Abstract
A case of influenza-associated acute necrotizing encephalitis (ANE) is described in an otherwise healthy adult. The patient was treated successfully with a combination of high-dose methylprednisolone and high-dose oseltamivir. The patient relapsed after discontinuing 150 mg twice daily oseltamivir but quickly improved and eventually recovered after reinitiation of high-dose oseltamivir for an additional 2 weeks. The clinical presentation, pathogenesis, and treatment of influenza-associated ANE is reviewed. The use of high-dose oseltamivir in combination with methylprednisolone may offer additional therapeutic benefit for this rare and poorly understood complication of influenza infection.
Keywords: acute necrotizing encephalitis, encephalitis, encephalopathy, influenza, oseltamivir
Acute necrotizing encephalitis (ANE) was first described in 1995 by Mizuguchi et al [1] after examining the records of Japanese children diagnosed with encephalitis associated with influenza infection in East Asia. During subsequent influenza outbreaks and pandemics, ANE was noted repeatedly in the pediatric populations, with a striking predominance among Japanese children [2, 3]. In contrast, there are only 4 published cases of influenza-associated ANE occurring in the adult population [4–7]. In this study, we report a successfully treated case of severe influenza-associated ANE in a previously healthy 46-year-old man.
METHODS
We describe the presentation, diagnosis, and treatment of an adult male with influenza-associated ANE. In addition, a narrative literature review was conducted regarding the diagnosis, pathogenesis, and treatment of ANE. Search terms using a PubMed query for articles published between1945 and 2016 included the following: influenza, encephalitis, encephalopathy, H1N1, acute necrotizing encephalitis, cytokines, oseltamivir, efficacy, influenza-associated encephalopathy. In addition, a manual search using references from identified articles was also reviewed for relevant publications. Selected articles were screened for inclusion based on relevance and rigor. The patient’s written consent was obtained for publication.
Case Report
A 46-year-old male patient with no prior medical history presented in March 2016 with acute onset dry cough, sore throat, myalgia, and fever that developed 48 hours before admission. Family members reported that he became drowsy during the first day of illness, then more confused and weak the day after. There was no witnessed seizure activity at home or en route to the facility.
The patient resided with his wife and 3 children in Buffalo, New York. All household members had an influenza-like illness during the week. The patient worked in an office setting. He had no recent travel, contact with travelers, or animal exposures.
He took no medications except acetaminophen for fevers. He was never vaccinated against influenza.
Upon presentation, all vital signs were within normal limits except for an oral temperature of 101°F. His neurological examination revealed generalized weakness and extreme drowsiness with moderate confusion. He demonstrated normal deep tendon reflexes and normal Babinski response bilaterally. There was no nuchal rigidity nor any other focal neurological deficits. The remainder of the physical examination was unremarkable.
His initial laboratory workup revealed a normal white blood cell count, hemoglobin, and platelets levels as well as normal electrolytes, liver function tests, coagulation parameters, and serum creatinine. A urine toxicology screen was negative for opiates, benzodiapines, cocaine, and barbituates. A rapid influenza antigen test obtained via nasopharyngeal swab was positive for influenza A (BinaxNOW; Alere Inc.). Lumbar puncture revealed an opening pressure of 12 cmH2O with clear, colorless cerebrospinal fluid (CSF). Analysis of CSF revealed an elevated protein at 78 mg/dL, 0 white cells, 0 red blood cells, and a glucose of 67 mg/dL.
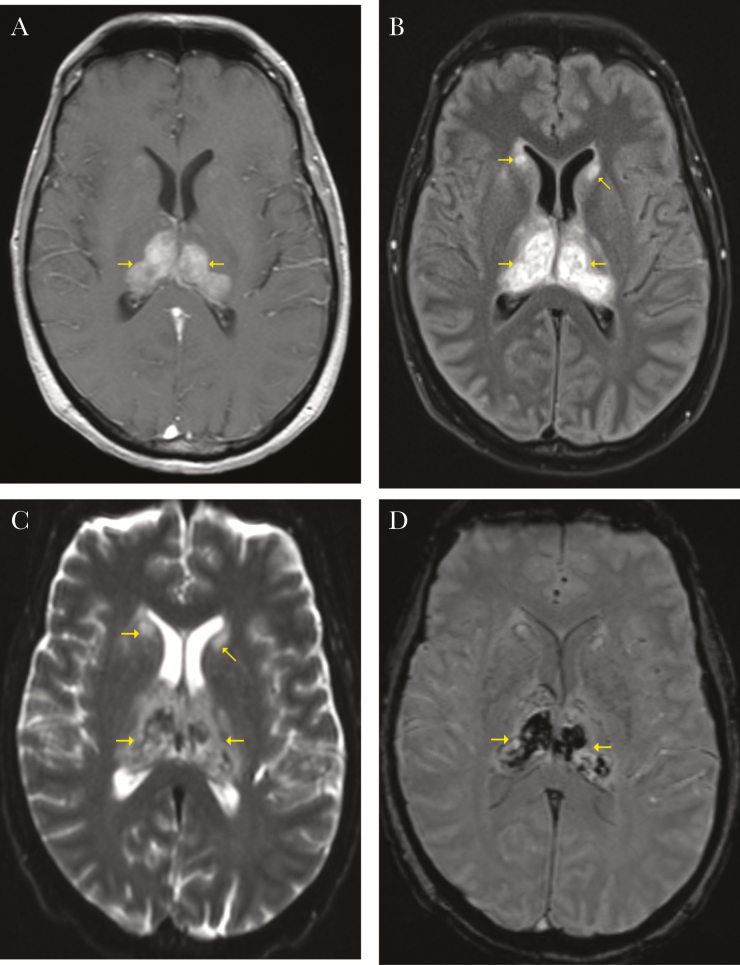
A chest x-ray and noncontrast computed tomography (CT) of the head obtained on admission were both normal. On hospital day 1, a noncontrast magnetic resonance image (MRI) of the brain showed bilateral symmetrical areas of hemorrhagic infarctions within thalami and heads of caudate nuclei, with associated edema (Figure 1). Brain magnetic resonance angiography and magnetic resonance venography showed normal vasculature without thrombosis.
Figure 1.
(A) T1, (B) T2, (C) diffusion weighted imaging, (D) susceptibility weighted imaging. Brain magnetic resonance images showing bilateral symmetrical areas of restricted diffusion and hemorrhagic infarctions within thalami and heads of caudate nuclei, with associated edema.
Later on, and within 24 hours of admission, the patient became completely unresponsive, requiring endotracheal intubation for airway protection. Chest imaging taken shortly after intubation revealed a new right lower lobe infiltrate, and treatment with levofloxacin and piperacillin-tazobactam was initiated for possible secondary bacterial versus aspiration pneumonia. There were no clinical or radiological signs of acute respiratory distress syndrome (ARDS) throughout the admission. Cerebrospinal fluid (CSF) and endotracheal aspirate fluid were submitted to the New York State Department of Health Laboratory (Wadsworth, NY) for multiple viral tests utilizing polymerase chain reaction (PCR). The CSF PCR panel was negative for herpes simplex virus (HSV)1, HSV2, varicella-zoster virus, cytomegalovirus, human herpesvirus 6 (HHV-6), adenovirus, Epstein-Barr virus, West Nile virus, and enteroviruses. His CSF was also negative for immunoglobulin (Ig)G and IgM for West Nile virus, cryptococcal antigen, and venereal disease research laboratory. Serum samples were also negative for human immunodeficiency virus, syphilis, antinuclear antibody, and antineutrophil cytoplasmic antibody. Cultures from CSF, blood, and respiratory secretions were all negative. Hypercoagulability screening was negative too. Serum thiamine and folate levels were normal.
Nasopharyngeal and endotracheal aspirate specimens were positive for pandemic 2009 H1N1 influenza by PCR. However, the CSF sample for influenza PCR was negative. Continuous electroencephalogram showed diffuse slowing but no seizure activity. There was no evidence of vasculitis on cerebral angiogram.
Based on the characteristic MRI findings, clinical presentation, and lack of other clear etiology, a diagnosis of influenza A-associated ANE was made, and treatment with oseltamivir 75 mg twice daily was initiated. Two days later, the patient was also started on methylprednisolone pulse therapy (1 gram intravenous daily for 3 days), which was followed by a tapering dose of prednisone. However, the patient remained unresponsive during the first 5 hospital days with ongoing fevers up to 102°F. Repeat blood and respiratory cultures remained negative. On day 5, he developed decerebrate posturing with brisk patellar and bicep reflexes and abnormal Babinski response. No nuchal rigidity was noted. Due to the severity of his condition and failure to improve with initial management, the dose of oseltamivir was increased to 150 mg twice daily on hospital day 5.
Within 24 hours his mentation improved and decerebrate posturing ceased. His alertness increased gradually over the next several days with improved mentation. By hospital day 10 he was following commands and was extubated. After extubation, he was noted to have dysarthria and a restricted affect with slow reaction times and generalized weakness. High-dose oseltamivir was discontinued after 5 days due to his improved status, but prednisone was continued for 2 more days.
However, his symptoms of lethargy recurred over the following 3 days. Brain MRI was repeated at this point and showed the same lesions identified on the previously MRI but were more defined. High-dose oseltamivir treatment (150 mg twice daily) was restarted without reintroduction of steroids, and his alertness improved again within 24 hours.
Thus, high-dose oseltamivir was continued for a total of 15 additional days. On the 19th hospital day, he was awake, responsive, and following all commands. He was functionally back to his baseline with some residual short-term memory loss and mild ataxia by the end of an acute physical rehabilitation stay, 49 days after his initial presentation. One year later, the patient had full recovery of his motor and over all functionality, except for infrequent episodes of difficulty finding words and decreased motivation.
DISCUSSION
Influenza virus infection is associated with a wide range of neurological complications including Reye syndrome, generalized encephalopathy, seizure, aseptic meningitis, and postinfectious acute disseminated encephalomyelitis [8, 9]. Acute necrotizing encephalitis is another distinct neurological complication of influenza infection with reported mortality rates of 30%–40% [1, 2, 10], second only to historical descriptions of encephalitis lethargica (60%) among neurologic complications of influenza [10].
Clinical Presentation, Diagnosis, and Outcome
Acute necrotizing encephalitis is almost exclusively reported in pediatric populations, beginning with Mizuguchi’s [1, 2] description of Japanese children in 1995 [3]. Reports of ANE seem to have 2 distinct patterns. In the first scenario, patients suddenly develop deterioration in mentation after presenting with severe influenza infection complicated by other organ dysfunction such as acute lung injury, disseminated intravascular coagulation (DIC), or shock [7]. In the second scenario, exemplified in this case report, systemic illness is less pronounced, and altered sensorium is often the initial manifestation leading to medical evaluation [11].
In many cases, the distinctive rapid onset of lethargy associated with ANE often appears within 2 days of influenza-like illness symptoms [3, 12, 13]. The clinical course is characterized by a rapidly progressive encephalopathy, with associated vomiting and lethargy. Seizures often develop early in the most affected patients [14]. Deep coma requiring mechanical ventilation usually ensues several hours to 1 day from start of neurological symptoms. In severe cases, widespread brain edema can be life-threatening [3, 11, 13]. Elevated opening pressures may be seen on lumbar puncture, but CSF analysis usually reveals normal cell counts and glucose levels, occasionally with mild elevation of protein levels [15, 16]. It is notable that influenza virus was not isolated from CSF or brain tissue in most reports [3, 4, 13].
Brain imaging demonstrates the hallmark findings of ANE. Initially, brain CT may show hypodense areas within thalamus bilaterally. Magnetic resonance imaging exam shows characteristic symmetrical bilateral hyperintense signals at both thalami, often most apparent within the caudate heads, and putamen with features of necrosis as time progresses. Involvement of other brain regions is less commonly reported, although cerebellar and brainstem lesions have been described [17].
The mortality of influenza-associated ANE is commonly reported to be approximately 30%–40% throughout literature [1, 2, 10]. Survivors are left with significant permanent neurological sequelae in approximately 40% of cases, with the residual neurologic deficits correlating with involved areas of the brain [2].
Pathogenesis
Acute necrotizing encephalitis is linked to influenza virus infection in many cases. Yet, in several reports, there were no causative pathogens identified. In a few cases, ANE was thought to have been caused by other pathogens such as HHV-6, measles, and mycoplasma [2, 18, 19].
The pathogenesis of ANE is not fully understood. Autopsy specimens show necrosis with petechial hemorrhages in the thalamus and tegmentum of the pons, as well as myelin pallor in the cerebral and cerebellar deep white matter [20]. Vascular endothelial pathology and surrounding vasogenic edema without visible vascular occlusion have also been reported [15]. The absence of influenza virus in central nervous system (CNS) specimens has led to the speculation that the triggering inflammatory insult originates outside the CNS. This was also suggested in other studies, which reported serum cytokines levels to be higher compared with levels measured within the CSF [21].
Some authors have hypothesized that disruption of the blood-brain barrier in the settings of systemic cytokine storm may be responsible for inducing the necrotic brain lesions observed in ANE [22]. Accordingly, influenza virus does not directly invade the CNS. Rather, significant inflammation elsewhere triggers an abundant immune response culminating in the disruption of the blood-brain barrier at localized areas, which eventually leads to the characteristic brain lesions seen in ANE [23–25]. At their physiologic levels, cytokines, especially interleukin-6 and tumor necrosis factor-α, exert a protective effect against invading pathogens. However, abnormally high cytokines levels (ie, cytokine storm) could have a paradoxical neurotoxic effect leading to disruption of the blood-brain barrier and subsequent injury [26]. Such levels have been reported in cases with influenza-associated encephalitis [4, 26, 27].
In contrast, conflicting observations from our case and from earlier literature may suggest a primary insult within the CNS in a subgroup of patients leading to the characteristic focal brain necrosis of influenza-associated ANE. Direct CNS invasion was found to occur via the olfactory nerve in 1 study [28]. This alternative pathogenesis theory goes along with several other reports in which influenza virus was detected in CSF or brain tissue by culture or PCR in some patients [10, 29–32].
In our case, several observations raise the possibility of a primary neurological insult from influenza infection. First, neurologic dysfunction ensued in the absence of other widespread organ dysfunction such as shock, multiple organ failure, DIC, or ARDS—making an overwhelming systemic insult an unlikely trigger for the rapid onset focal brain necrosis observed. Second, our patient failed to show improvement on standard doses of oseltamivir, but he improved shortly after using high-dose oseltamivir. Furthermore, symptoms recurred within 72 hours of discontinuation of high-dose oseltamivir, but they quickly resolved after restarting high-dose oseltamivir, which can indicate an ongoing viral brain insult. It is notable that the patient was still receiving steroids when the high-dose oseltamivir treatment was interrupted, but his second improvement phase occurred after restarting high-dose oseltamivir without reintroduction of steroids.
Management
At this time, there is no definitive treatment for ANE. Management relies largely on supportive care, including treatment of seizures, and increased intracranial pressure, if present. Intravenous corticosteroids, antivirals (eg, oseltamivir), and gammaglobulins are the most commonly reported therapies used [11, 18, 33]. The Research Organization for Influenza Encephalopathy Researchers in Japan described the benefits of methylprednisolone pulse therapy in patients with influenza-associated encephalitis. Although there was no significant difference in efficacy noted between methylprednisolone pulse therapy and intravenous dexamethasone, a clear correlation was established between better outcomes and the early administration (within 24 hours) of intravenous steroid therapy [34].
Several studies support the use of oseltamivir in complicated influenza infection, including those with neurological manifestations [35–37]. Oseltamivir’s ability to cross blood-brain barrier was found to be low in healthy volunteers [38]. The penetration of oseltamivir, particularly at higher doses, within inflamed and injured brain tissue is not described. Despite this, higher doses of oseltamivir have been used with benefit when treating influenza infections in animals [39]. One report noted favorable outcomes using prolonged high-dose oseltamivir treatment along with the use of intravenous steroid therapy and gammaglobulins in a case of influenza encephalitis [11].
Gammaglobulin and plasma exchange have been used to treat selected ANE cases with variable degrees of improvement [11, 34]. The use of other immunosuppressive medications are being studied for their potential benefit based on the hypothesis of ANE as an immune-mediated reaction. In addition, supportive treatment with hypothermia was proposed as an additional strategy for influenza-associated encephalitis [33].
CONCLUSIONS
Acute necrotizing encephalitis is a rare and serious neurologic complication of influenza infection characterized by rapid decline in mentation coupled with imaging findings of symmetric necrotic injury within the thalamus and adjacent structures. Along with supportive care, early use of high-dose corticosteroids and high-dose oseltamivir may lead to lower mortality with fewer long-term sequelae of disease. To the best of our knowledge, our patient is the fifth adult with influenza-associated ANE described in the literature, and the first adult to survive ANE in the United States.
Further studies are needed to characterize the specific pathogenesis of ANE and to better inform optimal management. Our experience suggested that the use of high-dose oseltamivir correlated with improved mentation and resolution of symptoms. The discontinuation of high-dose oseltamivir was followed by recurrence of symptoms with a second resolution after reinitiation of high-dose therapy. Based on these findings, we propose the use of high-dose oseltamivir for at least 14 days in patients with influenza-associated ANE, in addition to early use of corticosteroids and supportive care. Although vaccination against influenza virus has been shown to limit its morbidity and mortality in general [40], it has not been shown to directly affect morbidity and mortality from ANE specifically.
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Mizuguchi M, Abe J, Mikkaichi K et al. Acute necrotising encephalopathy of childhood: a new syndrome presenting with multifocal, symmetric brain lesions. J Neurol Neurosurg Psychiatry 1995; 58:555–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mizuguchi M. Acute necrotizing encephalopathy of childhood: a novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev 1997; 19:81–92. [DOI] [PubMed] [Google Scholar]
- 3. Morishima T, Togashi T, Yokota S et al. Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clin Infect Dis 2002; 35:512–7. [DOI] [PubMed] [Google Scholar]
- 4. Ishii N, Mochizuki H, Moriguchi-Goto S et al. An autopsy case of elderly-onset acute necrotizing encephalopathy secondary to influenza. J Neurol Sci 2015; 354:129–130. [DOI] [PubMed] [Google Scholar]
- 5. Iijima H, Wakasugi K, Ayabe M et al. A case of adult influenza A virus-associated encephalitis: magnetic resonance imaging findings. J Neuroimaging 2002; 12:273–5. [PubMed] [Google Scholar]
- 6. Jardine DL, Hurrell MA, Anderson TJ. A bad dose of the ‘flu. Lancet 2003; 362:1198. [DOI] [PubMed] [Google Scholar]
- 7. Lee YJ, Smith DS, Rao VA et al. Fatal H1N1-related acute necrotizing encephalopathy in an adult. Case Rep Crit Care 2011; 2011:562516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davis LE, Koster F, Cawthon A. Neurologic aspects of influenza viruses. Handb Clin Neurol 2014; 123:619–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corey L, Rubin RJ, Thompson TR et al. Influenza B-associated Reye’s syndrome: incidence in Michigan and potential for prevention. J Infect Dis 1977; 135:398–407. [DOI] [PubMed] [Google Scholar]
- 10. Togashi T, Matsuzono Y, Narita M. Epidemiology of influenza-associated encephalitis-encephalopathy in Hokkaido, the northernmost island of Japan. Pediatr Int 2000; 42:192–6. [DOI] [PubMed] [Google Scholar]
- 11. Akins PT, Belko J, Uyeki TM et al. H1N1 encephalitis with malignant edema and review of neurologic complications from influenza. Neurocrit Care 2010; 13:396–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Togashi T, Matsuzono Y, Anakura M, Nerome K. Acute encephalitis and encephalopathy at the height of influenza in childhood. Nihon Rinsho 1997; 55:2699–705. [PubMed] [Google Scholar]
- 13. Togashi T, Matsuzono Y, Narita M, Morishima T. Influenza-associated acute encephalopathy in Japanese children in 1994–2002. Virus Res 2004; 103:75–8. [DOI] [PubMed] [Google Scholar]
- 14. Voudris KA, Skaardoutsou A, Haronitis I et al. Brain MRI findings in influenza A-associated acute necrotizing encephalopathy of childhood. Eur J Paediatr Neurol 2001; 5:199–202. [DOI] [PubMed] [Google Scholar]
- 15. Fujimoto Y, Shibata M, Tsuyuki M et al. Influenza A virus encephalopathy with symmetrical thalamic lesions. Eur J Pediatr 2000; 159:319–21. [DOI] [PubMed] [Google Scholar]
- 16. Ormitti F, Ventura E, Summa A et al. Acute necrotizing encephalopathy in a child during the 2009 influenza A(H1N1) pandemia: MR imaging in diagnosis and follow-up. AJNR Am J Neuroradiol 2010; 31:396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mariotti P, Iorio R, Frisullo G et al. Acute necrotizing encephalopathy during novel influenza A (H1N1) virus infection. Ann Neurol 2010; 68:111–4. [DOI] [PubMed] [Google Scholar]
- 18. Mastroyianni SD, Gionnis D, Voudris K et al. Acute necrotizing encephalopathy of childhood in non-Asian patients: report of three cases and literature review. J Child Neurol 2006; 21:872–9. [DOI] [PubMed] [Google Scholar]
- 19. Wang HS, Huang SC. Acute necrotizing encephalopathy of childhood. Chang Gung Med J 2001; 24:1–10. [PubMed] [Google Scholar]
- 20. Nakai Y, Itoh M, Mizuguchi M et al. Apoptosis and microglial activation in influenza encephalopathy. Acta Neuropathol 2003; 105:233–9. [DOI] [PubMed] [Google Scholar]
- 21. Mizuguchi M, Yamanouchi H, Ichiyama T, Shiomi M. Acute encephalopathy associated with influenza and other viral infections. Acta Neurol Scand Suppl 2007; 186:45–56. [PubMed] [Google Scholar]
- 22. Toovey S. Influenza-associated central nervous system dysfunction: a literature review. Travel Med Infect Dis 2008; 6:114–24. [DOI] [PubMed] [Google Scholar]
- 23. Ito Y, Ichiyama T, Kimura H et al. Detection of influenza virus RNA by reverse transcription-PCR and proinflammatory cytokines in influenza-virus-associated encephalopathy. J Med Virol 1999; 58:420–5. [DOI] [PubMed] [Google Scholar]
- 24. Stanley ED, Jackson GG. Viremia in Asian influenza. Trans Assoc Am Physicians 1966; 79:376–87. [PubMed] [Google Scholar]
- 25. Mori I, Nagafuji H, Matsumoto K, Kimura Y. Use of the polymerase chain reaction for demonstration of influenza virus dissemination in children. Clin Infect Dis 1997; 24:736–7. [PubMed] [Google Scholar]
- 26. Ichiyama T, Endo S, Kaneko M et al. Serum cytokine concentrations of influenza-associated acute necrotizing encephalopathy. Pediatr Int 2003; 45:734–6. [DOI] [PubMed] [Google Scholar]
- 27. Togashi T, Matsuzono Y, Itakura O, Narita M. IL-6 and TNF-alpha in cerebrospinal fluid from infantile encephalitis-encephalopathy patients during influenza seasons. Nippon Shonika Gakkai Zasshi 1999; 16–9. [Google Scholar]
- 28. van Riel D, Verdijk R, Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol 2015; 235:277–87. [DOI] [PubMed] [Google Scholar]
- 29. Fujimoto S, Kobayashi M, Uemura O et al. PCR on cerebrospinal fluid to show influenza-associated acute encephalopathy or encephalitis. Lancet 1998; 352:873–5. [DOI] [PubMed] [Google Scholar]
- 30. One P, Of P, Report S et al. A pregnant woman with influenza A encephalopathy in whom influenza a/Hong Kong virus (H3) was isolated from cerebrospinal fluid. Arch Intern Med 2016; 172:60–9. [DOI] [PubMed] [Google Scholar]
- 31. Simon M, Hernu R, Cour M et al. Fatal influenza A(H1N1)pdm09 encephalopathy in immunocompetent man. Emerg Infect Dis 2013; 19:1005–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Jong MD, Bach VC, Phan TQ et al. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N Engl J Med 2005; 352:686–91. [DOI] [PubMed] [Google Scholar]
- 33. Morishima T, Yokota S (eds). Special Therapy of Influenza Encephalitis/Encephalopathy: A Proposal. Yokohama: Committee on the Treatment of Influenza Encephalitis/Encephalopathy [in Japanese] 2000; 1:34. [Google Scholar]
- 34. Okumura A, Mizuguchi M, Kidokoro H et al. Outcome of acute necrotizing encephalopathy in relation to treatment with corticosteroids and gammaglobulin. Brain Dev 2009; 31:221–7. [DOI] [PubMed] [Google Scholar]
- 35. Cooper NJ, Sutton AJ, Abrams KR et al. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. BMJ 2003; 326:1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kwon S, Kim S, Cho MH, Seo H. Neurologic complications and outcomes of pandemic (H1N1) 2009 in Korean children. J Korean Med Sci 2012; 27:402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tan K, Prerna A, Leo YS. Surveillance of H1N1-related neurological complications. Lancet Neurol 2010; 9:142–3. [DOI] [PubMed] [Google Scholar]
- 38. Jhee SS, Yen M, Ereshefsky L et al. Low penetration of oseltamivir and its carboxylate into cerebrospinal fluid in healthy Japanese and Caucasian volunteers. Antimicrob Agents Chemother 2008; 52:3687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Govorkova EA, Ilyushina NA, Boltz DA et al. Efficacy of oseltamivir therapy in ferrets inoculated with different clades of H5N1 influenza virus. Antimicrob Agents Chemother 2007; 51:1414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hannoun C, Megas F, Piercy J. Immunogenicity and protective efficacy of influenza vaccination. Virus Res 2004; 103:133–8. [DOI] [PubMed] [Google Scholar]