Abstract
Background
The species-specific risk of cardiac device-related infection (CDRI) among bacteremic patients is incompletely understood.
Methods
We conducted a prospective cohort study of hospitalized patients from October 2002 to December 2014 with a cardiac device (CD) and either Staphylococcus aureus bacteremia (SAB) or Gram-negative bacteremia (GNB). Cardiac devices were defined as either prosthetic heart valves (PHVs), including valvular support rings, permanent pacemakers (PPMs)/automatic implantable cardioverter defibrillators (AICDs), or left ventricular assist devices (LVADs).
Results
During the study period, a total of 284 patients with ≥1 CD developed either SAB (n = 152 patients) or GNB (n = 132 patients). Among the 284 patients, 150 (52.8%) had PPMs/AICDs, 72 (25.4%) had PHVs, 4 (1.4%) had LVADs, and 58 (20.4%) had >1 device present. Overall, 54.6% of patients with SAB and 16.7% of patients with GNB met criteria for definite CDRI (P < .0001). Multivariable logistic regression analysis revealed that 3 bacterial species were associated with an increased risk for CDRI: Staphylococcus aureus (odds ratio [OR] = 5.57; 95% confidence interval [CI], 2.16–14.36), Pseudomonas aeruginosa (OR = 50.28; 95% CI, 4.16–606.93), and Serratia marcescens (OR = 7.75; 95% CI, 1.48–40.48).
Conclusions
Risk of CDRI among patients with bacteremia varies by species. Cardiac device-related infection risk is highest in patients with bacteremia due to S aureus, P aeruginosa, or S marcescens. By contrast, it is lower in patients with bacteremia due to other species of Gram-negative bacilli. Patients with a CD who develop bacteremia due to either P aeruginosa or S marcescens should be considered for diagnostic imaging to evaluate for the presence of CDRI.
Keywords: cardiac device-related infection, Gram-negative bacteremia, pacemaker infection, prosthetic valve endocarditis, S aureus bacteremia
Infection is a devastating complication of cardiac device (CD) use [1–5]. Among patients with a CD who develop bacteremia, some but not all have underlying CD-related infection (CDRI). This distinction is important because treatment of CDRI generally requires surgical removal of the device. By contrast, bacteremia in patients with noninfected CDs can be treated with antibiotics alone [6–8].
Indirect evidence suggests that the risk of CDRI differs among bacterial species. For example, our group and others have documented the high rate of CDRI associated with Staphylococcus aureus bacteremia (SAB) [2, 9]. By contrast, available evidence suggests that the risk of CDRI in patients with Gram-negative bacteremia (GNB) is small [10, 11]. This impression has led to the recommendation that routine echocardiography to assess for CDRI in patients with a known alternative source of bacteremia due to any species of Gram-negative (GN) bacteria is not warranted [10, 12]. However, virtually nothing is known about whether the risk for CDRI is uniform across all GN bacterial species. In this study, we used a large cohort of prospectively enrolled patients to evaluate whether the risk of CDRI in a patient with a CD who develops bacteremia differs according to the species of the bloodstream pathogen.
METHODS
Study Population
All inpatients with either monomicrobial SAB or GNB from October 1, 2002 to December 31, 2014 at Duke Medical Center and Duke Regional Hospital were prospectively enrolled in the Bloodstream Infections Biorepository, which allowed for the collection of detailed clinical and microbiological information. Within this cohort, only patients with SAB or GNB and an implantable CD met the inclusion criteria for the present study and were further analyzed. Patients with candidemia were excluded from the study. The following CDs were included: permanent pacemakers (PPMs), automatic implantable cardioverter defibrillators (AICDs), prosthetic heart valves (PHVs) including valvular support rings, and left ventricular assist devices (LVADs). Because PPMs and AICDs are structurally similar, these patients were grouped together into a single category. Patients with ≥1 CD were classified as having multiple devices. Detailed clinical data, including patient characteristics, treatment patterns, and outcomes, were collected on a standardized case report form and entered into an electronic database (Microsoft Access 2016). This study had full approval from the Duke Institutional Review Board (IRB). Written informed consent was obtained from all patients or their legal representative. If a patient died before notification of blood culture results, the subjects were enrolled using an IRB-approved Notification of Decedent Research.
Definitions
As outlined previously [2], CDRI was considered definite or rejected. Definite CDRI was confirmed either clinically or microbiologically. Definite CDRI was clinically confirmed if (1) echocardiography demonstrated valvular or lead vegetations or the modified Duke criteria for infective endocarditis (IE) were met and/or (2) there was clinical evidence of erythema, warmth, fluctuance, wound dehiscence, erosion, or tenderness at the generator site [13]. Definite CDRI was microbiologically confirmed if cultures involving the CD were positive. Cardiac device-related infection was rejected if (1) the patient had no evidence of CDRI at the time of initial blood culture, the CD was not removed, there was no evidence of recurrent infection 12 weeks after the onset of bacteremia, and/or (2) no evidence of CDRI at autopsy. Cardiac device-related infections were defined as indeterminate if death occurred before CDRI was confirmed or rejected. In cases involving LVADs, only LVAD-specific infections were included according to definitions recognized by the International Society for Heart and Lung Transplantation [14].
Regarding the site of acquisition, the bacteremia was categorized as either hospital- or community-acquired [15]. Hospital-acquired bacteremia was defined as the bacteremia diagnosed ≥48 hours after hospital admission [16]. Community-acquired bacteremia was defined as the bacteremia diagnosed <48 hours after hospital admission. Community-acquired bacteremia was further subdivided into the following: (1) healthcare-associated (HCA) bacteremia and (2) non-HCA bacteremia. Healthcare-associated bacteremia, as modified by Friedman et al [16], was defined as a bacteremia diagnosed <48 hours after hospital admission in patients that meet 1 or more of the following criteria: hospitalized in past 90 days, resident of nursing home or long-term care facility, actively receiving home intravenous therapy, received wound care or specialized nursing care in previous 30 days, received hemodialysis in past 30 days, immunosuppressed (eg, presence of metastatic cancer, history of solid organ or hematological transplant, chemotherapy in last 30 days, currently on immunosuppressive medication for any reason), or surgery in last 180 days. Non-HCA bacteremia is any community-acquired bacteremia not meeting criteria for HCA bacteremia. Source of infection refers to the primary focus of the bacteremia. Patients with skin/soft tissue infection (eg, cellulitis) as the source of the bacteremia were grouped into a single source category (skin/soft tissue). Patients with gastrointestinal ([GI] eg, biliary tract) or genitourinary (GU) sources (eg, pyelonephritis) were grouped (GI/GU). An endovascular infection included sources such as central venous catheters, etc. Patients with bacteremia originating from the respiratory tract were grouped (respiratory/lung). Sources that did not fit into a predefined category were placed in “other.” Bacteremia with no identifiable source was placed in the “none/unknown” category. Bacteremia episodes were classified as early (<1 year after device implantation) or late (≥1year after device implantation) [2]. Persistent bacteremia was defined as ≥5 days of positive blood cultures. Patients were considered to have developed a complication from their bacteremia if they developed any of the following conditions: acute renal failure, acute lung injury/acute respiratory distress syndrome, disseminated intravascular coagulation, septic shock, IE, or prosthetic device infection. Acute Physiology and Chronic Health Evaluation (APACHE) II scores were calculated on the day of the index positive blood culture [17]. A chronic health point score (CHS) was calculated as part of the APACHE II score. The point system for this score is as follows: if a patient has severe organ system insufficiency (biopsy-proven cirrhosis, New York Heart Association Classification stage IV heart disease, dialysis, severe chronic obstructive pulmonary disease, or an immunosuppressive condition [eg, human immunodeficiency virus infection, organ transplant, chemotherapy]) and admitted for a nonoperative or after emergency surgery, they are assigned 5 points; if admitted for an elective surgery (and have severe end-organ disease), they receive 2 points; all others receive no points [17].
Bacterial isolates were speciated by the Duke Clinical Microbiology Laboratory using standard techniques. Minimum inhibitory concentration (MIC) values were determined using the MicroScan Walkaway system (microbroth dilution method) as described previously [18]. The MIC breakpoint values for each antibiotic were defined according to the most recent Clinical and Laboratory Standards Institute guidelines. Multidrug-resistant (MDR) phenotype was defined as nonsusceptible to at least 1 agent in ≥3 relevant antimicrobial categories [19]. For S aureus, Enterobacteriaceae, and Pseudomonas aeruginosa, appropriate antimicrobial categories have been previously defined [19].
Statistical Analysis
Baseline characteristics and clinical events are presented as means with standard deviation for continuous variables and frequencies with proportions for categorical variables. Statistical comparisons between groups for continuous variables were made with Student’s t test if the assumption of normality was satisfied; otherwise, the Wilcoxon rank-sum test was used. For categorical variables, comparisons were made using Pearson’s χ2 test when cell frequencies were sufficient; otherwise, Fisher’s exact test was used.
Multiple logistic regression models were fit to assess the association of clinical characteristics with definite CDRI. The backward variable selection technique was applied to arrive at a model containing only variables significantly associated with CDRI. Variables included in the combined model were selected based on clinical judgment and included patient demographics (eg, age), medical comorbidities (eg, chronic health points), source (eg, endovascular), and microbial species (eg, S aureus). The CHS of the APACHE II was used as an estimate of comorbid conditions in the cohorts. The reference group used to estimate the odds ratios (ORs) was the uninfected CD group. Cases of indeterminate CDRI were not included in the statistical analysis. Given the low number of CDRIs associated with GN bacilli, only P aeruginosa and Serratia marcescens were investigated as individual microbial species; the remainder of the GN bacilli were consolidated into a single “other” microbial species category for the model. Variables in the SAB subpopulation analysis included age, persistent bacteremia, source, and recent surgical procedure. Variables in the GNB subpopulation analysis included CHS, MDR, and microbial species.
For all tests, a P value < .05 was considered statistically significant. All analyses were performed using SAS 9.4 (Cary, NC).
RESULTS
Patient Demographics
A total of 2571 patients with monomicrobial bacteremia due to either SAB (N = 1039, 40.4%) or GNB (N = 1532, 59.6%) were prospectively enrolled during the 12-year study period. Of these patients, 284 (11.0%; SAB [N = 152, 53.5%]; GNB [N = 132, 46.5%]) had at least 1 CD in place at the time of their bacteremia (Table 1 and Supplemental A.1). Less than 1% of patients with a PHV had undergone transcatheter aortic or pulmonic valve replacement. In the CD-GNB cohort, 64 patients (48.5%) underwent some form of echocardiography compared with 142 (93.4%) patients in the CD-SAB cohort (Supplementary Table S1).
Table 1.
Clinical Characteristics of Patients With a Cardiac Device and Either Staphylococcus aureus Bacteremia or Gram-Negative Bacteremia From October 2002 to December 2014
| Characteristic | SAB | GNB | P Value |
|---|---|---|---|
| N = 152 | N = 132 | ||
| n (%) | n (%) | ||
| Age (mean) | 63.5 (15.2) | 66 (14.1) | .159 |
| >35 | 7 (4.6) | 2 (1.5) | |
| 35 to 65 | 72 (47.4) | 55 (41.7) | |
| >65 | 73 (48.0) | 75 (56.8) | |
| Female gender | 46 (30.3) | 43 (32.6) | .675 |
| Race | .760 | ||
| White | 106 (69.7) | 97 (73.5) | |
| Black | 43 (28.3) | 32 (24.2) | |
| Other | 3 (2.0) | 3 (2.3) | |
| Device Type | <.001 |
||
| PPM/AICD | 97 (63.8) | 53 (40.2) | |
| PHV | 33 (21.2) | 39 (29.6) | |
| LVAD | 1 (1.0) | 3 (2.3) | |
| Multiple | 21 (13.8) | 37 (28.0) | |
| Past Medical History | |||
| Corticosteroid use | 19 (12.6) | 21 (15.9) | .423 |
| Diabetes mellitus | 70 (46.1) | 62 (47.0) | .877 |
| Hemodialysis dependence | 25 (16.5) | 17 (12.9) | .398 |
| HIV positive | 0 | 1 (<1) | .470 |
| Injection drug use | 3 (2.0) | 3 (2.3) | 1.000 |
| Neoplasm | 16 (10.5) | 32 (24.2) | .002 |
| Recent surgery | 37 (24.5) | 50 (37.9) | .015 |
| Rheumatoid arthritis | 3 (2.0) | 5 (3.8) | .480 |
| Transplant | 5 (3.3) | 10 (7.6) | .107 |
| Site of Acquisition | <.001 | ||
| Hospital acquired | 27 (18.4) | 56 (42.4) | |
| Healthcare-associated community acquired | 96 (65.3) | 60 (45.4) | |
| Nonhealthcare-associated community acquired | 24 (16.3) | 16 (12.1) | |
| Source of Bacteremia | <.001 | ||
| Skin/soft tissue | 30 (19.7) | 15 (11.4) | |
| Endovascular | 27 (17.8) | 19 (14.4) | |
| Gastrointestinal/ Genitourinary | 0 | 50 (37.9) | |
| Respiratory/Lung | 11 (7.2) | 11 (8.3) | |
| Other | 43 (28.3) | 14 (10.6) | |
| None/Unknown | 41 (27.0) | 23 (17.4) | |
| Persistent bacteremia | 42 (27.6) | 6 (4.6) | <.001 |
| Early bacteremia | 59 (38.8) | 59 (45.4) | .214 |
| Length of Hospitalization | |||
| <9 days | 35 (23.2) | 45 (34.1) | |
| 9–14 days | 45 (29.8) | 23 (17.4) | |
| 15 to 20 days | 18 (11.9) | 7 (5.3) | |
| >20 days | 53 (35.1) | 57 (43.2) | |
| Complications | 112 (84.9) | 24 (21.2) | <.001 |
| APACHE II (mean) | 17 | 16 | .593 |
Abbreviations: AICD, automatic implantable cardioverter-defibrillator; APACHE II, Acute Physiology and Chronic Health Evaluation II; GNB, Gram-negative bacteremia; HIV, human immunodeficiency virus; LVAD, left ventricular assist device; PHV, prosthetic heart valve; PPM, permanent pacemakers; SAB, S aureus bacteremia.
NOTE: Approximately 10% of patients (N = 284) were outside hospital referrals.
The clinical characteristics of patients with SAB (CD-SAB) and GNB (CD-GNB) were significantly different. Patients with SAB had a higher proportion of PPMs/AICDs (63.8% vs 40.1%, P < .001), whereas patients with CD-GNB had a significantly higher percentage of multiple devices (28% vs 13.8%, P < .001) (Supplemental A.2). The CD-GNB patients had a significantly greater burden of chronic health comorbidities, including malignancy (24.2% vs 10.5%, P < .001) and surgery within the last 30 days from the index positive blood culture (37.9% vs 24.5%, P < .001) (Table 1). The CD-SAB patients were significantly more likely to have persistent bacteremia (27.6% vs 4.6%, P < .001) and complications arising from their bacteremia (84.9% vs 21.1%, P < .001). In addition, the route of infection was different, as CD-SAB patients were more likely to have a community-acquired source of bacteremia (65.3% vs 45.4%, P < .001), whereas CD-GNB patients more often had a hospital-acquired bacteremia (42.4% vs 18.4%, P < .001). There were also differences noted in bacteremia source. The CD-SAB patients more often had skin/soft tissue sources or sources (19.7% vs 11.4%, P < .001) (Table 1) that were classified in the other category (28.3% vs 10.6%, P < .001) (Table 1 and Supplementary Figure S1), whereas CD-GNB patients were more likely to have bacteremia originating from a GI/GU source (37.9% vs 0%, P < .001).
Microbiology and Infection Rates
The microbiological distribution of bacteremia in the CD-SAB and CD-GNB cohorts is presented in Table 2. The most common organisms isolated were methicillin-susceptible S aureus (83 cases [29.2%]), methicillin-resistant S aureus (69 cases [24.3%]), Escherichia coli (37 cases [13%]), and Klebsiella spp (27 cases [9.5%]). Combined, the remaining GN species made up less than 25% of the bacteremia cases (Table 2 and Supplementary Table S2).
Table 2.
Microbiologic Distribution of Bloodstream Infections in Patients With Cardiac Devices
| Organism | Infections, No. (%) |
|---|---|
| MSSA | 83 (29.2) |
| MRSA | 69 (24.3) |
| Escherichia coli | 37 (13.0) |
| Klebsiella species | 27 (9.5) |
| Miscellaneous species | 23 (8.1) |
| Enterobacter species | 18 (6.3) |
| Serratia marcescens | 14 (5.0) |
| Pseudomonas aeruginosa | 13 (4.6) |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible S aureus.
The risk of CDRI differed significantly among the S aureus and GN bacterial cohorts (Table 3). Of the 284 patients with bacteremia and a CD, 83 (54.6%) patients with SAB and 22 (16.7%) patients with GNB met criteria for definite CDRI (P < .0001). Cardiac device-related infection was rejected in 50 (32.9%) of CD-SAB patients and 69 (52.2%) of CD-GNB patients. Cardiac device-related infection remained indeterminate in 19 (12.5%) patients in the CD-SAB cohort and 41 (31.1%) patients in the CD-GNB cohort.
Table 3.
Comparison of Cardiac Device-Related Infection Cases Among 284 Cases of Bacteremia (P < .0001)
| Infection Status | Cases of BSI, No. (%) | |
|---|---|---|
| SAB | GNB | |
| (N = 152) | (N = 132) | |
| Definite | 83 (54.6) | 22 (16.7) |
| Indeterminate | 19 (12.5) | 41 (31.1) |
| Uninfected | 50 (32.9) | 69 (52.2) |
Abbreviations: BSI, bloodstream infection; GNB, Gram-negative bacteremia; SAB, Staphylococcus aureus bacteremia.
Definite Cardiac Device-Related Infection
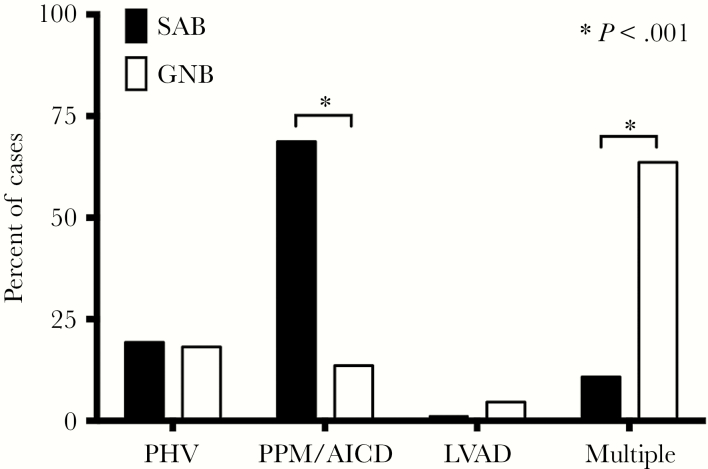
The types of infected CDs differed among patients with definite CDRI in the 2 cohorts (Figure 1). Patients with definite CDRI in the S aureus cohort were significantly more likely to have infected PPMs/AICDs (68.7% vs 13.6%, P < .001), whereas patients with definite CDRI in the GN cohort were more likely have to infection involving multiple CDs (63.6% vs 10.8%, P < .001) (Supplemental A.3).
Figure 1.
Rates of definite cardiac device-related infection by type in patients with Staphylococcus aureus bacteremia (SAB) and Gram-negative bacteremia (GNB). Abbreviations: AICD, automatic implantable cardioverter-defibrillators; Multiple, multiple cardiac devices; PHV, prosthetic heart valves; PPM, permanent pacemakers.
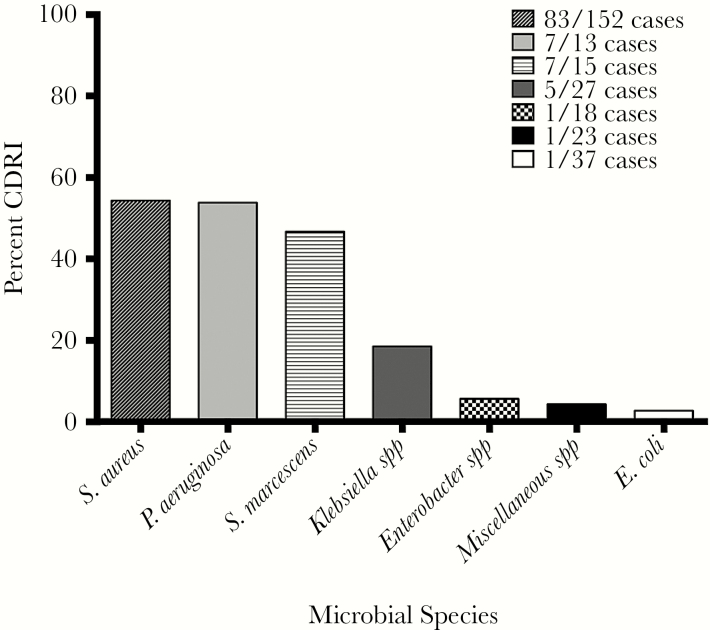
Definite CDRI rate was highest in patients with SAB (83 of 152 patients [54.6%]), P aeruginosa bacteremia (7 of 13 patients [53.8%]), and S marcescens bacteremia (7 of 15 patients, [46.7%]). By contrast, the rate of definite CDRI among patients with bacteremia due to other species of GN bacteria was low (8 of 105 patients [7.6%]) (Figure 2; see Supplementary Table S2 for complete list of species). In patients with a definite CDRI due to P aeruginosa or S marcescens, 5 patients (71.4%) patients and 4 patients (57.1%) had a concurrent LVAD, respectively (Supplemental A.4). Of the 83 cases of CDRI due to S aureus, 33 cases (~40%) were methicillin resistant (Supplementary Table S3). A total of 7 of 22 (31.8%) patients in the GNB cohort were infected with MDR pathogens (Supplementary Table S3). The source of the bacteremia for patients with CDRI in the GNB cohort varied (Supplementary Table S4).
Figure 2.
The risk of definite cardiac device-related infection (CDRI) by bacterial bloodstream pathogen. Miscellaneous species in study population = Bacteriodes spp (2 cases), Citrobacter spp (3 cases), Haemophilus spp (2 cases), Morganella morganii (2 cases), Proteus mirabilis (4 cases), Stenotrophomonas maltophilia (3 cases), and 1 case each for the following species: Acinetobacter spp, Aggregatibacter aphrophilus*, environmental Gram-negative rod, Capnocytophaga spp, Providencia stuartii, Salmonella. *, Specific miscellaneous species in patients with CDRI.
Multivariate Logistic Regression Analysis
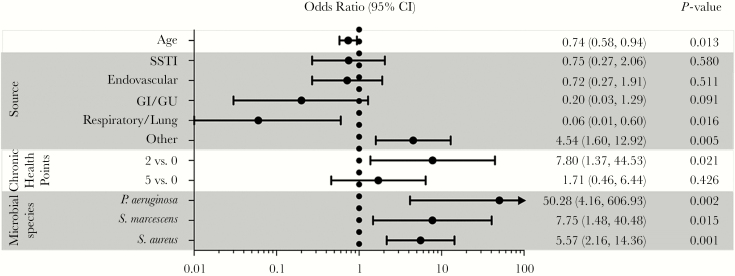
To identify risk factors associated with CDRI, multivariate logistic regression analyses were performed on the combined cohort of patients with SAB and GNB. Cardiac device- related infection was more likely in patients with pre-existing comorbid conditions (indicated by a score of 2 on the Chronic Health Component Score of APACHE II; OR = 7.80; 95% CI, 1.37–44.53; P = .021) and less likely among older patients (OR per 10-year increase = 0.74; 95% CI, 0.58–0.94; P = .013). Bloodstream bacterial species was also significantly associated with CDRI (Figure 3). Cardiac device patients with bacteremia due to S aureus (OR = 5.57; 95% CI, 2.16–14.36; P < .001), P aeruginosa (OR = 50.28; 95% CI, 4.16–606.93; P = .002), or S marcescens (OR = 7.75; 95% CI, 1.48–40.48; P = .015) were at increased risk of CDRI compared with patients with bacteremia due to all other species of GN bacilli.
Figure 3.
Odds ratio for variables associated with cardiac device-related infection in patients with Staphylococcus aureus or Gram-negative bacteremia. Abbreviations: CI, confidence interval; GI, gastrointestinal; GU, genitourinary; SSTI, skin/soft tissue infection.
Subgroup Analyses
Among patients with SAB, persistent bacteremia (OR = 3.34; 95% CI, 1.20–9.30; P = .021) and surgery within 30 days of the index positive blood culture (OR = 5.19; 95% CI, 1.23–21.92; P = .025) were associated with an increased risk for CDRI (Supplementary Figure S2). Gender, race, early/late bacteremia, device type (PPMs/AICDs vs PHVs), and methicillin resistance were not associated with CDRI in the CD-SAB cohort (data not shown). By contrast, the results supported an association between the species of the bloodstream pathogen and the risk of CDRI among patients with GNB (Supplementary Figure S3). Compared with patients with bacteremia due to “other” GN species, the results supported the finding of an increased risk of CDRI among patients with bacteremia due to S marcescens (OR = 7.77; 95% CI, 1.81–33.39; P = .006) or P aeruginosa (OR = 57.7; 95% CI, 5.39–619.01; P < .001).
DISCUSSION
Using a prospective cohort of bacteremic patients with CDs from 1 academic medical center, our findings suggest that the risk of definite CDRI in patients with GNB may vary according to species. Although the risk of CDRI is relatively low with organisms such as E coli, Klebsiella, and Enterobacter, the risk of CDRI among patients with bacteremia due to Pseudomonas or Serratia was similar to that of S aureus.
More than half of the patients with S aureus bacteremia in this investigation had definite CDRI. This finding is consistent with previous reports [2, 11, 20] and strengthens the generalizability of our results. However, in this study, we make the new discovery that the risk of definite CDRI associated with Pseudomonas or Serratia bacteremia was similar to that of S aureus. Although Pseudomonas and Serratia constituted less than 10% of GNB cases in this study, definite CDRI risk was high when they were present. By contrast, the risk for definite CDRI associated with other GN species was low (~7.6%). This low CDRI rate in non-Pseudomonas/Serratia GNB is consistent with prior reports [10, 11]. For example, Uslan et al [10] reported that that 6% of 49 patients with PPM/AICDs who developed GNB had either definite (2 patients) or possible (1 patient) CDRI. It is interesting to note that although less than 20% of patients in the Uslan et al [10] study had bacteremia due to either Pseudomonas (8 patients; 16%) or Serratia (1 patient; 2%), 2 of the 3 cases of definite or possible CDRI were caused by these pathogens.
These results suggest that CDRI risk with Pseudomonas or Serratia bacteremia is approaching the risk encountered in SAB, where echocardiography in patients is regarded as standard of care given the rate of IE [21, 22]. Almost all patients (93.4%) with SAB in the CD-SAB group underwent echocardiography compared with slightly less than half (48.5%) in the CD-GNB cohort. The results of this study suggest that patients with a CD who develop bacteremia due to either Pseudomonas or Serratia should be considered for echocardiography or other appropriate diagnostic studies to evaluate for CDRI.
Staphylococcus aureus, Pseudomonas, and Serratia have important epidemiologic and functional characteristics that are consistent with the elevated risk for CDRI that we observed in this investigation. Epidemiologically, S aureus is the leading cause of IE involving both native [23] and prosthetic [24] valves. Although IE due to enteric GNB is rare [25], outbreaks of IE due to both Pseudomonas [26] and Serratia [27] are well described. Functionally, all 3 species produce a variety of virulence factors that promote adhesion to host proteins and biofilm development. Staphylococcus aureus uses adhesive proteins collectively known as MSCRAMMs (microbial surface components reacting with adherence matrix molecules), forms biofilms, and produces persister cells that evade the host immune system and render antibiotics ineffective [28–31]. Pseudomonas and Serratia also possess a number of virulence factors, such as fimbriae or fimbria-like adhesins, which mediate surface attachment and biofilm formation [32–36].
Patients with CDs who developed SAB and GNB were significantly different. Patients with CD-GNB exhibited higher levels of comorbid conditions (as estimated by CHS, a component of the APACHE II score), with significantly higher rates of hospital-acquired infection, malignancy, and recent surgery [17]. Patients in the CD-GNB cohort were also more likely to have multiple devices than patients in the CD-SAB cohort. In many of these patients, at least 1 cardiac device was an LVAD. These findings are consistent with prior studies reporting a high prevalence of nosocomial GN LVAD infections [37, 38]. By contrast, patients with SAB were more likely to have community-acquired infection, a finding that is also in agreement with prior studies [39]. Cardiac device-SAB patients were more likely to exhibit persistent bacteremia or sustain complications, findings that are consistent with the inherent virulence of the organism and its inclination for metastatic seeding [40, 41]. Cardiac device-related infections in the CD-SAB cohort were more likely to involve PPMs/AICDs. Overall, these findings indicate that the patients in the CD-GNB cohort were more chronically ill relative to the CD-SAB cohort.
The current study has several limitations. First, the investigation was performed at a tertiary referral center and subject to referral bias. Approximately 10% of study patients were outside hospital referrals; however, study conclusions were unchanged when these patients were excluded from the analysis. Moreover, as a referral center, we were also limited in our ability to more precisely document the time from CD placement to bacteremia in approximately 25% of patients in the combined cohort. Second, mortality in patients categorized as having an indeterminate CDRI prevented the confirmation of infection status. To address the limitation of patient censoring because of early death, we repeated the analysis after excluding patients who died within 5 days of bacteremia (n = 4) and found no changes in study conclusions. Third, the only Gram-positive (GP) organism investigated was S aureus, and thus these findings cannot be extrapolated to other GP pathogens. Finally, the small number of patients with CDRIs in the CD-GNB cohort limited our ability to perform statistical modeling in this subpopulation, and we often encountered larger confidence intervals in this cohort. Consequently, we were unable to identify clinical risk factors associated with CDRI, to examine those patients with CDRI due to Pseudomonas or Serratia separately, or to remove patients with multiple devices or a concurrent LVAD. We appreciate that LVAD infections have independent algorithms for management [14]. However, as the numbers of patients with multiple CDs continue to increase, diagnostic and treatment algorithms will overlap. Studies that begin to incorporate instead of exclude this complex population represent a step forward in understanding how to best integrate these algorithms in the future, because these are increasingly the patients being encountered clinically.
CONCLUSIONS
Despite these limitations, the results of this investigation provide several key observations. Patients with CDs who develop SAB or GNB differ in their clinical characteristics, rates of infection, and the types of devices that are infected. Although P aeruginosa and S marcescens were infrequent causes of bacteremia in patients with CDs, they have comparable CDRI risk to that of S aureus when present. Imaging studies to evaluate for underlying CDRI should be considered in these patients. Future studies that include more patients and other institutions are needed to validate these findings.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Financial support. This work was funded by grants from the National Institutes of Health (5T32-AI052080-12 [to S. A. M.] and 2K24-AI093969, 2R01-AI068804, and R01-HL119648 [to V. G. F.]).
Potential conflicts of interest. V. G. F. was Chair Scientific Advisory Board for V710 S aureus vaccine and has or recently had consultancies with the following: Pfizer, Novartis, Galderma, Novadigm, Durata, Debiopharm, Genentech, Achaogen, Affinium, Medicines Co., Cerexa, Tetraphase, Trius, MedImmune, Bayer, Theravance, Cubist, Basilea, Affinergy, Janssen, xBiotech, Contrafect. V. G. F. has received grants to his institution from the following: US Food and Drug Administration, National Institutes of Health, Centers for Disease Control and Prevention, MedImmune, Cerexa/Forest/Actavis/Allergan, Pfizer, Advanced Liquid Logics, Theravance, Novartis, Cubist/Merck, Medical Biosurfaces, Locus, Affinergy, Contrafect, and Karius. V. G. F. has received Educational Fees from Green Cross, Cubist, Cerexa, Durata, Theravance, and Debiopharm. V. G. F. has received Royalties from UpToDate and has a Patent Pending for Sepsis Diagnostics.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Tarakji KG, Mittal S, Kennergren C et al. Worldwide randomized antibiotic envelope infection prevention trial (WRAP-IT). Am Heart J 2016; 180:12–21. [DOI] [PubMed] [Google Scholar]
- 2. Chamis AL, Peterson GE, Cabell CH et al. Staphylococcus aureus bacteremia in patients with permanent pacemakers or implantable cardioverter-defibrillators. Circulation 2001; 104:1029–33. [DOI] [PubMed] [Google Scholar]
- 3. Voigt A, Shalaby A, Saba S. Rising rates of cardiac rhythm management device infections in the United States: 1996 through 2003. J Am Coll Cardiol 2006; 48:590–1. [DOI] [PubMed] [Google Scholar]
- 4. Cabell CH, Heidenreich PA, Chu VH et al. Increasing rates of cardiac device infections among Medicare beneficiaries: 1990–1999. Am Heart J 2004; 147:582–6. [DOI] [PubMed] [Google Scholar]
- 5. Tarakji KG, Ellis CR, Defaye P, Kennergren C. Cardiac implantable electronic device infection in patients at risk. Arrhythm Electrophysiol Rev 2016; 5:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Voigt A, Shalaby A, Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol 2010; 33:414–9. [DOI] [PubMed] [Google Scholar]
- 7. LE KY, Sohail MR, Friedman PA et al. Clinical predictors of cardiovascular implantable electronic device-related infective endocarditis. Pacing Clin Electrophysiol 2011; 34:450–9. [DOI] [PubMed] [Google Scholar]
- 8. Baddour LM, Epstein AE, Erickson CC et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121:458–77. [DOI] [PubMed] [Google Scholar]
- 9. Sohail MR, Palraj BR, Khalid S et al. Predicting risk of endovascular device infection in patients with Staphylococcus aureus bacteremia (PREDICT-SAB). Circ Arrhythm Electrophysiol 2015; 8:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Uslan DZ, Sohail MR, Friedman PA et al. Frequency of permanent pacemaker or implantable cardioverter-defibrillator infection in patients with gram-negative bacteremia. Clin Infect Dis 2006; 43:731–6. [DOI] [PubMed] [Google Scholar]
- 11. Uslan DZ, Sohail MR, St Sauver JL et al. Permanent pacemaker and implantable cardioverter defibrillator infection: a population-based study. Arch Intern Med 2007; 167:669–75. [DOI] [PubMed] [Google Scholar]
- 12. DeSimone DC, Sohail MR. Management of bacteremia in patients living with cardiovascular implantable electronic devices. Heart Rhythm 2016; 13:2247–52. [DOI] [PubMed] [Google Scholar]
- 13. Li JS, Sexton DJ, Mick N et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30:633–8. [DOI] [PubMed] [Google Scholar]
- 14. Hannan MM, Husain S, Mattner F et al. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J Heart Lung Transplant 2011; 30:375–84. [DOI] [PubMed] [Google Scholar]
- 15. Thaden JT, Li Y, Ruffin F et al. Increased costs associated with bloodstream infections caused by multidrug-resistant Gram-negative bacteria are due primarily to patients with hospital-acquired infections. Antimicrob Agents Chemother 2017; 61:e01709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Friedman ND, Kaye KS, Stout JE et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 2002; 137:791–7. [DOI] [PubMed] [Google Scholar]
- 17. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13:818–29. [PubMed] [Google Scholar]
- 18. Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition. M07-A9. Wayne, PA; Clinical and Laboratory Standard Institute; 2012. [Google Scholar]
- 19. Magiorakos AP, Srinivasan A, Carey RB et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–81. [DOI] [PubMed] [Google Scholar]
- 20. Uslan DZ, Dowsley TF, Sohail MR et al. Cardiovascular implantable electronic device infection in patients with Staphylococcus aureus bacteremia. Pacing Clin Electrophysiol 2010; 33:407–13. [DOI] [PubMed] [Google Scholar]
- 21. Baddour LM, Wilson WR, Bayer AS et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American heart association. Circulation 2015; 132:1435–86. [DOI] [PubMed] [Google Scholar]
- 22. Holland TL, Arnold C, Fowler VG Jr. Clinical management of Staphylococcus aureus bacteremia: a review. JAMA 2014; 312:1330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fowler VG Jr, Miro JM, Hoen B et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 2005; 293:3012–21. [DOI] [PubMed] [Google Scholar]
- 24. Wang A, Athan E, Pappas PA et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 2007; 297:1354–61. [DOI] [PubMed] [Google Scholar]
- 25. Morpeth S, Murdoch D, Cabell CH et al. Non-HACEK Gram-negative bacillus endocarditis. Ann Intern Med 2007; 147:829–35. [DOI] [PubMed] [Google Scholar]
- 26. Reyes MP, Ali A, Mendes RE, Biedenbach DJ. Resurgence of Pseudomonas endocarditis in Detroit, 2006–2008. Medicine 2009; 88:294–301. [DOI] [PubMed] [Google Scholar]
- 27. Mills J, Drew D. Serratia marcescens endocarditis: a regional illness associated with intravenous drug abuse. Ann Intern Med 1976; 84:29–35. [DOI] [PubMed] [Google Scholar]
- 28. Lower SK, Lamlertthon S, Casillas-Ituarte NN et al. Polymorphisms in fibronectin binding protein A of Staphylococcus aureus are associated with infection of cardiovascular devices. Proc Natl Acad Sci U S A 2011; 108:18372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Conlon BP. Staphylococcus aureus chronic and relapsing infections: Evidence of a role for persister cells: an investigation of persister cells, their formation and their role in S. aureus disease. Bioessays 2014; 36:991–6. [DOI] [PubMed] [Google Scholar]
- 30. Nagpal A, Baddour LM, Sohail MR. Microbiology and pathogenesis of cardiovascular implantable electronic device infections. Circ Arrhythm Electrophysiol 2012; 5:433–41. [DOI] [PubMed] [Google Scholar]
- 31. Archer NK, Mazaitis MJ, Costerton JW et al. Staphylococcus aureus biofilms: properties, regulation, and roles in human disease. Virulence 2011; 2:445–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Donlan RM. Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis 2001; 33:1387–92. [DOI] [PubMed] [Google Scholar]
- 33. Rice SA, Koh KS, Queck SY et al. Biofilm formation and sloughing in Serratia marcescens are controlled by quorum sensing and nutrient cues. J Bacteriol 2005; 187:3477–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalivoda EJ, Brothers KM, Stella NA et al. Bacterial cyclic AMP-phosphodiesterase activity coordinates biofilm formation. PLoS One 2013; 8:e71267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laverty G, Gorman SP, Gilmore BF. Biomolecular mechanisms of Pseudomonas aeruginosa and Escherichia coli biofilm formation. Pathogens 2014; 3:596–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shanks RM, Stella NA, Kalivoda EJ et al. A Serratia marcescens OxyR homolog mediates surface attachment and biofilm formation. J Bacteriol 2007; 189:7262–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maniar S, Kondareddy S, Topkara VK. Left ventricular assist device-related infections: past, present and future. Expert Rev Med Devices 2011; 8:627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nienaber JJ, Kusne S, Riaz T et al. Clinical manifestations and management of left ventricular assist device-associated infections. Clin Infect Dis 2013; 57:1438–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Le KY, Sohail MR, Friedman PA et al. Clinical features and outcomes of cardiovascular implantable electronic device infections due to staphylococcal species. Am J Cardiol 2012; 110:1143–9. [DOI] [PubMed] [Google Scholar]
- 40. Naber CK. Staphylococcus aureus bacteremia: epidemiology, pathophysiology, and management strategies. Clin Infect Dis 2009; 48 (Suppl 4):S231–7. [DOI] [PubMed] [Google Scholar]
- 41. Fowler VG Jr, Sakoulas G, McIntyre LM et al. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis 2004; 190:1140–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.