Abstract
Molecular mechanisms of radiation dose-rate effects are not well understood. Among many possibilities, long-lasting sustained alterations in protein levels would provide critical information. To evaluate sustained effects after acute and chronic radiation exposure, we analyzed alterations in protein expression in the livers of mice. Acute exposure consisted of a lethal dose of 8 Gy and a sublethal dose of 4 Gy, with analysis conducted 6 days and 3 months after irradiation, respectively. Chronic irradiation consisted of a total dose of 8 Gy delivered over 400 days (20 mGy/day). Analyses following chronic irradiation were done immediately and at 3 months after the end of the exposure. Based on antibody arrays of protein expression following both acute lethal and sublethal dose exposures, common alterations in the expression of two proteins were detected. In the sublethal dose exposure, the expression of additional proteins was altered 3 months after irradiation. Immunohistochemical analysis showed that the increase in one of the two commonly altered proteins, MyD88, was observed around blood vessels in the liver. The alterations in protein expression after chronic radiation exposure were different from those caused by acute radiation exposures. Alterations in the expression of proteins related to inflammation and apoptosis, such as caspase 12, were observed even at 3 months after the end of the chronic radiation exposure. The alterations in protein expression depended on the dose, the dose rate, and the passage of time after irradiation. These changes could be involved in long-term effects of radiation in the liver.
Keywords: low-dose-rate irradiation effects, acute irradiation effects, protein expression, antibody array, mouse liver
INTRODUCTION
To understand the health effects of high-dose or low-dose radiation, many molecular biological analyses have been conducted. Currently, radiation is considered to influence living organisms in a dose-dependent manner, and the International Commission on Radiological Protection (ICRP) has established dose limits based on a linear no-threshold (LNT) model. However, the dose rate is an important factor in evaluating radiation risk to health. Indeed, there are cases of exposure to a low dose rate of radiation over the long term, such as at nuclear plant accident sites or during space exploration. In terms of radiation dose-rate effects, it is considered that the lower the rate for a given dose, the lower the effect induced by that dose [1]. However, specific responses have been shown to be induced by low-dose rate irradiation that are distinct from responses to acute radiation exposure [2–5]. In addition, in most cases, the effects observed are described in terms of mutation rates or cancer incidence, and the process remains unclear. Therefore, characterization of the effects of low-dose-rate radiation is indispensable for radiation protection and risk evaluation.
Radiation effects on living organisms have been researched at molecular levels such as at the level of mRNA and protein expression, as well as at the level of metabolic alterations. Analyses of protein expression in direct connection with biological functions provide useful information on health effects. Radiation effects on protein expression have been investigated in some systems [6–8]. Effects of low-dose radiation or low-dose-rate irradiation on protein expression have also been investigated in order to understand environmental or medical low-dose radiation [9, 10]. We previously reported changes in protein expression in the livers of mice irradiated at low dose rates over the long term [10]. Although attempts have been made to organize data regarding alterations in protein expression following irradiation in order to create a unified interpretation, such organization is difficult since most of the data have dealt with varying time points at different doses and in different experimental models [6]. In addition, information about protein expression at late times after irradiation seems to be lacking [9, 11]. To the best of our knowledge, comparative studies on dose-rate health effects for high-dose and low-dose radiation have not been conducted over significant time periods after irradiation in mouse models. Comparative studies between chronic and acute irradiation may offer critical information for understanding the processes that lead to the late effects of radiation.
Some effects of low-dose rate, long-term irradiation have been studied. A total dose of 8 Gy by chronic irradiation significantly increases neoplasm incidence and molecular alterations in mice [10, 12–15]. However, it remains unclear how this radiation exposure induces sustained effects that subsequently lead to health effects. If molecular markers can be detected in the process after irradiation, they would be useful in predicting and modifying radiation effects. To evaluate the alterations in protein expression in dose-dependent and dose-rate-dependent responses, protein expression in mice exposed to low-dose-rate, long-term radiation needs to be compared with that in mice exposed to acute radiation.
In the present study, a total dose of 8 Gy was used for both chronic and acute irradiation. In addition, to compare the effects of a lethal acute dose with the effects of a sublethal dose, a dose of 4 Gy was also evaluated. We then analyzed alterations in protein expression to better understand the processes that lead to radiation effects. We found specific responses to radiation in terms of changes in protein expression by comparative analysis, not only between sublethal and lethal doses, but also between acute and chronic irradiation.
MATERIALS AND METHODS
Mice and acute irradiation
For acute irradiation, 7-week-old male SPF C57BL/6J mice were obtained from CLEA Japan (Tokyo, Japan) and irradiated with 4 Gy of X-irradiation at 10 weeks of age. For 8 Gy X-irradiation, male SPF C57BL/6J mice from Japan SLC Co. (Hamamatsu, Japan) were irradiated at 24 weeks of age. The mice were irradiated with 8 Gy or 4 Gy at Tohoku University (dose rate: 0.72 Gy/min) or at the National Institute of Radiological Sciences (NIRS) (dose rate: 0.55 Gy/min). In the case of acute irradiation with 8 Gy, mice were sacrificed 6 days after irradiation. In the case of 4 Gy irradiation, the mice were sacrificed 3 months after irradiation. In all cases, livers were perfused with cold phosphate-buffered saline (PBS) and were stored at −80°C until use. All procedures were conducted in accordance with the relevant legal regulations of Japan under the Guidelines for Animal Experiments of the University of Occupational and Environmental Health and NIRS.
Continuous irradiation
For chronic irradiation, 7–8-week-old male SPF C57BL/6J mice were obtained from CLEA Japan and were irradiated at the Institute for Environmental Sciences in Rokkasho, Aomori, under the same conditions as described previously [13]. Briefly, continuous irradiation was carried out with 137Cs gamma rays for 400 days (22 h/day) at a dose rate of 20 mGy/day (total dose of 8000 mGy). Dosimetry and animal care under barrier conditions were carried out as described previously [12]. Mice were sacrificed immediately or at 3 months after chronic radiation exposure was completed, and the livers were perfused with cold PBS and stored at −80°C until use. All experiments were conducted in accordance with the relevant legal regulations of Japan, and under the Guidelines for Animal Experiments of the Institute for Environmental Sciences (IES).
Antibody array analysis
Comparative studies of protein expression in livers between non-irradiated and irradiated mice were performed using the Panorama® Antibody Microarray (XPRESS Profiler725 Kit) (Sigma-Aldrich Co., St Louis, MO). This antibody microarray contains 725 different antibodies. The antibodies represent families of proteins involved in a variety of functions such as apoptosis, cell cycle, cell stress, cytokines and gene regulation. Protein extraction from parts of frozen livers, antibody microarray hybridization, and imaging were performed at Filgen, Inc. (Nagoya, Japan) following the kit instructions. Slides were dried and scanned on a GenePix® 4000B scanner (Molecular Devices Co., Tokyo, Japan). Microarray images were analyzed with Array-Pro Analyzer® Ver. 4.5 (Media Cybernetics Inc., Bethesda, MD). Microarray data were analyzed with Microarray Data Analysis Tool Ver. 3.2 (Filgen, Inc.). Three pair comparisons were performed for each point. All of the antibody array data were shown in Supplementary Tables 1 and 2. Data of antibodies that have no reactivity with mouse proteins were excluded for subsequent analyses. In addition, as DcR3 does not seem to be identified in mice in spite of the antibody information from the manufacturer, the result was also excluded [16]. Antibody data that displayed increases or decreases of >2-fold in all three comparisons were selected and are presented in the figures and tables. When more than one kind of antibody to one protein were used, the most altered result is shown in the tables.
Sample preparation and western blot analysis
Liver tissues were homogenized in five volumes of a lysis solution (extraction buffer including Benzonase, protease inhibitors and phosphatase inhibitors) used in the Panorama® Antibody Microarray kit. Lysates were centrifuged (20 000 × g) at 2°C for 30 min. The supernatants were used for western blot analysis.
Protein concentration was determined using Bradford's method. Western blotting was performed as described elsewhere [10]. Equivalent amounts of protein were subjected to SDS-polyacrylamide gel electrophoresis (12%). Separated proteins were transferred electrophoretically to polyvinylidene difluoride (PVDF) membranes. The membrane was blocked with PBS-Tween (0.05%) solution containing 5% non-fat dry milk at 4°C overnight, followed by immunoblotting with antibodies against caspase 12 (C7611, Sigma) and actin (Santa Cruz Biotechnology, Inc., Dallas, TX) for 2 h at room temperature. After four washes with PBS-Tween solution, immune complexes were detected with the appropriate secondary antibodies (Santa Cruz Biotechnology, Inc.) and detection reagents (Luminata Crescendo Western HRP substrate, Merck Millipore, Darmstadt, Germany). Signal bands were analyzed using an LAS-1000 analysis system (Fuji Film, Tokyo, Japan).
Immunohistochemical staining analysis
Tissue samples were fixed in 10% neutral buffered formalin. The samples were then placed in ethanol and paraffin-blocked. Paraffin embedded samples were deparaffinized and rehydrated with graded alcohols and were used for immunohistochemical staining analysis. Antibodies against MyD88 (MAB2745, Abnova, Taipei, Taiwan) and Laminin2 α2 (L0663, Sigma) were used, and the samples were analyzed at the Biopathology Institute Co., Ltd (Oita, Japan).
Statistical analysis
Statistical analysis was performed using Student's t-test, and P-values are indicated in Tables 1 and 2.
Table 1.
Alterations in protein expression in mouse livers resulting from acute irradiation
| Protein | Fold change (mean ± SD) | P | Related functions |
|---|---|---|---|
| 8 Gy | |||
| MyD88 | 5.6 ± 3.6 | <0.05 | Toll-like receptor signaling |
| Parkin | 2.4 ± 0.5 | <0.01 | Cancer suppressor |
| Bcl-xL | 0.44 ± 0.02 | <0.01 | Apoptosis |
| 4 Gy | |||
| MyD88 | 3.5 ± 0.59 | <0.01 | Toll-like receptor signaling |
| MAP1b | 2.5 ± 0.26 | <0.01 | Microtubule association, autophagy |
| DcR1 | 2.2 ± 0.04 | <0.01 | Apoptosis |
| Bcl-xL | 0.34 ± 0.05 | <0.01 | Apoptosis |
| P34cdc2 | 0.38 ± 0.1 | <0.01 | Cell cycle |
Expression of the proteins in boldface type is altered in the case of both 8 Gy and 4 Gy irradiation at 6 days and 3 months after irradiation, respectively.
Table 2.
Alterations in protein expression by protracted low-dose-rate irradiation of mouse livers
| Protein | Fold change (mean ± SD) | P | Related functions |
|---|---|---|---|
| Immediately after total dose irradiation with 8 Gy | |||
| MBNL1 | 3.3 ± 0.2 | <0.01 | Gene expression and regulation |
| Laminin2 α2 | 2.6 ± 0.4 | <0.01 | Basement membrane |
| DRAK1 | 0.23 ± 0.09 | <0.01 | Apoptosis |
| PUMA/bbc3 | 0.36 ± 0.11 | <0.01 | Apoptosis |
| BID | 0.27 ± 0.05 | <0.01 | Apoptosis |
| cRaf (pSer621) | 0.4 ± 0.09 | <0.01 | MEK signaling |
| Bim | 0.41 ± 0.07 | <0.01 | Apoptosis |
| Bmf | 0.34 ± 0.09 | <0.01 | Apoptosis |
| SynCAM | 0.45 ± 0.05 | <0.01 | Cell–Cell adhesion |
| ILP2 | 0.43 ± 0.02 | <0.01 | Apoptosis |
| 3 months after total dose irradiation with 8 Gy | |||
| Laminin2 α2 | 4.3 ± 2.9 | 0.063 | Basement membrane |
| NFκB | 16 ± 20 | 0.131 | Gene expression and regulation |
| Caspase12 | 3.3 ± 0.9 | <0.01 | Apoptosis |
| Phosphor-β-catenin (pSer45) | 2.7 ± 1.0 | <0.05 | Cytoskeleton |
| DRAK1 | 0.29 ± 0.1 | <0.01 | Apoptosis |
| BID | 0.31 ± 0.04 | <0.01 | Apoptosis |
| Bim | 0.35 ± 0.07 | <0.01 | Apoptosis |
| Bmf | 0.33 ± 0.06 | <0.01 | Apoptosis |
| Apaf1 | 0.34 ± 0.06 | <0.01 | Apoptosis |
Alterations were observed in expression of the proteins in boldface type both immediately and at 3 months after irradiation.
RESULTS
Acute lethal and sublethal radiation effects on protein expression in the liver
In order to evaluate differences between the effects of chronic and acute irradiation on protein expression, an antibody array approach was adopted. First, mice were acutely irradiated with 8 Gy or 4 Gy. Six days after 8 Gy irradiation, the proteins MyD88 and Parkin displayed increased expression, whereas Bcl-xL was the only protein to show a decrease in expression (Table 1).
Acute irradiation with 4 Gy is sublethal, and most mice can survive long term after such radiation. At this dose, alterations in protein expression were observed at 3 months after the irradiation (Table 1). Two proteins, MyD88 and Bcl-xL, were commonly altered in mouse livers acutely irradiated with either 8 Gy or 4 Gy. The expression of some other proteins, including p34cdc2, DcR1 and MAP1b, was altered in livers 3 months after irradiation with 4 Gy.
MyD88 expression was immunohistochemically stained in livers 3 months after acute irradiation (Fig. 1A and B). Higher MyD88 signals in endothelial cells around blood vessels 3 months after irradiation were observed, as compared with controls.
Fig. 1.
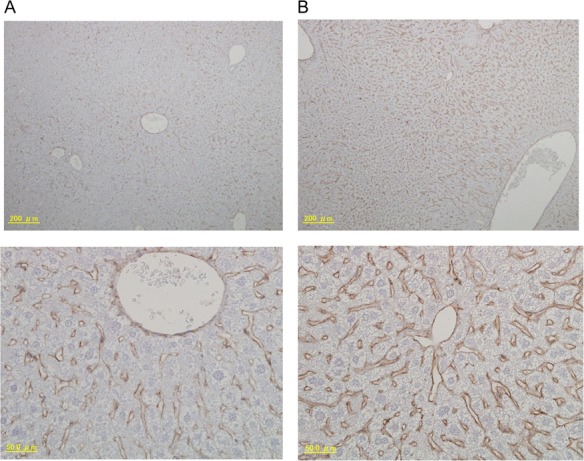
Immunohistochemical staining analyses of MyD88 in mouse livers 3 months after acute irradiation. (A) Control; (B) 3 months after acute irradiation. Upper panels are ×100, and lower panels are ×400.
Alterations in protein expression induced by chronic irradiation
The effects of chronic irradiation on protein expression were also tested using these antibody arrays. Irradiation with a low dose rate of 20 mGy/day was performed at the IES (Aomori, Japan). This dose rate induces hepatocellular adenomas as a late effect [13]. Mice irradiated with this dose rate for 400 days (a total dose of 8 Gy) were used for the analysis. Alterations in protein expression in livers immediately after irradiation were observed (Table 2). Proteins whose expression was altered were related to the inflammatory response and apoptosis regulation. Protein expression was also evaluated at 3 months after chronic irradiation. Interestingly, new induction of proteins even at 3 months after irradiation was detected. Many of these proteins were related to apoptosis. Laminin2 α2 (the one protein whose expression was altered both immediately and at 3 months after chronic irradiation) and caspase 12 (whose expression was altered at 3 months after chronic irradiation), for which little or no information is available related to radiation response, were further examined. Laminin2 α2 expression in livers at 3 months after irradiation was evaluated using immunohistochemical staining (Fig. 2). The protein was detected mainly in bile duct epithelial cells (Fig. 2A and D); however, strong signals were also observed in some areas in bile duct epithelial cells in control samples (Fig. 2C). An attempt to analyze the alteration of Laminin2 α2 by western blot was not successful. This was probably because the antibody used had low affinity to denatured Laminin2 α2 [17]. Although strong signals were detected in some inflammatory lesions at 3 months after chronic irradiation (Fig. 2F), large differences were not detected between control and irradiated samples (Fig. 2A, C, D and E). In contrast, increased caspase 12 expression in irradiated samples compared with control was clearly confirmed by western blotting (Fig. 3). This finding suggests that increases in apoptosis-related proteins can be sustained for long periods after chronic irradiation.
Fig. 2.
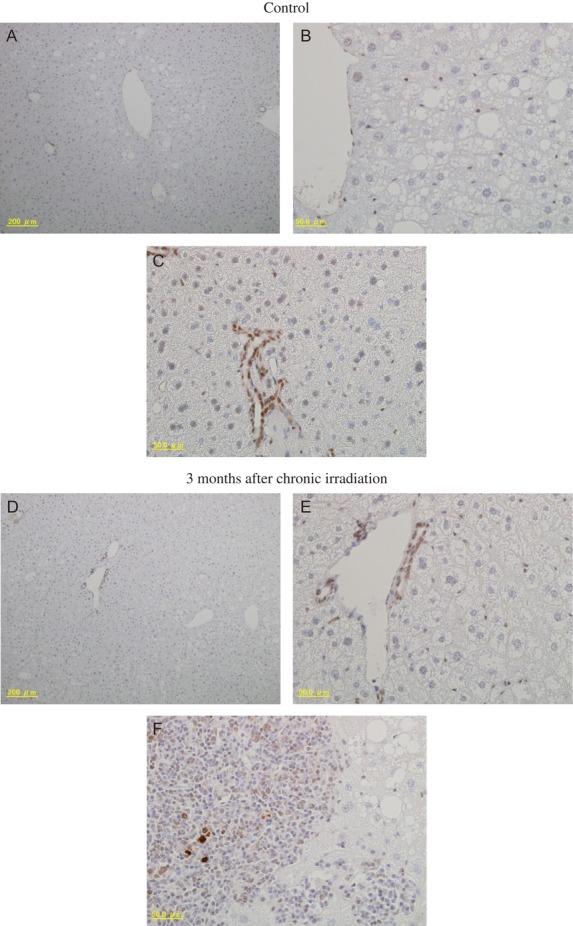
Immunohistochemical staining analyses of Laminin2 α2 in mouse livers 3 months after chronic irradiation. Control (A–C) and 3 months after chronic irradiation (D–F). Panels (A) and (D) are ×100 magnification, and panels (B, C, E) and (F) are ×400.
Figure 3.
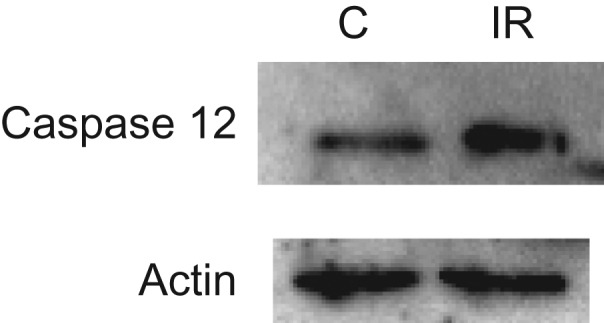
Caspase 12 expression in mouse livers assessed by western blotting at 3 months after chronic irradiation. C = Control, IR = 3 months after irradiation (30 μg/lane). Actin was assayed as a loading control.
DISCUSSION
It is necessary to understand how radiation exposure causes acute effects, late effects, recovery, or death in order to establish strategies for radiation protection. We looked for differences in protein expression between acutely and chronically irradiated mice so as to better understand late alterations in biological processes after irradiation. A total dose of 8 Gy, which induces cancer even with chronic irradiation (20 mGy/day) [13], was used for experiments to compare acute and chronic irradiation. In the case of acute irradiation, a sublethal dose of 4 Gy was also used as a reference to analyze the process. One protein whose expression was increased, and one whose expression was decreased, were common between both 8 Gy and 4 Gy irradiated groups. The protein whose expression was decreased was Bcl-xL. Bcl-xL is a Bcl-x splicing variant and has anti-apoptotic ability [18]. Indeed, Bcl-xL overexpression reduces gamma irradiation–induced apoptosis, and BH4 peptides derived from Bcl-xL have been demonstrated to protect mice from death due to high-dose radiation [19, 20]. Because BH4 forms part of the N-terminal of Bcl-xL, and because the antibody used in the present study recognizes 3–14 amino acids of Bcl-xL, these findings suggest that high and acute irradiation suppresses Bcl-xL expression, indicating the promotion of radiation damage, that is, apoptosis induction. On the other hand, the protein that showed an increase in expression upon radiation, MyD88, is related to innate host defense pathways. It has been reported that MyD88 is induced by high-dose irradiation [21, 22]. MyD88 also functions in the Toll-like receptor (TLR) signaling pathway, which is related to radiation protection [23–25]. Indeed, MyD88 is required for induction of the radioprotective activity of Flagellin, a radioprotector [23]. The levels of Parkin were increased only with acute irradiation with 8 Gy. Parkin has been identified as a protein related to Parkinson's disease and is considered to be a cancer suppressor gene. Parkin functions in the Warburg effect, and its induction after irradiation is considered to play a role in suppressing cancer [26]. This protein is induced by p53 expression, and its increase promotes respiration in mitochondria, while its suppression promotes the Warburg effect and tumorigenesis. In addition, as Parkin has a role inducing mitochondrial autophagy for mitochondrial quality control, autophagy control might be related to radiation-induced Parkin exression [27].
Immunohistochemical staining studies indicated that, after irradiation at 4 Gy, MyD88 was distributed around blood vessels. This finding supports the hypothesis that inflammatory reactions induced by blood cells are sustained in the liver. On the other hand, in the case of irradiation at 4 Gy, the proteins DcR1 and MAP1b were induced at 3 months after irradiation. An increase in MAP1b might promote inflammation via suppression of autophagy [28]. DcR1 is a decoy receptor for the negative regulation of apoptosis via death receptors [29–31]. DcR1 has been reported to be induced by DNA damage [31]. It has also been reported that Bcl-xL knockdown promotes TNF-induced apoptosis [32]. Bcl-xL and receptor signaling via TNF receptor or death receptors in apoptosis seem to be linked. After irradiation with 4 Gy, p34cdc2, which is related to cell cycle regulation, is decreased. Cdc2 is considered to be downstream of cdc25. Though the significance of altering cdc2 expression remains unclarified, its suppression seems to be related to cell cycle arrest [33, 34]. It has been demonstrated that acute, high-dose irradiation upregulates or downregulates cell cycle–related genes, and, in particular, the induction of cyclin-dependent kinase inhibitor 1A (cdkn1A) persists in the livers of mice for 10 weeks after irradiation [11]. The present results support previous findings that cell cycle responses to acute, high-dose irradiation persist long after the irradiation. Several studies have demonstrated that high-dose irradiation induces protein expression in mice for a short time; however, little is known about how protein levels are altered several months after irradiation [6, 11]. The present study demonstrated that the effects of acute high-dose irradiation persist in mice for >3 months, even though their phenotypic appearance is normal.
In addition, we found that the alterations in protein expression induced after chronic irradiation are different from the alterations induced by acute irradiation. The level of one protein, Laminin2 α2, was found to be increased by the antibody array in both analyses immediately after, and at 3 months after, chronic irradiation. The function of this protein remains unclear, but it has been shown that it can be used as a serum biomarker for liver fibrosis [35]. Indeed, immunohistochemical analysis suggests that Laminin2 α2 is distributed in the damaged parts of blood vessels. In addition, Laminin α2 has been reported to be related to tumorigenesis [36]. Laminin α2 facilitates tumor growth as well as increased DNA repair after radiation. Each laminin is a glycoprotein heterotrimer that is located mainly in basement membranes, and the α2 chain is one of the common subunits [37]. As the antibody used in the microarray analysis recognizes the N-terminal portion of the α2 chain of laminin, this protein might also be related to radiation-induced carcinogenesis. The participation of Laminin2 α2 in a sustained radiation response needs to be confirmed by further studies. The proteins whose levels were decreased by radiation (Bim, BID, Bmf and DRAK1) were all decreased at both time points after chronic irradiation, and all participate in apoptosis regulation. Bim, BID, Bmf and DRAK1 promote apoptosis [38–40], while Bim and BID participate in the mitochondrial regulation of apoptosis. Puma was decreased immediately after chronic irradiation, and a decrease in Apaf1 was detected only at 3 months after chronic radiation; these proteins are also related to apoptosis [41, 42]. Apoptosis suppression can allow damaged cells to persist, and suppression of both Bim and PUMA is considered to be related to carcinogenesis [42]. On the other hand, an increase in the level of caspase 12 was detected at 3 months after chronic irradiation. Caspase 12, an ER-stress signaling protein, may be related to apoptosis and inflammatory responses [43–45], and is also involved in ROS signaling [46]. In addition, caspase 12 has been reported to be induced by acute irradiation [47]. The induction of caspase 12 at a late time point after chronic irradiation might be the key to understanding radiation-induced carcinogenesis. Chronic irradiation of 20 mGy/day is sufficient to increase the incidence of neoplasms. The effects on the liver induced by this irradiation are considered to be related to carcinogenesis. Indeed, the incidence rate for hepatocellular adenomas has been shown to increase after chronic irradiation [13].
Here, we analyzed proteins that were increased or decreased >2-fold, as representative altered proteins. In fact, proteins changed <2-fold might be also important. Further studies would be interesting using the present antibody array data (see Supplementary Tables 1 and 2).
The proteins whose levels were altered after chronic irradiation largely mediate apoptosis signaling and are very different from the proteins that were induced by acute irradiation. In acute irradiation, proteins involved in defense pathways and inflammatory reactions were induced. In particular, it is very interesting that apoptosis signaling in the mitochondria and microsomes was involved in the altered signaling pathways after chronic irradiation as these apoptosis pathways seem to be related to each other [48]. Understanding how these signaling pathways are affected after chronic irradiation would contribute to evaluation of radiation damage, protection against radiation risk, and medical treatment for radiation exposure.
SUPPLEMENTARY DATA
Supplementary data are available at Journal of Radiation Research online.
FUNDING
This work was supported in part by the Budget for Nuclear Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan based on screening and counseling by the Atomic Energy Commission and under contract with the Aomori Prefectural Government, Japan.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Ms Kyoko Sakuma, Ms Hiromi Arai and Ms Yasuko Morimoto for their expert technical assistance and administrative support.
REFERENCES
- 1. United Nations Scientific Committee on the Effects of Atomic Radiation Influence of dose and dose rate on stochastic effects of radiation In: Sources and Effects of Ionizing Radiation. New York: UNSCEAR; United Nations, 1993. [Google Scholar]
- 2. Kovalchuk O, Ponton A, Filkowski J, et al. Dissimilar genome response to acute and chronic low-dose radiation in male and female mice. Mutat Res 2004;550:59–72. [DOI] [PubMed] [Google Scholar]
- 3. Sugihara T, Magae J, Wadhwa R, et al. Dose and dose-rate effects of low-dose ionizing radiation on activation of Trp53 in immortalized murine cells. Radiat Res 2004;162:296–307. [DOI] [PubMed] [Google Scholar]
- 4. Tomita M, Morohoshi F, Matsumoto Y, et al. Role of DNA double-strand break repair genes in cell proliferation under low dose-rate irradiation conditions. J Radiat Res 2008;49:557–64. [DOI] [PubMed] [Google Scholar]
- 5. Cao L, Kawai H, Sasatani M, et al. A novel ATM/TP53/p21-mediated checkpoint only activated by chronic γ-irradiation. PLoS One 2014;9:e104279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marchetti F, Coleman MA, Jones IM, et al. Candidate protein biodosimeters of human exposure to ionizing radiation. Int J Radiat Biol 2006;82:605–39. [DOI] [PubMed] [Google Scholar]
- 7. Tapio S, Hornhardt S, Gomolka M, et al. Use of proteomics in radiological research: current state of the art. Radiat Environ Biophys 2010;49:1–4. [DOI] [PubMed] [Google Scholar]
- 8. Leszczynski D. Radiation proteomics: a brief overview. Proteomics 2014;14:481–8. [DOI] [PubMed] [Google Scholar]
- 9. Bakshi MV, Azimzadeh O, Barjaktarovic Z, et al. Total body exposure to low-dose ionizing radiation induces long-term alterations to the liver proteome of neonatally exposed mice. J Proteome Res 2015;14:366–73. [DOI] [PubMed] [Google Scholar]
- 10. Nakajima T, Taki K, Wang B, et al. Induction of rhodanese, a detoxification enzyme, in livers from mice after long-term irradiation with low-dose-rate gamma-rays. J Radiat Res 2008;49:661–6. [DOI] [PubMed] [Google Scholar]
- 11. Pawlik A, Delmar P, Bosse S, et al. Changes in transcriptome after in vivo exposure to ionising radiation reveal a highly specialised liver response. Int J Radiat Biol 2009;85:656–71. [DOI] [PubMed] [Google Scholar]
- 12. Tanaka S, Tanaka IB III, Sasagawa S, et al. No lengthening of life span in mice continuously exposed to gamma rays at very low dose rates. Radiat Res 2003;160:376–9. [DOI] [PubMed] [Google Scholar]
- 13. Tanaka IB III, Tanaka S, Ichinohe K, et al. Cause of death and neoplasia in mice continuously exposed to very low dose rates of gamma rays. Radiat Res 2007;167:417–37. [DOI] [PubMed] [Google Scholar]
- 14. Uehara Y, Ito Y, Taki K, et al. Gene expression profiles in mouse liver after long-term low-dose-rate irradiation with gamma rays. Radiat Res 2010;174:611–7. [DOI] [PubMed] [Google Scholar]
- 15. Taki K, Wang B, Nakajima T, et al. Microarray analysis of differentially expressed genes in the kidneys and testes of mice after long-term irradiation with low-dose-rate gamma-rays. J Radiat Res 2009;50:241–52. [DOI] [PubMed] [Google Scholar]
- 16. You RI, Chang YC, Chen PM, et al. Apoptosis of dendritic cells induced by decoy receptor 3 (DcR3). Blood 2008;111:1480–8. [DOI] [PubMed] [Google Scholar]
- 17. Schuler F, Sorokin LM. Expression of laminin isoforms in mouse myogenic cells in vitro and in vivo. J Cell Sci 1995;108:3795–805. [DOI] [PubMed] [Google Scholar]
- 18. Huang DCS, Adams JM, Cory S.. The conserved N-terminal BH4 domain of Bcl-2 homologues is essential for inhibition of apoptosis and interaction with CED-4. EMBO J 1998;17:1029–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Z-B, Zhang Y, Liu Y-Q, et al. Bcl-xL overexpression restricts γ-radiation-induced apoptosis. Cell Biol Int 2006;30:15–20. [DOI] [PubMed] [Google Scholar]
- 20. McDunn JE, Muenzer JT, Dunne B, et al. An anti-apoptotic peptide improves survival in lethal total body irradiation. Biochem Biophys Res Commun 2009;382:657–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madhusoodhanan R, Natarajan M, Veeraraghavan J, et al. NFκB signaling related molecular alterations in human neuroblastoma cells after fractionated irradiation. J Radiat Res 2009;50:311–24. [DOI] [PubMed] [Google Scholar]
- 22. Sreekumar A, Nyati MK, Varambally S, et al. Profiling of cancer cells using protein microarrays: discovery of novel radiation-regulated proteins. Cancer Res 2001;61:7585–93. [PubMed] [Google Scholar]
- 23. Vijay-Kumar M, Aitken JD, Sanders CJ, et al. Flagellin treatment protects against chemicals, bacteria, viruses, and radiation. J Immunol 2008;180:8280–5. [DOI] [PubMed] [Google Scholar]
- 24. Saha S, Bhanja P, Liu L, et al. TLR9 agonist protects mice from radiation-induced gastrointestinal syndrome. PLoS One 2012;7:e29357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gao F, Zhang C, Zhou C, et al. A critical role of toll-like receptor 2 (TLR2) and its’ in vivo ligands in radio-resistance. Sci Rep 2015;5:13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang C, Lin M, Wu R, et al. Parkin, a p53 target gene, mediates the role of p53 in glucose metabolism and the Warburg effect. Proc Natl Acad Sci USA 2011;108:16259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Narendra D, Tanaka A, Suen DF, et al. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 2008;183:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Poulose SM, Bielinski DF, Carrihill-Knoll K, et al. Exposure to 16O-particle radiation causes aging-like decrements in rats through increased oxidative stress, inflammation and loss of autophagy. Radiat Res 2011;176:761–9. [DOI] [PubMed] [Google Scholar]
- 29. Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 2001;104:487–501. [DOI] [PubMed] [Google Scholar]
- 30. Ashkenazi A, Dixit VM. Apoptosis control by death and decoy receptors. Curr Opin Cell Biol 1999;11:255–60. [DOI] [PubMed] [Google Scholar]
- 31. Sheikh MS, Huang Y, Fernandez-Salas EA, et al. The antiapoptotic decoy receptor TRID/TRAIL-R3 is a p53-regulated DNA damage–inducible gene that is overexpressed in primary tumors of the gastrointestinal tract. Oncogene 1999;18:4153–9. [DOI] [PubMed] [Google Scholar]
- 32. Bai J, Sui J, Demirjian A, et al. Predominant Bcl-xL knockdown disables antiapoptotic mechanisms: tumor necrosis factor–related apoptosis-inducing ligand-based triple chemotherapy overcomes chemoresistance in pancreatic cancer cells in vitro. Cancer Res 2005;65:2344–52. [DOI] [PubMed] [Google Scholar]
- 33. Xiao Z, Chen Z, Gunasekera AH, et al. Chk1 mediates S and G2 arrests through Cdc25A degradation in response to DNA-damaging agents. J Biol Chem 2003;278:21767–73. [DOI] [PubMed] [Google Scholar]
- 34. Kim MJ, Lee JY, Lee SJ. Transient suppression of nuclear Cdc2 activity in response to ionizing radiation. Oncol Rep 2008;19:1323–9. [PubMed] [Google Scholar]
- 35. Parsian H, Rahimipour A, Nouri M, et al. Assessment of liver fibrosis development in chronic hepatitis B patients by serum hyaluronic acid and laminin levels. Acta Clin Croat 2010;49:257–65. [PubMed] [Google Scholar]
- 36. Lathia JD, Li M, Hall PE, et al. Laminin alpha 2 enables glioblastoma stem cell growth. Ann Neurol 2012;72:766–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol 2004;20:255–84. [DOI] [PubMed] [Google Scholar]
- 38. Ren D, Tu HC, Kim H, et al. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science 2010;330:1390–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Labi V, Erlacher M, Kiessling S, et al. Loss of the BH3-only protein Bmf impairs B cell homeostasis and accelerates γ irradiation–induced thymic lymphoma development. J Exp Med 2008;205:641–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bandres E, Catalan V, Sola I, et al. Dysregulation of apoptosis is a major mechanism in the lymph node involvement in colorectal carcinoma. Oncol Rep 2004;12:287–92. [PubMed] [Google Scholar]
- 41. Paik SS, Jang KS, Song YS, et al. Reduced expression of Apaf-1 in colorectal adenocarcinoma correlates with tumor progression and aggressive phenotype. Ann Surg Oncol 2007;14:3453–9. [DOI] [PubMed] [Google Scholar]
- 42. Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene 2008;27Suppl 1:S71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yoneda T, Imaizumi K, Oono K, et al. Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2–dependent mechanism in response to the ER stress. J Biol Chem 2001;276:13935–40. [DOI] [PubMed] [Google Scholar]
- 44. Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science 1998;281:1305–8. [DOI] [PubMed] [Google Scholar]
- 45. Saleh M, Mathison JC, Wolinski MK, et al. Enhanced bacterial clearance and sepsis resistance in caspase-12–deficient mice. Nature 2006;440:1064–8. [DOI] [PubMed] [Google Scholar]
- 46. Brezniceanu ML, Lau CJ, Godin N, et al. Reactive oxygen species promote caspase-12 expression and tubular apoptosis in diabetic nephropathy. J Am Soc Nephrol 2010;21:943–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Claro S, Oshiro ME, Freymuller E, et al. γ-Radiation induces apoptosis via sarcoplasmatic reticulum in guinea pig ileum smooth muscle cells. Eur J Pharmacol 2008;590:20–8. [DOI] [PubMed] [Google Scholar]
- 48. Breckenridge DG, Germain M, Mathai JP, et al. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene 2003;22:8608–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


