Abstract
This paper investigates intrinsic and extrinsic influences on otariid energetics. We conducted metabolic experiments with three species of Australian otariid and found that sex and water temperature, but not species, significantly influenced metabolic rates. These findings have implications for understanding the physiological capability of otariids to adapt to changing environments.
Keywords: Metabolic rate, otariid, sex, water temperature
Abstract
The study of marine mammal energetics can shed light on how these animals might adapt to changing environments. Their physiological potential to adapt will be influenced by extrinsic factors, such as temperature, and by intrinsic factors, such as sex and reproduction. We measured the standard metabolic rate (SMR) of males and females of three Australian otariid species (two Australian fur seals, three New Zealand fur seals and seven Australian sea lions). Mean SMR ranged from 0.47 to 1.05 l O2 min−1, which when adjusted for mass was from 5.33 to 7.44 ml O2 min−1 kg−1. We found that Australian sea lion mass-specific SMR (sSMR; in millilitres of oxygen per minute per kilogram) varied little in response to time of year or moult, but was significantly influenced by sex and water temperature. Likewise, sSMR of Australian and New Zealand fur seals was also influenced by sex and water temperature, but also by time of year (pre-moult, moult or post-moult). During the moult, fur seals had significantly higher sSMR than at other times of the year, whereas there was no discernible effect of moult for sea lions. For both groups, females had higher sSMR than males, but sea lions and fur seals showed different responses to changes in water temperature. The sSMR of fur seals increased with increasing water temperature, whereas sSMR of sea lions decreased with increasing water temperature. There were no species differences when comparing animals of the same sex. Our study suggests that fur seals have more flexibility in their physiology than sea lions, perhaps implying that they will be more resilient in a changing environment.
Introduction
Predicted global climate change is already altering the marine environment and will subsequently affect the animals that live and hunt within its bounds (Simmonds and Isaac, 2007). Some of the changes expected include increasing ocean temperatures and changes to seasonal oceanic processes that will be likely to affect the distribution of fish assemblages within the marine environment (Learmonth et al., 2006; Schumann et al., 2013). Pinnipeds may be particularly susceptible to these changes if their prey distribution alters such that they have to travel further or dive deeper to obtain food (Staniland et al., 2007) or if the marine environment warms to such an extent that they cannot thermoregulate effectively (Boyles et al., 2011). Thus, in order to predict how changes in environmental conditions might impact on pinnipeds it is important to understand how different groups use their energy stores over a range of environmental conditions (Canale and Henry, 2010). Understanding how much flexibility pinnipeds have in order to adapt to the changing conditions can be, in part, met through studying their energetics (Geiser and Turbill, 2009; Canale and Henry, 2010).
The study of energetics provides information about the needs of pinnipeds and the cost of satisfying those needs (Williams and Yeates, 2004). Survival requires the maintenance of an overall positive energy balance, satisfied by obtaining more energy than is expended. Energy expenditure is most accurately estimated by determining metabolic rates, and these can vary over seasons and years, with body mass accounting for most of this variation for mammals (Kleiber, 1947; McNab, 2008). Pinniped energy expenditure is also influenced by intrinsic factors, such as activity, reproduction (preparation for and recovery from the energetic demands of the breeding season), moult and sex, and extrinsic factors, such as temperature and photoperiod, can also contribute to some of this variation. These factors have been investigated in a wide range of phocid (e.g. Rosen and Renouf, 1995; Boily and Lavigne, 1997; Ochoa-Acuña et al., 1998; Sparling et al., 2006) and otariid seals (e.g. Costa and Gales, 2003; Williams et al., 2007; Ladds et al., 2016) but have not shown any consistent relationships among species.
Harbour seals (Phoca vitulina) demonstrate sex and age variation, with metabolic rates declining with age, females faster than males, and they experience metabolic depression during pre- and post-moult stages (Rosen and Renouf, 1995). In contrast, grey seals (Halichoerus grypus) have their highest metabolic rate during winter and they increase, rather than depress, their metabolic rate during the moult (Boily and Lavigne, 1997). Within otariids there appear to be clear seasonal patterns in metabolic rate of fur seals (Dalton et al., 2015), although no effect of reproduction or season has been found for sea lions (Williams et al., 2007). The processes that underlie these variations in response to changing environmental conditions are not well understood, and it is clear that the responses vary greatly between and within pinniped species.
Fur seals and sea lions differ greatly in their thermoregulatory strategies. Fur seals rely on two thick layers of fur to thermoregulate, trapping a layer of air between their fur layers to support its insulation (Liwanag et al., 2012a). The fur seal blubber layer is metabolically inert and is primarily used for energy storage (Liwanag et al., 2012a; Dalton et al., 2014a). Sea lions, on the other hand, rely on a thick blubber layer interspersed with layers of muscle (Mellish et al., 2004) to protect themselves from cold water (Mellish et al., 2007; Williams et al., 2007; Liwanag et al., 2012b). Sea lions maintain two blubber layers, one for energy storage, which maintains a constant thickness throughout the year, and one for thermal insulation, which responds to changes in temperature (Williams et al., 2007).
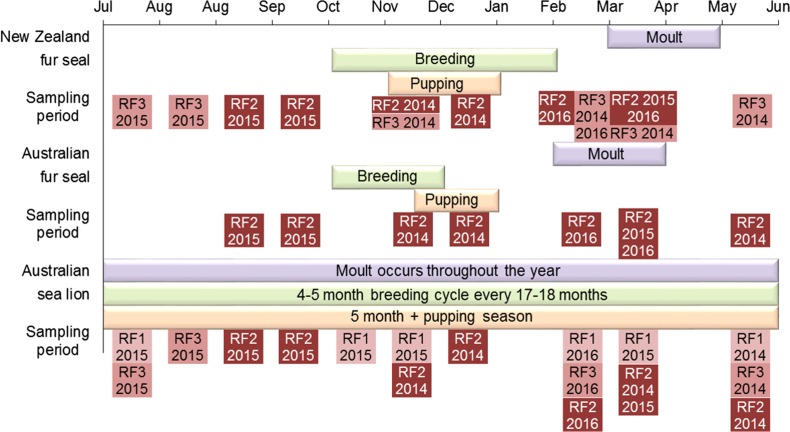
The three otariid species that occupy Australian waters present an interesting comparison of how marine mammals may respond to ecosystem changes, as they have different reproductive cycles, thermoregulatory methods and foraging strategies. The Australian fur seal (Arctocephalus pusillus doriferus) and the New Zealand fur seal (Arctocephalus forsteri) have an annual breeding and moulting cycle typical of pinnipeds (Fig. 1; Goldsworthy and Shaughnessy, 1994; Gibbens and Arnould, 2009). In contrast, the Australian sea lion (Neophoca cinerea) breeds asynchronously every 17–18 months (Higgins, 1993; Gales et al., 1994) and has an extended moult that can occur at any time of the year. Both the Australian fur seal (Arnould and Hindell, 2001; Kirkwood et al., 2006) and the Australian sea lion are predominantly benthic foragers (Costa and Gales, 2003; Lowther et al., 2013), whereas the New Zealand fur seals are typically pelagic foragers (Harcourt et al., 2002).
Figure 1:
Moulting, breeding and pupping time line of Australian fur seals, New Zealand fur seals and Australian sea lions and timetable of experiments conducted at three Australian marine facilities over 3 years. Shaded boxes indicate that trials were conducted during that month in the respective facility. RF1 is located in a temperate to sub-tropical region, RF2 is located in a sub-tropical region, and RF3 is located in a temperate region.
The habitats of the Australian fur seal and the Australian sea lion do not overlap, but the New Zealand fur seal occurs across both the feeding and breeding ranges of the other two species (Page et al., 2005a; Campbell et al., 2014). The ranges of the Australian fur seal and the New Zealand fur seal are currently expanding as they begin to reoccupy territory they held before commercial sealing (Goldsworthy et al., 2003), whereas the Australian sea lion is listed as endangered, and the population continues to decline (McIntosh et al., 2013). By investigating how marine mammals occupying similar habitats but using different reproductive and foraging strategies vary their primary energy expenditure over the course of a year, we can begin to understand how they might respond to environmental changes. Therefore, it was the aim of this study to explore the intrinsic and extrinsic influences on metabolic rate in a sample of fur seals and sea lions.
Materials and methods
Animals
We conducted experiments to measure the metabolic rates of captive otariids (n = 12) in three Australian marine facilities: Dolphin Marine Magic, Coffs Harbour (RF1: 30°17′S, 153°8′E); Underwater World, Sunshine Coast (RF2: 25°40′S, 153°7′E); and Taronga Zoo, Sydney (RF3: 33°50′S, 151°14′E). Experiments were conducted at various times of year from 2013 to 2015. Owing to logistical constraints, it was not possible to measure all otariids in the same month of the same year (data collection periods are shown in Fig. 1). Rather, for fur seals we ensured that sampling was spread over the year but included each significant stage of their annual cycle (analogous to moult, post-moult and prior to the moult, but before breeding; Fig. 1). Australian sea lions were measured at the same time as the fur seals because we could not determine their moulting and breeding cycles. During each visit to the marine facility, the animals were measured between one and four times. We used three New Zealand fur seals, two Australian fur seals and seven Australian sea lions, all of which were not reproducing at the time of experiments, were on permanent display at their respective facilities and were cared for under the husbandry guidelines of that facility. The study was approved by Macquarie University ethics committee (ARA-2012_064) and Taronga Conservation Society Australia ethics committee (4c/10/13). All Australian sea lions that participated in the study were born as a part of an ongoing captive breeding programme in Australian aquaria, whereas all fur seals came into captivity as juveniles after having been found in poor health or injured and were considered unsuitable for release back into the wild after rehabilitation. Fur seal ages were estimated from their size and condition when they were introduced to their facility, and they are now all subadults or adults. Otariids were weighed once per week as a part of their normal routine.
Metabolic rate measurements
We measured the standard metabolic rate (SMR) of otariids using open-flow respirometry. Standard metabolic rate was used because otariids were measured in water and they did not adhere to all of the standards of Kleiber for measuring basal metabolic rate (Kleiber, 1975; Hurley and Costa, 2001). Otariids had not fed for at least 10 h before each trial to ensure they were post-absorptive (Rosen and Trites, 1997), and no animals were pregnant or lactating. Otariids were quiescent (not sleeping) during measurement and reached steady-states of oxygen consumption in 5 min or less. Otariids were measured early in the morning, before they had become active (i.e. swimming), and participated in trials only if they were found to be dry in their enclosure. Measurements of metabolic rate were recorded for up to 15 min, with the lowest, consistent 3 min (minimum) being used for analysis.
We measured SMR when otariids were sitting upright and still in water under a moulded acrylic hood (80 litres). This behaviour was reinforced with small amounts of food (fish and squid), which were reduced as each otariid's capacity to remain inactive improved with training. This amount of food would not have influenced metabolic rate (Rosen and Renouf, 1997; Rosen et al., 2015). The hood was connected to an open-flow respirometry system (Sable Systems International, Inc., Henderson, NV, USA), where air was pulled from the hood with a Sable Systems Mass Flow pump at an adjustable flow rate ranging from 300 to 350 l min−1. We adjusted and monitored the flow for each individual to ensure that the oxygen inside the hood remained >20%. A continuous sub-sample was drawn into the analyser from the pump at ~1200 ml min−1, pushed through the analyser and measured for water vapour then dried (magnesium perchlorate) and scrubbed of carbon dioxide (soda lime) before entering an FC-1 oxygen analyser. We monitored the scrubbers using the built-in CO2 analyser and an external water vapour analyser for fluctuations above 1 and 5%, respectively. The percentage of oxygen in the expired air was measured continuously with Sable Systems ExpeData software and recorded at five samples per second. Oxygen consumption was calculated using equations from Withers (1977), assuming a respiratory quotient (RQ) of 0.77 (Feldkamp, 1987; Boyd et al., 1995b).
We calibrated the system every 2–3 days using nitrogen (N2) and ambient air, following the method of Fedak et al. (1987). Nitrogen gas was passed through a flowmeter at a known rate using a Sable Systems FoxBox. The predicted values of the N2 flow were 400 and 500 ml min−1. Values were within ±5% of predicted values.
Analyses
Before analysis, we examined the suitability of the data for analysis using linear models. We used a linear regression to investigate the relationship between mass (in kilograms) and SMR (in litres of oxygen per minute). Owing to the large range, mass was logarithmically transformed, and we used mass-specific SMR (henceforth, sSMR; in millilitres of oxygen per minute per kilogram) to make statistically relevant comparisons across fur seal and sea lion groups. We identified outliers in the continuous response variables (SMR and sSMR) using exploratory graphical techniques and removed any that corresponded to a behavioural anomaly. We also assessed collinearity-correlation among explanatory variables [mass, sex, age, moult (presence/absence), animal identity, month, ambient temperature and water temperature] via multiple pair-wise scatterplots (pair plots; Zuur et al., 2009b, 2010). We examined the response variables for normality visually using a histogram, and any factor explanatory variables were tested for equal variances across the response variable (Bartlett's test).
We measured the metabolic rate of a subset of six otariids (NFM1, AFM1, ASM2, ASF2, ASF3 and ASF5) in the same month, 1 year apart and used Student's paired t-tests to look for differences in mean sSMR in order to test for a training effect. As there were no significant differences (P > 0.05) in mean sSMR for any of the six otariids between the two years, training effects were not considered further.
Fur seal and sea lion sSMR data were analysed separately. We used multiple linear mixed-effects models (LME) with restricted maximum likelihood (REML) estimation to evaluate which sources of variation best explained changes in sSMR (in millilitres of oxygen per minute per kilogram; NLME package in R; Pinheiro et al., 2014). Using sSMR as the response variable, we first ran a null model (no random effects) to find a baseline from which we could evaluate the influence of the random effect on the models. We then ran LMEs with animal identity as the random effect to account for repeated measures. The predictor variables for sea lions were as follows: sex, age, month of the year, moult (absence/presence) and water temperature. We did not use ambient temperature in the models as it was highly collinearly related to water temperature, which was used in preference because the animals were measured in the water. As month is a cyclical variable, we transformed it to sine [sin(360/11) × month] or cosine [cos(360/11) × month], as in the study of Sparling et al. (2006), and both were tested in the model. The predictor variables for fur seals were as follows: sex, age, species, season (pre-moult, moult, post-moult) and water temperature. We chose to use an information-theoretical approach to build candidate models because stepwise model selection can produce unreliable results (Whittingham et al., 2006). The models were run with all combinations of predictor variables using dredge from the R package MuMIn (Bartoń, 2013). Models were ranked using ‘model.sel’ from the R package MuMIn, and Akaike model weights were used to rank the models.
Model selection was based on a combination of Akaike information criteria (AICc), log likelihoods (logLik) and R2. The amount of variance explained by the random effect was assessed through the difference of the marginal (fixed effect only) and conditional (all model variables) R2 (rsquared.glmm function). The assumptions of homoscedasticity, normality, homogeneity and independence were investigated by plotting predicted vs. fitted residuals, QQ-plots, Cleveland dot-plots and ACF plots (Zuur et al., 2009a). Where possible, we tested for significant differences in sSMR and factorial predictor variables across classes of otariids using a post hoc general linear hypothesis and multiple comparisons test via the Tukey method with the function glht from the package multcomp (Hothorn et al., 2013). All analysis was completed in R (version 3.1.3; R Core Development Team, 2015), and values are reported as means ± SD.
Results
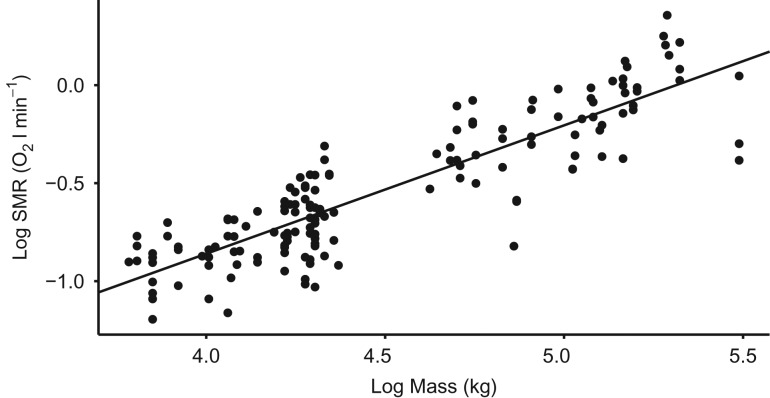
Metabolic rates measured at rest in water were collected for 12 otariids at semi-regular intervals over 3 years (Fig. 2). A total of 155 measurements were collected, with 153 used for analysis because two observations were excluded as they were identified as outliers from behavioural anomalies. There was a strong positive relationship between SMR (in litres of oxygen per minute) and log mass (in kilograms) for all 12 otariids expressed by the following equation: log(SMR) = −3.48 + 0.66log(mass) (logLik = 57.78, R2 = 0.769, P < 0.001; Fig. 2). The mean SMR for all otariids ranged from 0.34 to 1.31 l O2 min−1, and sSMR ranged from 3.06 to 9.71 ml O2 min−1 kg−1 (Table 1).
Figure 2:
Logarithm of metabolic rate while resting in water (SMR; in litres of oxygen per minute) as a function of the logarithm of body mass (in kilograms) for one female Australian fur seal (n = 13), one male Australian fur seal (n = 16), three male New Zealand furseals (n = 31), five female Australian sea lions (n = 68) and two male Australian sea lions (n = 26). The line plotted is the fitted equation: log(SMR) = −3.48 + 0.66log(mass).
Table 1:
Mean ± SD of standard metabolic rate (in litres of oxygen per minute) and mass-specific standard metabolic rate (in millilitres of oxygen per minute per kilogram), multiples of basal metabolic rate and the age, mass range and sample sizes for Australian fur seals, New Zealand fur seals and Australian sea lions
| Species and sex | n | Age range (years) | Mass range (kg) | Total trials | SMR (l O2 min−1) | sSMR (ml O2 min−1 kg−1) | BMR multiple |
|---|---|---|---|---|---|---|---|
| Australian fur seal | |||||||
| Female | 1 | 17.8–19.1 | 69–79 | 13 | 0.49 ± 0.06 | 6.63 ± 1.04 | 2.0 |
| Male | 1 | 15.1–17.1 | 175–242 | 16 | 1.05 ± 0.20 | 5.33 ± 1.18 | 2.1 |
| New Zealand fur seal | |||||||
| Male | 3 | 7.5–14.0 | 47–161 | 31 | 0.62 ± 0.18 | 6.42 ± 1.66 | 2.2 |
| Australian sea lion | |||||||
| Female | 5 | 5.1–26.4 | 44–76 | 68 | 0.47 ± 0.08 | 7.44 ± 1.16 | 2.1 |
| Male | 2 | 9.0–14.3 | 108–177 | 25 | 0.84 ± 0.13 | 5.94 ± 1.09 | 2.0 |
Abbreviations: BMR, basal metabolic rate; SMR, standard metabolic rate; sSMR, mass-specific standard metabolic rate.
Fur seals
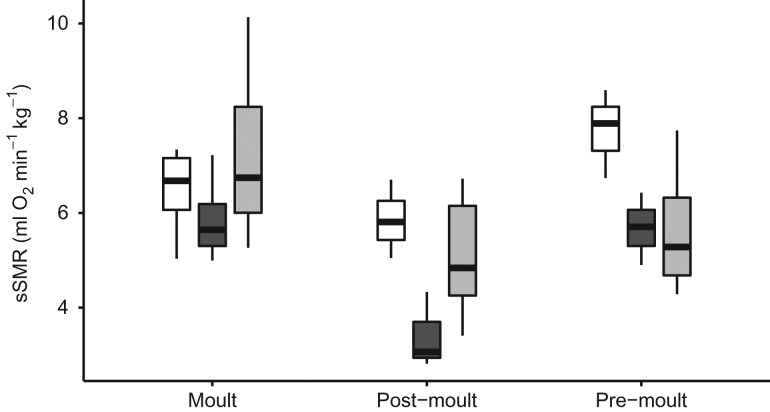
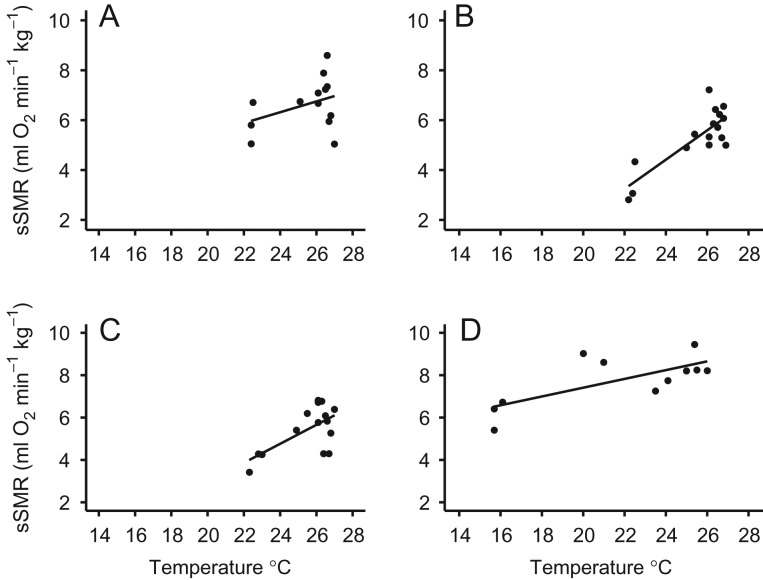
Australian fur seals and New Zealand fur seals have an annual moult and breeding season that occur at similar times of year (Fig. 1). The model that best explained the variation in sSMR for fur seals included an interaction between season (pre-moult, moult or post-moult) and sex, and there was a large effect of animal identity [LME: AICc = 154.8; logLik = −69.38, R2 (conditional) = 0.267; R2 (marginal) = 0.839]. There was no significant effect of age, water temperature or species. The sSMR for both males and females was lowest during post-moult (Fig. 3). For males, sSMR was highest during the annual moult, whereas for females the sSMR was highest during the pre-moult (Fig. 3). Although season was able to explain more of the variance in the model than water temperature, there was a positive linear relationship between water temperature and sSMR for each of the four fur seals (Fig. 4A–D).
Figure 3:
Median, interquartile range (box) and range (bars) of mass-specific standard metabolic rate (sSMR; in millilitres of oxygen per minute per kilogram) for an Australian fur seal male (black box, n = 1) and female (white box, n = 1) and New Zealand fur seal males (grey box, n = 3) during the moult, post-moult and pre-moult periods.
Figure 4:
Median, interquartile range (box) and range (bars) of mass-specific standard metabolic rate (sSMR; in millilitres of oxygen per minute per kilogram) for male (grey box, n = 2) and female (white box, n = 5) Australian sea lions over the course of the year.
Sea lions
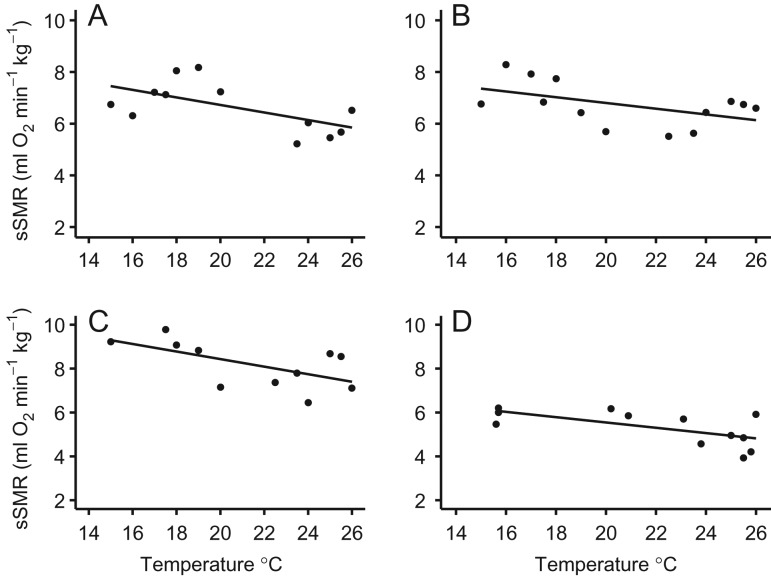
Australian sea lion moult and breeding can occur at any time of the year, so it was not possible to examine the effect of season on metabolic rate. Instead, we tested the effect of sine and cosine of month. The final model for sea lions included water temperature and sex as fixed effects, with individual as the random effect. Animal identity had an effect on the model, as the variance explained was improved [LME: AICc = 281.0, logLik = −134.01, R2 (conditional) = 0.284, R2 (marginal) = 0.515]. Females had higher sSMR than males (Fig. 5), although sine month, cosine month, moult and age did not contribute to the final model. Sea lions housed at RF1 and RF3 were exposed to a wide range of water temperatures (16–27°C), and there was a negative relationship between sSMR and water temperature (Fig. 6A–D). Sea lions from RF2 were measured in water temperatures of 22–27°C, but there was no relationship between sSMR and water temperature.
Figure 5:
Relationship between mass-specific standard metabolic rate (sSMR; in millilitres of oxygen per minute per kilogram) and water temperature (WT; in degrees Celsius) for four individual fur seals. (A) Female Australian fur seal (sSMR = 1.12 + 0.21 × WT, logLik = −17.42, R2 = 0.140, P = 0.207, n = 13). (B) Male Australian fur seal (sSMR = −9.70 + 0.59 × WT, logLik = −15.63, R2 = 0.683, P < 0.001, n = 16). (C) Male New Zealand fur seal (sSMR = −5.99 + 0.45 × WT, logLik = −18.36, R2 = 0.404, P = 0.011, n = 15). (D) Male New Zealand fur seal (sSMR = 1.95 + 0.29 × WT, logLik = −14.70, R2 = 0.587, P = 0.003, n = 12).
Figure 6:
Relationship between mass-specific standard metabolic rate (sSMR; in millilitres of oxygen per minute per kilogram) and water temperature (WT; in degrees Celsius) for Australian sea lions. (A) Adult male (ASM1; sSMR = 9.79 − 0.16 × WT, logLik = −12.40, R2 = 0.450, P = 0.017, n = 12). (B) Adult female (ASF4; sSMR = 9.26 − 0.12 × WT, logLik = −13.16, R2 = 0.348, P = 0.034, n = 13). (C) Adult female (ASF2; sSMR = 12.67 − 0.20 × WT, logLik = −14.38, R2 = 0.497, P = 0.011, n = 12). (D) Adult male (ASM2; sSMR = 7.96 − 0.12 × WT, logLik = −11.73, R2 = 0.336, P = 0.038, n = 13).
Discussion
Standard metabolic rate
Measuring pinnipeds in captivity provides an excellent proxy for estimating the energy expenditure of wild populations. Respirometry is considered the most accurate method of measuring metabolic rate, but is inherently difficult to use in the wild (Boyd, 2002; Halsey et al., 2009; Dalton et al., 2014b). Therefore, these types of experiments allow us to develop our understanding of the physiology of target species, with minimal impact on populations and using the most accurate technology available. We measured the SMR of three species of otariid (n = 12) at rest in water during significant times of their annual cycle. We found that the mean sSMR for the otariids in this study were 2–2.2 times (Table 1) that predicted by Kleiber (1975), which falls within the range predicted for a marine mammal (Williams et al., 2001) and is lower than the in-air resting metabolic rate of juvenile northern fur seals (2.9 times predicted; Dalton et al., 2015) and than the average daily metabolic rate of lactating northern fur seals (3.2 times predicted; Costa and Gentry, 1986).
Few studies have successfully measured the true basal metabolic rate of pinnipeds in the strict conditions of Kleiber (1975). The captive otariids were adult, non-reproductive, quiescent and post-absorptive, but they were measured for a relatively short time and in water. We measured captive otariids in the morning, before they became active, and only if they were dry, suggesting that they had been resting and not swimming before measurement. We measured them in water because they were habituated to this experimental set-up and were noticeably calm during experiments, corresponding to their relatively low metabolic rate, despite not meeting all of the conditions of Kleiber (1975). The range of average sSMR for the animals in this study was 5.3–7.4 ml O2 min−1 kg−1, which was within the range of resting metabolic rate from Southern sea lions (Otaria flavescens) of a similar size (4.3–9.1 ml O2 min−1 kg−1; Dassis et al., 2012). In that study, a single captive animal had resting metabolic rate within the range of the wild animals that were measured simultaneously. We therefore consider our results to be a good estimate of resting metabolic rate in these species, and our measurement of sSMR is probably approaching basal metabolic rate.
Influence of the annual cycle on metabolic rates
The stages of the annual cycle that are energetically costly for otariids are the preparation for and the recovery from annual breeding, including the annual moult. Thus, it is expected that the moult and breeding will have the greatest influence on the variation in the metabolic rate of pinnipeds (Costa and Trillmich, 1988; Rosen and Renouf, 1995). Australian sea lions have different reproductive and moulting strategies from every other otariid, whereas the Australian fur seal and New Zealand fur seal have typical yearly cycles of pinnipeds. Australian fur seals and New Zealand fur seals are similar, with breeding and pupping occurring during the Austral summer, followed by a moult (Goldsworthy and Shaughnessy, 1994; Gibbens and Arnould, 2009). In contrast, Australian sea lions have an asynchronous breeding and moulting cycle, where females come into oestrus every 17–18 months and moulting can occur year round for 3–4 months (Higgins, 1993; Gales et al., 1994). This lack of synchronization was evident in the sSMR of the sea lions, as there were no significant changes over the course of the year, whereas fur seals had distinct changes in their sSMR coinciding with the moult and the build-up of body condition before the breeding season.
Fur seals
In the preparation for and recovery from breeding, male and female fur seals have different motivations for fat accumulation, although their physiological responses appear similar. Females allocate their energy resources to fat stores for gestation and milk production that must be maintained year round if the female is pregnant or lactating (Costa, 1991). Females are usually pregnant during the pre-moult period and must be in good condition to birth and feed a new pup (Boyd et al., 1995a). Females in better condition more often give birth to larger pups that have a higher chance of survival (Guinet et al., 1998). Dominant males require a large body mass during the breeding season in order to establish and defend territory and reproduce successfully, whereas during the non-breeding season they generally maintain a lower body mass when they leave the breeding areas to forage (Boyd and Duck, 1991; Carey, 1991). This is an energetically costly endeavour that only large, healthy males can achieve (Boyd and Duck, 1991). We found that the rate of energy expenditure from male and female fur seals was consistent with that expected for wild fur seals (Costa and Gentry, 1986). Male sSMR was highest during the moult and lowest during the post-moult, after which it increased again before the breeding season. In females, resting metabolic rate was much more consistent, increasing from the post-moult (lowest) to the pre-moult (highest). These metabolic changes align with body conditions of wild Australian fur seals, where the blubber distribution of females does not change over the course of the year, whereas males undergo large seasonal shifts (Carey, 1991; Arnould and Warneke, 2002).
We found that for both fur seal species, sSMR was the lowest during the post-moult (Austral autumn and winter) period. The reduction in sSMR during this period is likely to be a strategy to maintain body condition during periods of reduced prey availability and increased thermoregulatory demands. For both fur seal species, the post-moult period corresponds to the lowest sea-surface temperatures and, presumably, the time of lowest productivity within their home ranges (Harris et al., 1991; Baylis et al., 2008b). Lactating Australian and New Zealand fur seals tend to undertake their longest foraging trips (Arnould and Hindell, 2001; Harcourt et al., 2002; Page et al., 2005b; Baylis et al., 2008a; Kirkwood and Arnould, 2011) and seals tend to maintain a lower body mass (corresponding to a low metabolic rate) after the moulting and breeding season (Arnould and Warneke, 2002; Beck et al., 2003; Sparling et al., 2006).
Sometime before the breeding season, male fur seals increase their metabolic rate from the post-moult period (Fig. 3). The female Australian fur seal also showed an increase, although not as pronounced as for males. It may be important that this event is synchronized for males and females such that they reach sexual maturity simultaneously each year (Boyd, 1991). The timing also corresponds to the accumulation of fat; as the fur seals get fatter, their metabolic rate increases (Beck et al., 2003). As we were unable to measure the fur seals year round, the exact timing of this phenomenon is unknown, although it is likely to be consistent with the onset of spermatogenesis for males, which begins 3–4 months before the breeding season (Stewardson et al., 1998; Stewardson, 2007). Spermatogenesis is energetically expensive, particularly for large mammals with low metabolic rates (Gomendio et al., 2011).
Males and females have different energy requirements at different times that can be achieved by either eating more or reducing energy use. Our results demonstrate that fur seals decrease their energy use during times of fat accumulation, and anecdotal evidence from captivity suggests that the quantity of food increases during this time for fur seals (A. Tolley, M. Ryan and R. Tate, personal communication). In the wild, New Zealand fur seals target higher-energy prey close to the breeding season (Page et al., 2005a), and Australian fur seals make longer foraging trips (Arnould and Hindell, 2001), but neither increase their foraging effort (Kirkwood et al., 2006). Therefore, it is possible that to aid fat accumulation without an increase in foraging effort, fur seals depress their metabolism and, possibly, encounter more prey items as a result of an increase in prey availability.
Sea lions
Australian sea lions show little variation in metabolic rate throughout the year, as demonstrated by the lack of significance of month in the overall model. This is consistent with results of Williams et al. (2007), who found that the resting metabolic rate of Californian sea lions (Zalophus californianus) showed little change across seasons. Sustaining a consistent sSMR may be a strategy for sea lions to maintain their asynchronous breeding cycle. The lack of seasonal variation in the metabolic rates of Australian sea lions is reflected in their temporally stable and geographically fixed foraging patterns (Lowther et al., 2011, 2013). Despite substantial individual variation in foraging strategies, Australian sea lions forage at the same trophic level in the same regions over seasons and years (Lowther et al., 2011, 2013). There were no seasonal changes in metabolic rates observed (present study) or foraging strategies (Lowther et al., 2011), and the availability of sea lion prey is consistent, even if low, year round (McIntosh et al., 2006; Peters et al., 2015). This means that Australian sea lions are likely to adopt other behaviour strategies, such as increasing their food intake, to cope with additional energetic costs throughout the year (e.g. lactation; Williams et al., 2007).
As male Australian sea lions are not able to use seasonal cues in their environment to predict the onset of the breeding cycle, we contend that they maintain a constant sSMR and a static foraging strategy, remaining close to the breeding colonies, in order to be prepared for breeding with females at any time of year (Lowther et al., 2013; Ahonen et al., 2016). This is likely to be an adaption to a low-productivity environment that is fairly constant (McKenzie et al., 2005; Villegas-Amtmann et al., 2009). The breeding period of Australian sea lions lasts for 120 days, suggesting that males must have an extended period of spermatogenesis (Ahonen et al., 2016). Males conserve energy by ‘mate guarding’, i.e. choosing a single female to mate with from when they haul out until they go into oestrus (Higgins, 1990). After mating, they leave to forage or to mate at another nearby colony, and therefore may not have the option of layering additional blubber before the next period when spermatogenesis and mate guarding occur (Ahonen et al., 2016).
Temperature
We show that some of the variation in metabolic rates of otariids can be explained by changes in natural fluctuations in water temperature within each facility (Figs 5 and 6). Although we did not measure the sSMR of otariids in water <16°C, there appears to be an increase of sSMR with increasing water temperature for fur seals (Fig. 5A–D) and a decrease in sSMR with increasing water temperature for sea lions (Fig. 6A–D). Sea lions that were housed at the highest latitude (RF3) did not demonstrate variations in sSMR from 22 to 26°C. The different responses to temperature are likely to result from differences in thermoregulatory strategies. Fur seals rely on a thick layer of fur to thermoregulate, because the blubber layer they maintain is metabolically inert and used primarily for energy storage (Liwanag et al., 2012a; Dalton et al., 2014a). Sea lions rely on a thicker blubber layer to protect themselves from cold water (Mellish et al., 2007; Williams et al., 2007; Liwanag et al., 2012b), which is interspersed with layers of muscle (Mellish et al., 2004). It is possible that the metabolic rate of sea lions declines during warmer temperatures as they use their metabolically active blubber layer through blood perfusion—dilating blood vessels to allow blood to flow through and be warmed by the outside temperature (Meagher et al., 2008; Liwanag et al., 2009)—thus reducing the metabolic costs of maintaining a constant body temperature.
Maintenance of these thermoregulatory strategies is correspondingly different in the two families, each with its own energetic cost. Fur seals use a layer of air trapped between their fur layers to insulate their body. This allows the skin to be maintained at body temperature, but requires that fur seals spend a significant amount of time grooming their pelage (Battaile et al., 2015). This is an energetically expensive tactic (Liwanag, 2010), but could be complementary in cold water because it would raise metabolic rate. At warm temperatures, fur seals increase their metabolic rate in order to encourage blood flow to the flippers that are unprotected by hair to cool down (Dalton et al., 2014a), whereas in cool temperatures fur seals restrict blood flow to these areas in order to minimize heat loss (Mostman-Liwanag, 2008). As sea lions rely solely on their blubber to remain warm, they must retain a thicker layer than fur seals to compensate (Scholander et al., 1950), which can be maintained only by consuming large amounts of energy. Sea lion blubber thickness appears to remain constant throughout the year (Mellish et al., 2007), which may be why the metabolic rate of sea lions remains relatively constant across months but declines when water temperature increases. Despite the substantial differences in the thermoregulatory strategies of otariids, there was little difference in their overall sSMR, suggesting that these strategies have complementary costs.
Sex
A significant effect of sex on sSMR was found for both sea lions and fur seals, where females had higher sSMR than males. This same effect has been found in other species of adult pinniped, including Californian sea lions (Hurley and Costa, 2001), grey seals (Beck et al., 2003) and Antarctic fur seals (Arctocephalus gazella) (Boyd and Duck, 1991; Boyd and Croxall, 1996). Pinniped juveniles and pups do not show any significant sex differences in their metabolic rates, instead maintaining a consistently elevated metabolic rate associated with the cost of growth (Fowler et al., 2007; Verrier et al., 2011). As they age, morphological and physiological differences arise, including extreme sexual dimorphism and an elevated sSMR in the female (Hurley and Costa, 2001) that does not change depending on reproductive status (Williams et al., 2007). Females are usually in a stage of reproduction throughout the year (lactating or pregnant), whereas males spend some of the year removed from reproductive constraints. By measuring females that were non-breeding and non-lactating we removed the effect of reproduction, yet females still had elevated sSMR in comparison to the males. Therefore, the higher sSMR that we observed was probably related to the ongoing costs of reproduction. As there is no evidence that the metabolic rate of otariids varies between reproductive and non-reproductive cycles (Costa and Gentry, 1986; Williams et al., 2007), these differences in sSMR are likely to be attributable to allometry (Kleiber, 1975; McNab, 2008).
Moult
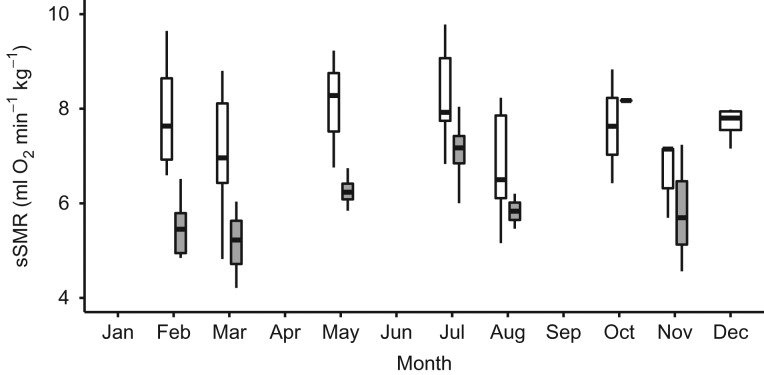
In pinnipeds, the moult usually occurs following the breeding period, either immediately after breeding or following a brief foraging period (Scheffer and Johnson, 1963). For Australian and New Zealand fur seals the moult occurs early in the year for ~2 months. Australian sea lions can moult at any time of year, and the moult is generally extended over 3–5 months. Metabolic responses to this phenomenon differ across species, and the energetic processes behind the moult are not well understood. In our study, the male fur seals increased sSMR during the moult, but there was no consistent effect of moult on the sSMR for any of the other otariids. Harbour, spotted (Phoca largha) and northern elephant seals (Mirounga angustirostris) have low resting metabolic rates during their moult (Ashwell-Erickson et al., 1986; Worthy et al., 1992). The metabolic rate of northern fur seals (Callorhinus ursinus) was highest during autumn and lowest in the winter, which corresponded to the beginning and the end of the moult (Dalton et al., 2015). Grey seals and non-reproductive Californian sea lion females showed increased metabolic rate during the moult; juveniles significantly more than adults (Boily, 1996; Boily and Lavigne, 1997; Beck et al., 2003; Williams et al., 2007). Increasing metabolic rate during the moult is proposed to aid in thermoregulation for fur seals while some of the insulating layer is lost and the energy invested into the growth of new hair (Boyd et al., 1993). Decreasing metabolic rate is proposed to delay fat loss while hauled out during the moult (Beck et al., 2003).
As sea lions do not rely on their fur layer for thermoregulation, their energetic response to the moult is likely to differ from that of the fur seals. During the moult, the blubber layer and lipid content of Californian sea lions is at its lowest, suggesting that an increase in metabolism is required to maintain body temperature within the thermoregulatory range (Williams et al., 2007). As the sea lions in our study were housed in warm water for the duration of their moult, the effect of the moult may have been masked. The female sea lions displayed no discernible pattern in sSMR during the moult (Table 2). The two male sea lions in this study moulted at different times of the year, one during the warmest water period (25–26°C), when we recorded his highest sSMR (ASM1; Table 2), and the other during moderate water temperatures (19–20°C), when we recorded his lowest sSMR (ASM2). If the sea lions do indeed use perfusion to cool during warm temperatures, this effect may have been exacerbated by the moult, allowing the body to cool more quickly and slowing their metabolism. During the period of moderate water temperatures, the sea lions may need to increase their metabolic rate to cope with the cooler water and hair loss. Seal moult generally occurs in summer to maximize skin surface temperature for the promotion of hair growth (Paterson et al., 2012) and because they are unable to thermoregulate efficiently (Feltz and Fay, 1966).
Table 2:
Mean ± SD and n of mass-specific standard metabolic rate (in millilitres of oxygen per minute per kilogram) and multiples of Kleiber's (1975) predicted basal metabolic rate (BMR*) for an Australian fur seal female and male, three New Zealand fur seal males, five Australian sea lion females and two Australian sea lion males measured in different months
| Month | Measure | Feb | Mar | May | Jul | Aug | Sep | Oct | Nov | Dec |
|---|---|---|---|---|---|---|---|---|---|---|
| Australian fur seal | ||||||||||
| AFF1 | sSMR | 7.0 ± 0.2 | 6.1 ± 0.5 | NA | NA | 6.3 ± 0.6 | 5.0 | NA | 6.7 | 8.2 ± 0.5 |
| BMR* | 2.1 | 1.8 | 1.9 | 1.5 | 1.5 | 2.0 | ||||
| n | 3 | 4 | 2 | 1 | 1 | 2 | ||||
| AFM1 | sSMR | 6.7 ± 0.5 | 5.5 ± 0.4 | NA | NA | 3.6 ± 0.8 | 3.1 | NA | 4.9 | 6.1 ± 0.5 |
| BMR* | 2.6 | 2.1 | 1.4 | 1.2 | 1.8 | 2.3 | ||||
| n | 3 | 7 | 2 | 1 | 1 | 2 | ||||
| New Zealand fur seal | ||||||||||
| NFM1 | sSMR | 6.5 ± 0.3 | 6.1 ± 0.2 | NA | NA | 4.0 ± 0.3 | NA | NA | 5.4 | 4.3 ± 0.00 |
| BMR* | 2.3 | 2.0 | 1.4 | 2.0 | 1.5 | |||||
| n | 3 | 6 | 3 | 1 | 2 | |||||
| NFM2 | sSMR | NA | NA | 5.8 ± 0.2 | NA | NA | NA | NA | 5.2 ± 0.1 | NA |
| BMR* | 1.9 | 1.9 | ||||||||
| n | 2 | 2 | ||||||||
| NFM3 | sSMR | 8.2 ± 0.02 | 9.8 ± 0.4 | 8.8 ± 0.5 | 5.9 ± 0.2 | 6.7 | NA | NA | 7.5 ± 0.2 | NA |
| BMR* | 2.5 | 2.7 | 2.4 | 1.7 | 1.9 | 2.1 | ||||
| n | 3 | 2 | 2 | 2 | 1 | 2 | ||||
| Australian sea lion | ||||||||||
| ASF1 | sSMR | 8.1 ± 0.5 | 7.2 ± 0.4 | 9.2 | 9.7 ± 0.4 | NA | NA | 8.8 | 7.1 | NA |
| BMR* | 2.2 | 1.9 | 2.4 | 2.6 | 2.4 | 1.9 | ||||
| n | 3 | 3 | 1 | 3 | 1 | 1 | ||||
| ASF2 | sSMR | 7.9 ± 0.4 | 7.8 ± 0.4 | NA | NA | 7.0 ± 0.9 | 7.6 | NA | 7.1 | 7.9 ± 0.1 |
| BMR* | 2.2 | 2.2 | 1.9 | 2.1 | 2.0 | 2.3 | ||||
| n | 3 | 6 | 2 | 1 | 1 | 2 | ||||
| ASF3 | sSMR | 7.6 ± 0.6 | 6.3 ± 0.3 | 7.0 ± 0.6 | 7.2 | 7.6 ± 0.4 | ||||
| BMR* | 2.3 | 1.9 | NA | NA | 2.1 | NA | NA | 2.2 | ||
| n | 3 | 6 | 3 | 1 | 2 | |||||
| ASF4 | sSMR | 6.7 ± 0.1 | 5.9 ± 0.3 | 7.5 ± 0.8 | 7.5 ± 0.3 | 6.4 | 5.7 | |||
| BMR* | 2.2 | 2.2 | 2.0 | 2.1 | NA | NA | 2.0 | 2.3 | NA | |
| n | 3 | 3 | 2 | 3 | 1 | 1 | ||||
| ASF5 | sSMR | 9.3 ± 0.3 | 8.1 ± 0.3 | 6.4 ± 0.9 | 6.3 | 7.6 ± 0.2 | ||||
| BMR* | 2.8 | 2.4 | NA | NA | 1.9 | NA | NA | 1.9 | 2.3 | |
| n | 2 | 5 | 3 | 1 | 2 | |||||
| ASM1 | sSMR | 5.9 ± 0.3 | 5.6 ± 0.4 | 6.5 ± 0.2 | 7.5 ± 0.3 | 8.2 | 7.2 | |||
| BMR* | 2.1 | 1.9 | 2.2 | 2.5 | NA | NA | 2.7 | 2.4 | NA | |
| n | 3 | 2 | 2 | 3 | 1 | 1 | ||||
| ASM2 | sSMR | 4.9 ± 0.3 | 4.2 | 6.0 ± 0.2 | 6.0 | 5.8 ± 0.4 | 5.1 ± 0.6 | |||
| BMR* | 1.8 | 1.6 | 2.2 | 2.2 | 2.2 | NA | NA | 1.9 | NA | |
| n | 5 | 1 | 2 | 1 | 2 | 2 | ||||
Abbreviations: AFF, Australian fur seal female; AFM, Australian fur seal male; ASF, Australian sea lion female; ASM, Australian sea lion male; BMR, basal metabolic rate; NFM, New Zealand fur seal male; SMR, standard metabolic rate; sSMR, mass-specific standard metabolic rate. The number following the species and sex identity is an individual identifier. Bold values indicate months when the seal was moulting. NA indicates a month when that individual was not measured.
Implications for a changing environment
Australian sea lions typically forage in temperatures of 12–22°C in South Australia (Lowther et al., 2013). Male New Zealand fur seals forage in waters around Macquarie Island (54°S, 159°E), where the water temperature can be as low as 2°C, to Montague Island (36°S, 150°E) and across to western Australia (33°S, 114°E), where the water can reach 24°C (Campbell et al., 2014; McIntosh et al., 2014). Australian fur seals are found predominantly in the Bass Strait, southern Australia, where water temperatures have a much smaller range of 12.6–19.3°C (Kirkwood et al., 2006; McIntosh et al., 2014). Therefore, the fur seals and sea lions in our study were exposed to a range of temperatures that were at the upper limit of what they would experience in the wild. Despite prolonged exposure to water temperatures higher than those that seals would experience in the wild, metabolic rates were not outside those expected for a marine mammal (Williams et al., 2000). It is possible, therefore, that the fur seals and sea lions housed in captivity have acclimated to warm water. South-east Australia is expected to have some of the largest increase in sea surface temperature globally with 0.7–1.4°C warming by 2030 (Ridgway and Hill, 2012; Carroll et al., 2016), and the present study presents evidence that the fur seals and sea lions that occupy this area have the physiological capacity to adapt to these changes.
Conclusions
Pinnipeds that have a limited ability to adjust their energy storage and usage may be more susceptible to environmental change. Maximizing fitness can, in part, be achieved through adjusting metabolic rates in response to changes in environmental conditions. Flexibility in physiological and morphological traits is important to survival, because mammals that have static metabolic rates and core body temperatures are more likely to become extinct (Geiser and Turbill, 2009). Australian fur seals and New Zealand fur seals demonstrated annual variations in their standard metabolic rates, which corresponded to their annual breeding and moulting cycle. Australian sea lions showed very little variation in metabolic rate over the year or in response to the moult, but metabolic rate reduced in response to increasing water temperatures. Otariids in the present study have demonstrated adaptations to warming water, a trait that might enhance their survival in a changing environment. Fur seal numbers in Australia are increasing, whereas sea lions are in decline and classified as endangered. Sea lions may compensate for living in a low-productivity environment by using an 18 month breeding cycle and a static foraging strategy and energy usage (Lowther and Goldsworthy, 2011; Ahonen et al., 2016). It is unclear at this stage whether the sea lion strategy means that they are ready to withstand further change, or they may not persist under more extreme pressures. In contrast, fur seals may be buffered by their potential to use their pelagic diving abilities to move offshore and exploit cold upwellings.
Acknowledgements
We thank all of the curatorial staff at Dolphin Marine Magic, Underwater World Mooloolaba and Taronga Zoo for their invaluable assistance with data collection, training the seals and ongoing commitment to this project. All experiments were conducted under the current laws of Australia authorised under New South Wales Office of Environment and Heritage Scientific Licence SL100746 to R.G.H.
Funding
This project was funded by Australian Research Council Linkage Grant (grant number LP110200603) to R.G.H. and D.J.S., with support from Taronga Conservation Society Australia. M.A.L. is a recipient of a Macquarie University Research Excellence Scholarship.
References
- Ahonen H, Lowther AD, Harcourt RG, Goldsworthy SD, Charrier I, Stow AJ (2016) The limits of dispersal: fine scale spatial genetic structure in Australian sea lions. Front Mar Sci 3: 65 doi:10.3389/fmars.2016.00065. [Google Scholar]
- Arnould JP, Hindell MA (2001) Dive behaviour, foraging locations, and maternal-attendance patterns of Australian fur seals (Arctocephalus pusillus doriferus). Can J Zool 79: 35–48. [Google Scholar]
- Arnould JP, Warneke RM (2002) Growth and condition in Australian fur seals (Arctocephalus pusillus doriferus) (Carnivora: Pinnipedia). Aust J Zool 50: 53–66. [Google Scholar]
- Ashwell-Erickson S, Fay FH, Elsner R, Wartzok D (1986) Metabolic and hormonal correlates of molting and regeneration of pelage in Alaskan harbor and spotted seals (Phoca vitulina and Phoca largha). Can J Zool 64: 1086–1094. [Google Scholar]
- Bartoń K. (2013) MuMIn: multi-model inference, R package version 1.9.13.
- Battaile BC, Sakamoto KQ, Nordstrom CA, Rosen DA, Trites AW (2015) Accelerometers identify new behaviors and show little difference in the activity budgets of lactating northern fur seals (Callorhinus ursinus) between breeding islands and foraging habitats in the Eastern Bering Sea. PLoS One 10: e0118761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis AMM, Page B, Goldsworthy SD (2008. a) Colony-specific foraging areas of lactating New Zealand fur seals. Mar Ecol Prog Ser 361: 279–290. [Google Scholar]
- Baylis AMM, Page B, Goldsworthy SD (2008. b) Effect of seasonal changes in upwelling activity on the foraging locations of a wide-ranging central-place forager, the New Zealand fur seal. Can J Zool 86: 774–789. [Google Scholar]
- Beck CA, Bowen WD, Iverson SJ (2003) Sex differences in the seasonal patterns of energy storage and expenditure in a phocid seal. J Anim Ecol 72: 280–291. [Google Scholar]
- Boily P. (1996) Metabolic and hormonal changes during the molt of captive gray seals (Halichoerus grypus). Am J Physiol Regul Integr Comp Physiol 270: R1051–R1058. [DOI] [PubMed] [Google Scholar]
- Boily P, Lavigne DM (1997) Developmental and seasonal changes in resting metabolic rates of captive female grey seals. Can J Zool 75: 1781–1789. [Google Scholar]
- Boyd IL. (1991) Environmental and physiological factors controlling the reproductive cycles of pinnipeds. Can J Zool 69: 1135–1148. [Google Scholar]
- Boyd IL. (2002) Energetics: consequences for fitness In Hoelzel AR, ed, Marine Mammal Biology: an Evolutionary Approach. Blackwell Science Ltd, Oxford, UK, pp 247–277. [Google Scholar]
- Boyd IL, Croxall JP (1996) Dive durations in pinnipeds and seabirds. Can J Zool 74: 1696–1705. [Google Scholar]
- Boyd IL, Duck CD (1991) Mass changes and metabolism in territorial male Antarctic fur seals (Arctocephalus gazella). Physiol Zool 64: 375–392. [Google Scholar]
- Boyd I, Arnbom T, Fedak M (1993) Water flux, body composition, and metabolic rate during molt in female southern elephant seals (Mirounga leonina). Physiol Zool 66: 43–60. [Google Scholar]
- Boyd I, Reid K, Bevan R (1995. a) Swimming speed and allocation of time during the dive cycle in Antarctic fur seals. Anim Behav 50: 769–784. [Google Scholar]
- Boyd I, Woakes A, Butler P, Davis R, Williams T (1995. b) Validation of heart rate and doubly labelled water as measures of metabolic rate during swimming in California sea lions. Funct Ecol 9: 151–160. [Google Scholar]
- Boyles JG, Seebacher F, Smit B, McKechnie AE (2011) Adaptive thermoregulation in endotherms may alter responses to climate change. Proceedings of Annual Meeting of the Society for Integrative and Comparative Biology. Society for Integrative and Comparative Biology, Salt Lake City, UT, USA, pp 1–15. [DOI] [PubMed] [Google Scholar]
- Campbell R, Holley D, Collins P, Armstrong S (2014) Changes in the abundance and distribution of the New Zealand fur seal (Arctocephalus forsteri) in Western Australia: are they approaching carrying capacity. Aust J Zool 62: 261–267. [Google Scholar]
- Canale CI, Henry P-Y (2010) Adaptive phenotypic plasticity and resilience of vertebrates to increasing climatic unpredictability. Clim Res 43: 135–147. [Google Scholar]
- Carey PW. (1991) Resource-defense polygyny and male territory quality in the New Zealand fur seal. Ethology 88: 63–79. [Google Scholar]
- Carroll G, Everett JD, Harcourt R, Slip D, Jonsen I (2016) High sea surface temperatures driven by a strengthening current reduce foraging success by penguins. Sci Rep 6: 22236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa DP. (1991) Reproductive and foraging energetics of high latitude penguins, albatrosses and pinnipeds: implications for life history patterns. Am Zool 31: 111–130. [Google Scholar]
- Costa DP, Gales NJ (2003) Energetics of a benthic diver: seasonal foraging ecology of the Australian sea lion, Neophoca cinerea. Ecol Monogr 73: 27–43. [Google Scholar]
- Costa DP, Gentry RL (1986) Free-ranging energetics of northern fur seals In Costa DP, Gentry RL, eds, Fur Seals: Maternal Strategies on Land and at Sea. Princeton University Press, Princeton, NJ, USA, pp 79–101. [Google Scholar]
- Costa DP, Trillmich F (1988) Mass changes and metabolism during the perinatal fast: a comparison between Antarctic (Arctocephalus gazella) and Galapagos fur seals (Arctocephalus galapagoensis). Physiol Zool 61: 160–169. [Google Scholar]
- Dalton AJM, Rosen DAS, Trites AW (2014. a) Broad thermal capacity facilitates the primarily pelagic existence of northern fur seals (Callorhinus ursinus). Mar Mamm Sci 30: 994–1013. [Google Scholar]
- Dalton AJM, Rosen DAS, Trites AW (2014. b) Season and time of day affect the ability of accelerometry and the doubly labeled water methods to measure energy expenditure in northern fur seals (Callorhinus ursinus). J Exp Mar Biol Ecol 452: 125–136. [Google Scholar]
- Dalton AJM, Rosen DAS, Trites AW (2015) Resting metabolic rate and activity: key components of seasonal variation in daily energy expenditure for the northern fur seal (Callorhinus ursinus). Can J Zool 93: 635–644. [Google Scholar]
- Dassis M, Rodríguez DH, Ieno EN, Davis RW (2012) Submerged swimming and resting metabolic rates in Southern sea lions. J Exp Mar Biol Ecol 432–433: 106–112. [Google Scholar]
- Fedak M, Pullen M, Kanwisher J (1987) Circulatory responses of seals to periodic breathing: heart rate and breathing during exercise and diving in the laboratory and open sea. Can J Zool 66: 53–60. [Google Scholar]
- Feldkamp S. (1987) Swimming in the California sea lion: morphometrics, drag and energetics. J Exp Biol 131: 117–135. [DOI] [PubMed] [Google Scholar]
- Feltz ET, Fay FH (1966) Thermal requirements in vitro of epidermal cells from seals. Cryobiology 3: 261–264. [DOI] [PubMed] [Google Scholar]
- Fowler SL, Costa DP, Arnould JP, Gales NJ, Burns JM (2007) Ontogeny of oxygen stores and physiological diving capability in Australian sea lions. Funct Ecol 21: 922–935. [Google Scholar]
- Gales NJ, Shaughnessy PD, Dennis TE (1994) Distribution, abundance and breeding cycle of the Australian sea lion Neophoca cinerea (Mammalia: Pinnipedia). J Zool 234: 353–370. [Google Scholar]
- Geiser F, Turbill C (2009) Hibernation and daily torpor minimize mammalian extinctions. Naturwissenschaften 96: 1235–1240. [DOI] [PubMed] [Google Scholar]
- Gibbens J, Arnould JPY (2009) Interannual variation in pup production and the timing of breeding in benthic foraging Australian fur seals. Mar Mamm Sci 25: 573–587. [Google Scholar]
- Goldsworthy SD, Shaughnessy PD (1994) Breeding biology and haul-out pattern of the New Zealand fur seal, Arctopehalus forsteri, at Cape Gantheaume, South Australia. Wildl Res 21: 365–375. [Google Scholar]
- Goldsworthy SD, Bulman C, He X, Larcome J, Littan CL (2003) Trophic interactions between marine mammals and Australian fisheries: an ecosystem approach In Gales N, Hindell M, Kirkwood R, eds, Marine Mammals: Fisheries, Tourism and Management Issues. CSIRO Publications, Melbourne, Victoria, Australia, pp 62–99. [Google Scholar]
- Gomendio M, Tourmente M, Roldan ER (2011) Why mammalian lineages respond differently to sexual selection: metabolic rate constrains the evolution of sperm size. Proc Biol Sci 278: rspb20110275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinet C, Roux JP, Bonnet M, Mison V (1998) Effect of body size, body mass, and body condition on reproduction of female South African fur seals (Arctocephalus pusillus) in Namibia. Can J Zool 76: 1418–1424. [Google Scholar]
- Halsey LG, Green JA, Wilson RP, Frappell PB (2009) Accelerometry to estimate energy expenditure during activity: best practice with data loggers. Physiol Biochem Zool 82: 396–404. [DOI] [PubMed] [Google Scholar]
- Harcourt RG, Bradshaw CJA, Dickson K, Davis LS (2002) Foraging ecology of a generalist predator, the female New Zealand fur seal. Mar Ecol Prog Ser 227: 11–24. [Google Scholar]
- Harris GP, Griffiths FB, Clementson LA, Lyne V, van der Doe H (1991) Seasonal and interannual variability in physical processes, nutrient cycling and the structure of the food chain in Tasmanian shelf waters. J Plankton Res 13: 109–131. [Google Scholar]
- Higgins LV. (1990) Reproductive behavior and maternal investment of Australian sea lions. PhD thesis; University of California, Santa Cruz, CA, USA. [Google Scholar]
- Higgins LV. (1993) The nonannual, nonseasonal breeding cycle of the Australian sea lion, Neophoca cinerea. J Mammal 74: 270–274. [Google Scholar]
- Hothorn T, Bretz F, Westfall P, Heiberger R, Schuetzenmeister A, Scheibe S (2013) multcomp: Simultaneous inference in general parametric models. Version 1.3.0, R Foundation for Statistical Computing, Vienna, Austria.
- Hurley J, Costa DP (2001) Standard metabolic rate at the surface and during trained submersions in adult California sea lions (Zalophus californianus). J Exp Biol 204: 3273–3281. [DOI] [PubMed] [Google Scholar]
- Kirkwood R, Arnould JPY (2011) Foraging trip strategies and habitat use during late pup rearing by lactating Australian fur seals. Aust J Zool 59: 216–226. [Google Scholar]
- Kirkwood R, Lynch M, Gales N, Dann P, Sumner M (2006) At-sea movements and habitat use of adult male Australian fur seals (Arctocephalus pusillus doriferus). Can J Zool 84: 1781–1788. [Google Scholar]
- Kleiber M. (1947) Body size and metabolic rate. Physiol Rev 27: 511–541. [DOI] [PubMed] [Google Scholar]
- Kleiber M. (1975) Metabolic turnover rate: a physiological meaning of the metabolic rate per unit body weight. J Theor Biol 53: 199–204. [DOI] [PubMed] [Google Scholar]
- Ladds M, Slip D, Harcourt R (2016) Swimming metabolic rates vary by sex and development stage, but not by species, in three species of Australian otariid seals. J Comp Physiol B in press. doi:10.1007/s00360-016-1046-5. [DOI] [PubMed] [Google Scholar]
- Learmonth JA, MacLeod CD, Santos MB, Pierce GJ, Crick HQP, Robinson RA (2006) Potential effects of climate change on marine mammals. Oceanogr Mar Sci 44: 431–464. [Google Scholar]
- Liwanag HEM. (2010) Energetic costs and thermoregulation in northern fur seal (Callorhinus ursinus) pups: the importance of behavioral strategies for thermal balance in furred marine mammals. Physiol Biochem Zool 83: 898–910. [DOI] [PubMed] [Google Scholar]
- Liwanag HEM, Williams TM, Costa D, Kanatous S, Davis R, Boyd I (2009) The effects of water temperature on the energetic costs of juvenile and adult California sea lions (Zalophus californianus): the importance of skeletal muscle thermogenesis for thermal balance. J Exp Biol 212: 3977–3984. [DOI] [PubMed] [Google Scholar]
- Liwanag HEM, Berta A, Costa DP, Abney M, Williams TM (2012. a) Morphological and thermal properties of mammalian insulation: the evolution of fur for aquatic living. Biol J Linn Soc 106: 926–939. [Google Scholar]
- Liwanag HEM, Berta A, Costa DP, Budge SM, Williams TM (2012. b) Morphological and thermal properties of mammalian insulation: the evolutionary transition to blubber in pinnipeds. Biol J Linn Soc 107: 774–787. [Google Scholar]
- Lowther AD, Goldsworthy SD (2011) Maternal strategies of the Australian sea lion (Neophoca cinerea) at Dangerous Reef, South Australia. Aust J Zool 59: 54–62. [Google Scholar]
- Lowther AD, Harcourt RG, Hamer DJ, Goldsworthy SD (2011) Creatures of habit: foraging habitat fidelity of adult female Australian sea lions. Mar Ecol Prog Ser 443: 249–263. [Google Scholar]
- Lowther AD, Harcourt RG, Page B, Goldsworthy SD (2013) Steady as he goes: at-sea movement of adult male Australian sea lions in a dynamic marine environment. PLoS One 8: e74348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh RR, Page B, Goldsworthy SD (2006) Dietary analysis of regurgitates and stomach samples from free-living Australian sea lions. Wildlife Res 33: 661–669. [Google Scholar]
- McIntosh RR, Arthur AD, Dennis T, Berris M, Goldsworthy SD, Shaughnessy PD, Teixeira CEP (2013) Survival estimates for the Australian sea lion: negative correlation of sea surface temperature with cohort survival to weaning. Mar Mamm Sci 29: 84–108. doi:10.1111/j.1748-7692.2011.00558.x. [Google Scholar]
- McIntosh R, Kirkwood R, Thalman S, Alderman R, Arnould JPY, Mitchell T, Kirkman SP, Salton M, Slip DJ (2014). Pup estimates for Australian and New Zealand fur seals in Victoria, Tasmania and New South Wales between 2007 and 2013, Unpublished report to Australian Government Department of the Environment, Phillip Island Nature Parks and Department of the Environment, Melbourne, Victoria, Australia, pp 94. [Google Scholar]
- McKenzie J, Parry LJ, Page B, Simon DG (2005) Estimation of pregnancy rates and reproductive failure in New Zealand fur seals (Arctocephalus forsteri). J Mammal 86: 1237–1246. [Google Scholar]
- McNab B. (2008) An analysis of the factors that influence the level and scaling of mammalian BMR. Comp Biochem Physiol A Mol Integr Physiol 151: 5–28. [DOI] [PubMed] [Google Scholar]
- Meagher EM, McLellan WA, Westgate AJ, Wells RS, Blum JE, Pabst DA (2008) Seasonal patterns of heat loss in wild bottlenose dolphins (Tursiops truncatus). J Comp Physiol B 178: 529–543. [DOI] [PubMed] [Google Scholar]
- Mellish JE, Tuomi PA, Horning M (2004) Assessment of ultrasound imaging as a noninvasive measure of blubber thickness in pinnipeds. J Zoo Wildl Med 35: 116–118. [DOI] [PubMed] [Google Scholar]
- Mellish JE, Horning M, York AE (2007) Seasonal and spatial blubber depth changes in captive harbor seals (Phoca vitulina) and Steller's sea lions (Eumetopias jubatus). J Mammal 88: 408–414. [Google Scholar]
- Mostman-Liwanag HE. (2008) Fur versus blubber: a comparative look at marine mammal insulation and its metabolic and behavioral consequences. PhD thesis; University of California, Santa Cruz, CA, USA. [Google Scholar]
- Ochoa-Acuña H, Francis JM, Boness DJ (1998) Interannual variation in birth mass and postnatal growth rate of Juan Fernández fur seals. Can J Zool 76: 978–983. [Google Scholar]
- Page B, McKenzie J, Goldsworthy SD (2005. a) Dietary resource partitioning among sympatric New Zealand and Australian fur seals. Mar Ecol Prog Ser 293: 283–302. [Google Scholar]
- Page B, McKenzie J, Goldsworthy SD (2005. b) Inter-sexual differences in New Zealand fur seal diving behaviour. Mar Ecol Prog Ser 304: 249–264. [Google Scholar]
- Paterson W, Sparling CE, Thompson D, Pomeroy PP, Currie JI, McCafferty DJ (2012) Seals like it hot: changes in surface temperature of harbour seals (Phoca vitulina) from late pregnancy to moult. J Therm Biol 37: 454–461. [Google Scholar]
- Peters KJ, Ophelkeller K, Bott NJ, Deagle BE, Jarman SN, Goldsworthy SD (2015) Fine-scale diet of the Australian sea lion (Neophoca cinerea) using DNA-based analysis of faeces. Mar Ecol 36: 347–367. [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D (2014) nlme: linear and nonlinear mixed effects models, R package version 3.1-117, R Foundation for Statistical Computing, Vienna, Austria.
- R Core Development Team (2015) R: A language and environment for statistical computing., R version 3.3.1, Ed R package version 3.2.3 R Foundation for Statistical Computing, Vienna, Austria.
- Ridgway K, Hill K (2012). East Australian Current In Poloczanska ES, Hobday AJ, Richardson AJ, eds, A Marine Climate Change Impacts and Adaptation Report for Australia. CSIRO Marine and Atmospheric Research and Integrated Marine Observing System, Tasmania, Australia. [Google Scholar]
- Rosen D, Renouf D (1995) Variation in the metabolic rates of captive harbour seals. Dev Mar Biol 4: 393–399. [Google Scholar]
- Rosen DA, Renouf D (1997) Seasonal changes in blubber distribution in Atlantic harbor seals: indications of thermodynamic considerations. Mar Mamm Sci 13: 229–240. [Google Scholar]
- Rosen DAS, Trites AW (1997) Heat increment of feeding in Steller sea lions, Eumetopias jubatus. Comp Biochem Physiol A Physiol 118: 877–881. [DOI] [PubMed] [Google Scholar]
- Rosen DAS, Gerlinsky CD, Trites AW (2015) Evidence of partial deferment of digestion during diving in Steller sea lions (Eumetopias jubatus). J Exp Mar Biol Ecol 469: 93–97. [Google Scholar]
- Scheffer VB, Johnson AM (1963) Molt in the northern fur seal. US Department of the Interior, Fish and Wildlife Service, Bureau of Commercial Fisheries, Washinton, DC. [Google Scholar]
- Schumann N, Gales NJ, Harcourt RG, Arnould JPY (2013) Impacts of climate change on Australian marine mammals. Aust J Zool 61: 146–159. [Google Scholar]
- Simmonds MP, Isaac SJ (2007) The impacts of climate change on marine mammals: early signs of significant problems. Oryx 41: 19–26. [Google Scholar]
- Sparling CE, Speakman JR, Fedak MA (2006) Seasonal variation in the metabolic rate and body composition of female grey seals: fat conservation prior to high-cost reproduction in a capital breeder. J Comp Physiol B 176: 505–512. [DOI] [PubMed] [Google Scholar]
- Staniland IJ, Boyd IL, Reid K (2007) An energy–distance trade-off in a central-place forager, the Antarctic fur seal (Arctocephalus gazella). Mar Biol 152: 233–241. [Google Scholar]
- Stewardson C. (2007). National Assessment of Interactions between Humans and Seals: Fisheries, Aquaculture and Tourism. Department of Agriculture, Fisheries and Forestry, Canberra, ACT, Australia, pp 142. [Google Scholar]
- Stewardson CL, Bester MN, Oosthuizen WH (1998) Reproduction in the male Cape fur seal Arctocephalus pusillus pusillus: age at puberty and annual cycle of the testis. J Zool 246: 63–74. [Google Scholar]
- Verrier D, Guinet C, Authier M, Tremblay Y, Shaffer S, Costa DP, Groscolas R, Arnould JPY (2011) The ontogeny of diving abilities in subantarctic fur seal pups: developmental trade-off in response to extreme fasting. Funct Ecol 25: 818–828. [Google Scholar]
- Villegas-Amtmann S, Atkinson S, Costa D (2009) Low synchrony in the breeding cycle of Galapagos sea lions revealed by seasonal progesterone concentrations. J Mammal 90: 1232–1237. [Google Scholar]
- Whittingham MJ, Stephens PA, Bradbury RB, Freckleton RP (2006) Why do we still use stepwise modelling in ecology and behaviour. J Anim Ecol 75: 1182–1189. [DOI] [PubMed] [Google Scholar]
- Williams TM, Yeates L (2004) The energetics of foraging in large mammals: a comparison of marine and terrestrial predators. Int Congr Ser 1275: 351–358. [Google Scholar]
- Williams TM, Davis RW, Fuiman LA, Francis J, Le Boeuf BJ, Horning M, Calambokidis J, Croll DA (2000) Sink or swim: strategies for cost-efficient diving by marine mammals. Science 288: 133–136. [DOI] [PubMed] [Google Scholar]
- Williams TM, Haun J, Davis RW, Fuiman LA, Kohin S (2001) A killer appetite: metabolic consequences of carnivory in marine mammals. Comp Biochem Physiol A Mol Integr Physiol 129: 785–796. [DOI] [PubMed] [Google Scholar]
- Williams TM, Rutishauser M, Long B, Fink T, Gafney J, Mostman‐Liwanag H, Casper D (2007) Seasonal variability in otariid energetics: implications for the effects of predators on localized prey resources. Physiol Biochem Zool 80: 433–443. [DOI] [PubMed] [Google Scholar]
- Withers PC. (1977) Measurement of VO2, VCO2, and evaporative water loss with a flow-through mask. J Appl Physiol Respir Environ Exerc Physiol 42: 120–123. [DOI] [PubMed] [Google Scholar]
- Worthy GAJ, Morris PA, Costa DP, Boeuf BJL (1992) Moult energetics of the northern elephant seal (Mirounga angustirostris). J Zool 227: 257–265. [Google Scholar]
- Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1: 3–14. doi:10.1111/j.2041-210X.2009.00001.x. [Google Scholar]
- Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM (2009. a) Mixed effects models and extensions in ecology with R. Springer, New York, USA. [Google Scholar]
- Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM (2009. b) Limitations of linear regression applied on ecological data Mixed effects models and extensions in ecology with R. Springer, pp 11–33. [Google Scholar]