.x. [DOI] [PubMed] [Google Scholar]
- Olsen R. W., Tobin A. J. Molecular biology of GABAA receptors. FASEB J. 1990 Mar;4(5):1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- Pfeiffer F., Betz H. Solubilization of the glycine receptor from rat spinal cord. Brain Res. 1981 Dec 7;226(1-2):273–279. doi: 10.1016/0006-8993(81)91099-4. [DOI] [PubMed] [Google Scholar]
- Pfeiffer F., Graham D., Betz H. Purification by affinity chromatography of the glycine receptor of rat spinal cord. J Biol Chem. 1982 Aug 25;257(16):9389–9393. [PubMed] [Google Scholar]
- Pfeiffer F., Simler R., Grenningloh G., Betz H. Monoclonal antibodies and peptide mapping reveal structural similarities between the subunits of the glycine receptor of rat spinal cord. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7224–7227. doi: 10.1073/pnas.81.22.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revah F., Bertrand D., Galzi J. L., Devillers-Thiéry A., Mulle C., Hussy N., Bertrand S., Ballivet M., Changeux J. P. Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor. Nature. 1991 Oct 31;353(6347):846–849. doi: 10.1038/353846a0. [DOI] [PubMed] [Google Scholar]
- Revah F., Galzi J. L., Giraudat J., Haumont P. Y., Lederer F., Changeux J. P. The noncompetitive blocker [3H]chlorpromazine labels three amino acids of the acetylcholine receptor gamma subunit: implications for the alpha-helical organization of regions MII and for the structure of the ion channel. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4675–4679. doi: 10.1073/pnas.87.12.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Gómez A., Morato E., García-Calvo M., Valdivieso F., Mayor F., Jr Localization of the strychnine binding site on the 48-kilodalton subunit of the glycine receptor. Biochemistry. 1990 Jul 31;29(30):7033–7040. doi: 10.1021/bi00482a012. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieden V., Grenningloh G., Schofield P. R., Betz H. Functional expression in Xenopus oocytes of the strychnine binding 48 kd subunit of the glycine receptor. EMBO J. 1989 Mar;8(3):695–700. doi: 10.1002/j.1460-2075.1989.tb03428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieden V., Kuhse J., Betz H. Agonist pharmacology of neonatal and adult glycine receptor alpha subunits: identification of amino acid residues involved in taurine activation. EMBO J. 1992 Jun;11(6):2025–2032. doi: 10.1002/j.1460-2075.1992.tb05259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt B., Knaus P., Becker C. M., Betz H. The Mr 93,000 polypeptide of the postsynaptic glycine receptor complex is a peripheral membrane protein. Biochemistry. 1987 Feb 10;26(3):805–811. doi: 10.1021/bi00377a022. [DOI] [PubMed] [Google Scholar]
- Schofield P. R., Darlison M. G., Fujita N., Burt D. R., Stephenson F. A., Rodriguez H., Rhee L. M., Ramachandran J., Reale V., Glencorse T. A. Sequence and functional expression of the GABA A receptor shows a ligand-gated receptor super-family. Nature. 1987 Jul 16;328(6127):221–227. doi: 10.1038/328221a0. [DOI] [PubMed] [Google Scholar]
- Schröder S., Hoch W., Becker C. M., Grenningloh G., Betz H. Mapping of antigenic epitopes on the alpha 1 subunit of the inhibitory glycine receptor. Biochemistry. 1991 Jan 8;30(1):42–47. doi: 10.1021/bi00215a007. [DOI] [PubMed] [Google Scholar]
- Sieghart W., Eichinger A., Richards J. G., Möhler H. Photoaffinity labeling of benzodiazepine receptor proteins with the partial inverse agonist [3H]Ro 15-4513: a biochemical and autoradiographic study. J Neurochem. 1987 Jan;48(1):46–52. doi: 10.1111/j.1471-4159.1987.tb13125.x. [DOI] [PubMed] [Google Scholar]
- Soffe S. R. Ionic and pharmacological properties of reciprocal inhibition in Xenopus embryo motoneurones. J Physiol. 1987 Jan;382:463–473. doi: 10.1113/jphysiol.1987.sp016378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B., Seeburg P. H. Glutamate receptor channels: novel properties and new clones. Trends Pharmacol Sci. 1992 Jul;13(7):291–296. doi: 10.1016/0165-6147(92)90088-n. [DOI] [PubMed] [Google Scholar]
- Sontheimer H., Becker C. M., Pritchett D. B., Schofield P. R., Grenningloh G., Kettenmann H., Betz H., Seeburg P. H. Functional chloride channels by mammalian cell expression of rat glycine receptor subunit. Neuron. 1989 May;2(5):1491–1497. doi: 10.1016/0896-6273(89)90195-5. [DOI] [PubMed] [Google Scholar]
- Ticku M. K., Olsen R. W. Interaction of barbiturates with dihydropicrotoxinin binding sites related to the GABA receptor-ionophore system. Life Sci. 1978 May 8;22(18):1643–1651. doi: 10.1016/0024-3205(78)90061-9. [DOI] [PubMed] [Google Scholar]
- Unwin N. The structure of ion channels in membranes of excitable cells. Neuron. 1989 Dec;3(6):665–676. doi: 10.1016/0896-6273(89)90235-3. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A., Burnashev N., Monyer H., Seeburg P. H., Sakmann B. Structural determinants of ion flow through recombinant glutamate receptor channels. Science. 1991 Jun 21;252(5013):1715–1718. doi: 10.1126/science.1710829. [DOI] [PubMed] [Google Scholar]



