Abstract
CD2 is a T cell surface glycoprotein that mediates cellular adhesion and can participate in signal transduction. It is expressed early in thymocyte ontogeny and consequently has been proposed to participate in T cell development. To study the in vivo function of CD2, the murine gene was inactivated using the technique of homologous recombination in embryonic stem cells. Homozygous mutant mice are healthy and have an apparently normal complement of lymphocytes. They mount effective immune responses similar to those of wild type controls. In particular, the generation and function of cytotoxic T cells was found to be normal as was the production of antibodies following immunization. Selection of thymocytes expressing either MHC class I- or class II-restricted transgenic T cell receptors was also grossly normal in the absence of CD2. Thus, CD2 may be dispensable for the development and function of T cells. Within the context of other targetted mutations, these mice should be useful in defining the precise roles of various cell surface molecules involved in T cell responses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard A., Gelin C., Raynal B., Pham D., Gosse C., Boumsell L. Phenomenon of human T cells rosetting with sheep erythrocytes analyzed with monoclonal antibodies. "Modulation" of a partially hidden epitope determining the conditions of interaction between T cells and erythrocytes. J Exp Med. 1982 May 1;155(5):1317–1333. doi: 10.1084/jem.155.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyers A. D., Spruyt L. L., Williams A. F. Molecular associations between the T-lymphocyte antigen receptor complex and the surface antigens CD2, CD4, or CD8 and CD5. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2945–2949. doi: 10.1073/pnas.89.7.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. H., Cantrell D. A., Brattsand G., Crumpton M. J., Gullberg M. The CD2 antigen associates with the T-cell antigen receptor CD3 antigen complex on the surface of human T lymphocytes. Nature. 1989 Jun 15;339(6225):551–553. doi: 10.1038/339551a0. [DOI] [PubMed] [Google Scholar]
- Burgers P. M., Percival K. J. Transformation of yeast spheroplasts without cell fusion. Anal Biochem. 1987 Jun;163(2):391–397. doi: 10.1016/0003-2697(87)90240-5. [DOI] [PubMed] [Google Scholar]
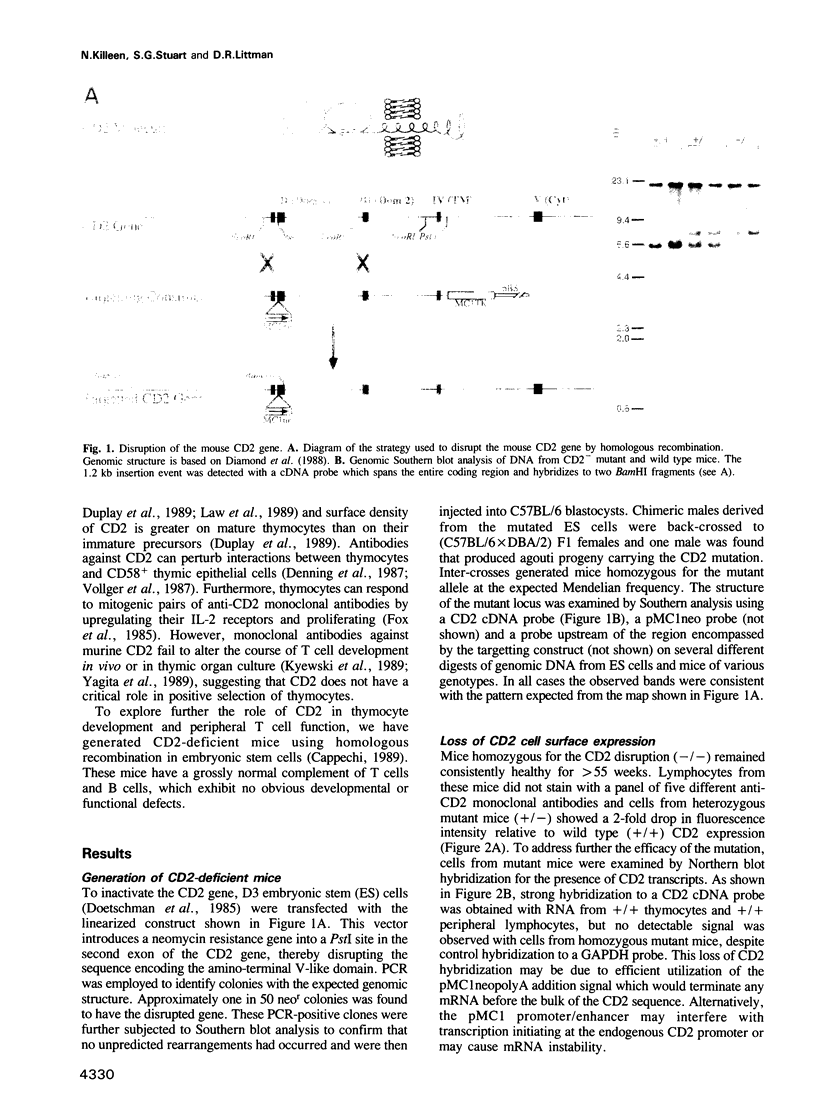
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clark S. J., Law D. A., Paterson D. J., Puklavec M., Williams A. F. Activation of rat T lymphocytes by anti-CD2 monoclonal antibodies. J Exp Med. 1988 Jun 1;167(6):1861–1872. doi: 10.1084/jem.167.6.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J Immunol. 1986 Feb 1;136(3):949–954. [PubMed] [Google Scholar]
- Denning S. M., Tuck D. T., Vollger L. W., Springer T. A., Singer K. H., Haynes B. F. Monoclonal antibodies to CD2 and lymphocyte function-associated antigen 3 inhibit human thymic epithelial cell-dependent mature thymocyte activation. J Immunol. 1987 Oct 15;139(8):2573–2578. [PubMed] [Google Scholar]
- Diamond D. J., Clayton L. K., Sayre P. H., Reinherz E. L. Exon-intron organization and sequence comparison of human and murine T11 (CD2) genes. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1615–1619. doi: 10.1073/pnas.85.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dianzani U., Redoglia V., Malavasi F., Bragardo M., Pileri A., Janeway C. A., Jr, Bottomly K. Isoform-specific associations of CD45 with accessory molecules in human T lymphocytes. Eur J Immunol. 1992 Feb;22(2):365–371. doi: 10.1002/eji.1830220212. [DOI] [PubMed] [Google Scholar]
- Doetschman T. C., Eistetter H., Katz M., Schmidt W., Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985 Jun;87:27–45. [PubMed] [Google Scholar]
- Duplay P., Lancki D., Allison J. P. Distribution and ontogeny of CD2 expression by murine T cells. J Immunol. 1989 May 1;142(9):2998–3005. [PubMed] [Google Scholar]
- Dustin M. L., Sanders M. E., Shaw S., Springer T. A. Purified lymphocyte function-associated antigen 3 binds to CD2 and mediates T lymphocyte adhesion. J Exp Med. 1987 Mar 1;165(3):677–692. doi: 10.1084/jem.165.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Role of lymphocyte adhesion receptors in transient interactions and cell locomotion. Annu Rev Immunol. 1991;9:27–66. doi: 10.1146/annurev.iy.09.040191.000331. [DOI] [PubMed] [Google Scholar]
- Fox D. A., Hussey R. E., Fitzgerald K. A., Bensussan A., Daley J. F., Schlossman S. F., Reinherz E. L. Activation of human thymocytes via the 50KD T11 sheep erythrocyte binding protein induces the expression of interleukin 2 receptors on both T3+ and T3- populations. J Immunol. 1985 Jan;134(1):330–335. [PubMed] [Google Scholar]
- Frankel W. N., Rudy C., Coffin J. M., Huber B. T. Linkage of Mls genes to endogenous mammary tumour viruses of inbred mice. Nature. 1991 Feb 7;349(6309):526–528. doi: 10.1038/349526a0. [DOI] [PubMed] [Google Scholar]
- Gückel B., Berek C., Lutz M., Altevogt P., Schirrmacher V., Kyewski B. A. Anti-CD2 antibodies induce T cell unresponsiveness in vivo. J Exp Med. 1991 Nov 1;174(5):957–967. doi: 10.1084/jem.174.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn W. C., Menu E., Bothwell A. L., Sims P. J., Bierer B. E. Overlapping but nonidentical binding sites on CD2 for CD58 and a second ligand CD59. Science. 1992 Jun 26;256(5065):1805–1807. doi: 10.1126/science.1377404. [DOI] [PubMed] [Google Scholar]
- Holter W., Fischer G. F., Majdic O., Stockinger H., Knapp W. T cell stimulation via the erythrocyte receptor. Synergism between monoclonal antibodies and phorbol myristate acetate without changes of free cytoplasmic Ca++ levels. J Exp Med. 1986 Mar 1;163(3):654–664. doi: 10.1084/jem.163.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard F. D., Ledbetter J. A., Wong J., Bieber C. P., Stinson E. B., Herzenberg L. A. A human T lymphocyte differentiation marker defined by monoclonal antibodies that block E-rosette formation. J Immunol. 1981 Jun;126(6):2117–2122. [PubMed] [Google Scholar]
- Hünig T. The cell surface molecule recognized by the erythrocyte receptor of T lymphocytes. Identification and partial characterization using a monoclonal antibody. J Exp Med. 1985 Sep 1;162(3):890–901. doi: 10.1084/jem.162.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hünig T., Tiefenthaler G., Meyer zum Büschenfelde K. H., Meuer S. C. Alternative pathway activation of T cells by binding of CD2 to its cell-surface ligand. Nature. 1987 Mar 19;326(6110):298–301. doi: 10.1038/326298a0. [DOI] [PubMed] [Google Scholar]
- Kamoun M., Martin P. J., Hansen J. A., Brown M. A., Siadak A. W., Nowinski R. C. Identification of a human T lymphocyte surface protein associated with the E-rosette receptor. J Exp Med. 1981 Jan 1;153(1):207–212. doi: 10.1084/jem.153.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye J., Hsu M. L., Sauron M. E., Jameson S. C., Gascoigne N. R., Hedrick S. M. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 1989 Oct 26;341(6244):746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- Kisielow P., Blüthmann H., Staerz U. D., Steinmetz M., von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988 Jun 23;333(6175):742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- Kyewski B. A., Jenkinson E. J., Kingston R., Altevogt P., Owen M. J., Owen J. J. The effects of anti-CD2 antibodies on the differentiation of mouse thymocytes. Eur J Immunol. 1989 May;19(5):951–954. doi: 10.1002/eji.1830190526. [DOI] [PubMed] [Google Scholar]
- Law D. A., Spruyt L. L., Paterson D. J., Williams A. F. Subsets of thymopoietic rat thymocytes defined by expression of the CD2 antigen and the MRC OX-22 determinant of the leukocyte-common antigen CD45. Eur J Immunol. 1989 Dec;19(12):2289–2295. doi: 10.1002/eji.1830191217. [DOI] [PubMed] [Google Scholar]
- Ley S. C., Davies A. A., Druker B., Crumpton M. J. The T cell receptor/CD3 complex and CD2 stimulate the tyrosine phosphorylation of indistinguishable patterns of polypeptides in the human T leukemic cell line Jurkat. Eur J Immunol. 1991 Sep;21(9):2203–2209. doi: 10.1002/eji.1830210931. [DOI] [PubMed] [Google Scholar]
- Makni H., Malter J. S., Reed J. C., Nobuhiko S., Lang G., Kioussis D., Trinchieri G., Kamoun M. Reconstitution of an active surface CD2 by DNA transfer in CD2-CD3+ Jurkat cells facilitates CD3-T cell receptor-mediated IL-2 production. J Immunol. 1991 Apr 15;146(8):2522–2529. [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Moingeon P., Chang H. C., Sayre P. H., Clayton L. K., Alcover A., Gardner P., Reinherz E. L. The structural biology of CD2. Immunol Rev. 1989 Oct;111:111–144. doi: 10.1111/j.1600-065x.1989.tb00544.x. [DOI] [PubMed] [Google Scholar]
- Moingeon P., Lucich J. L., McConkey D. J., Letourneur F., Malissen B., Kochan J., Chang H. C., Rodewald H. R., Reinherz E. L. CD3 zeta dependence of the CD2 pathway of activation in T lymphocytes and natural killer cells. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1492–1496. doi: 10.1073/pnas.89.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Takahashi K., Fukazawa T., Koyanagi M., Yokoyama A., Kato H., Yagita H., Okumura K. Relative contribution of CD2 and LFA-1 to murine T and natural killer cell functions. J Immunol. 1990 Dec 1;145(11):3628–3634. [PubMed] [Google Scholar]
- Ohno H., Nakamura T., Yagita H., Okumura K., Taniguchi M., Saito T. Induction of negative signal through CD2 during antigen-specific T cell activation. J Immunol. 1991 Oct 1;147(7):2100–2106. [PubMed] [Google Scholar]
- Ohno H., Ushiyama C., Taniguchi M., Germain R. N., Saito T. CD2 can mediate TCR/CD3-independent T cell activation. J Immunol. 1991 Jun 1;146(11):3742–3746. [PubMed] [Google Scholar]
- Owen M. J., Jenkinson E. J., Brown M. H., Sewell W. A., Krissansen G. W., Crumpton M. J., Owen J. J. Murine CD2 gene expression during fetal thymus ontogeny. Eur J Immunol. 1988 Jan;18(1):187–189. doi: 10.1002/eji.1830180129. [DOI] [PubMed] [Google Scholar]
- Rajewsky K. A phenotype or not: targeting genes in the immune system. Science. 1992 Apr 24;256(5056):483–483. doi: 10.1126/science.1570513. [DOI] [PubMed] [Google Scholar]
- Rutschmann R., Karjalainen K. Mouse LFA-3 studied with chimeric soluble CD2 shows preferential expression on lymphoid cells. Eur J Immunol. 1991 Jun;21(6):1379–1384. doi: 10.1002/eji.1830210608. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraven B., Samstag Y., Altevogt P., Meuer S. C. Association of CD2 and CD45 on human T lymphocytes. Nature. 1990 May 3;345(6270):71–74. doi: 10.1038/345071a0. [DOI] [PubMed] [Google Scholar]
- Selvaraj P., Dustin M. L., Mitnacht R., Hünig T., Springer T. A., Plunkett M. L. Rosetting of human T lymphocytes with sheep and human erythrocytes. Comparison of human and sheep ligand binding using purified E receptor. J Immunol. 1987 Oct 15;139(8):2690–2695. [PubMed] [Google Scholar]
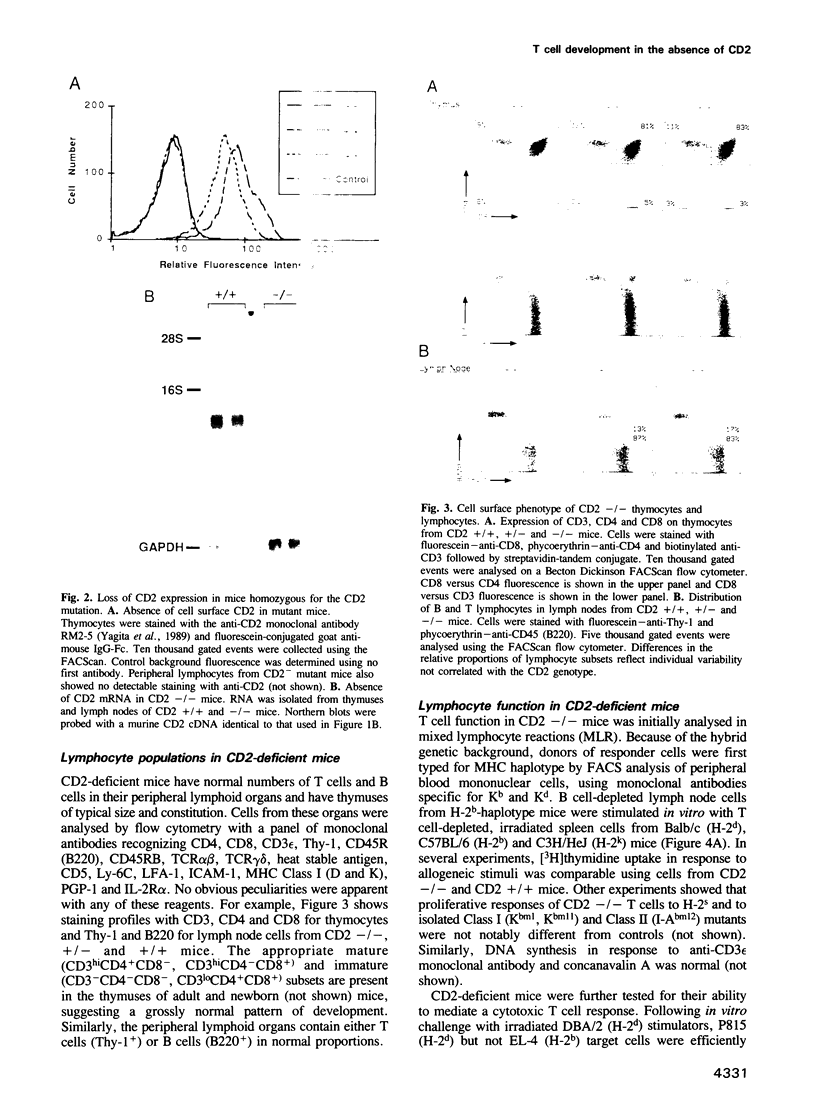
- Selvaraj P., Plunkett M. L., Dustin M., Sanders M. E., Shaw S., Springer T. A. The T lymphocyte glycoprotein CD2 binds the cell surface ligand LFA-3. 1987 Mar 26-Apr 1Nature. 326(6111):400–403. doi: 10.1038/326400a0. [DOI] [PubMed] [Google Scholar]
- Sewell W. A., Brown M. H., Owen M. J., Fink P. J., Kozak C. A., Crumpton M. J. The murine homologue of the T lymphocyte CD2 antigen: molecular cloning, chromosome assignment and cell surface expression. Eur J Immunol. 1987 Jul;17(7):1015–1020. doi: 10.1002/eji.1830170718. [DOI] [PubMed] [Google Scholar]
- Springer T. A., Dustin M. L., Kishimoto T. K., Marlin S. D. The lymphocyte function-associated LFA-1, CD2, and LFA-3 molecules: cell adhesion receptors of the immune system. Annu Rev Immunol. 1987;5:223–252. doi: 10.1146/annurev.iy.05.040187.001255. [DOI] [PubMed] [Google Scholar]
- Spruyt L. L., Glennie M. J., Beyers A. D., Williams A. F. Signal transduction by the CD2 antigen in T cells and natural killer cells: requirement for expression of a functional T cell receptor or binding of antibody Fc to the Fc receptor, Fc gamma RIIIA (CD16). J Exp Med. 1991 Dec 1;174(6):1407–1415. doi: 10.1084/jem.174.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh H. S., Kisielow P., Scott B., Kishi H., Uematsu Y., Blüthmann H., von Boehmer H. Thymic major histocompatibility complex antigens and the alpha beta T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature. 1988 Sep 15;335(6187):229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
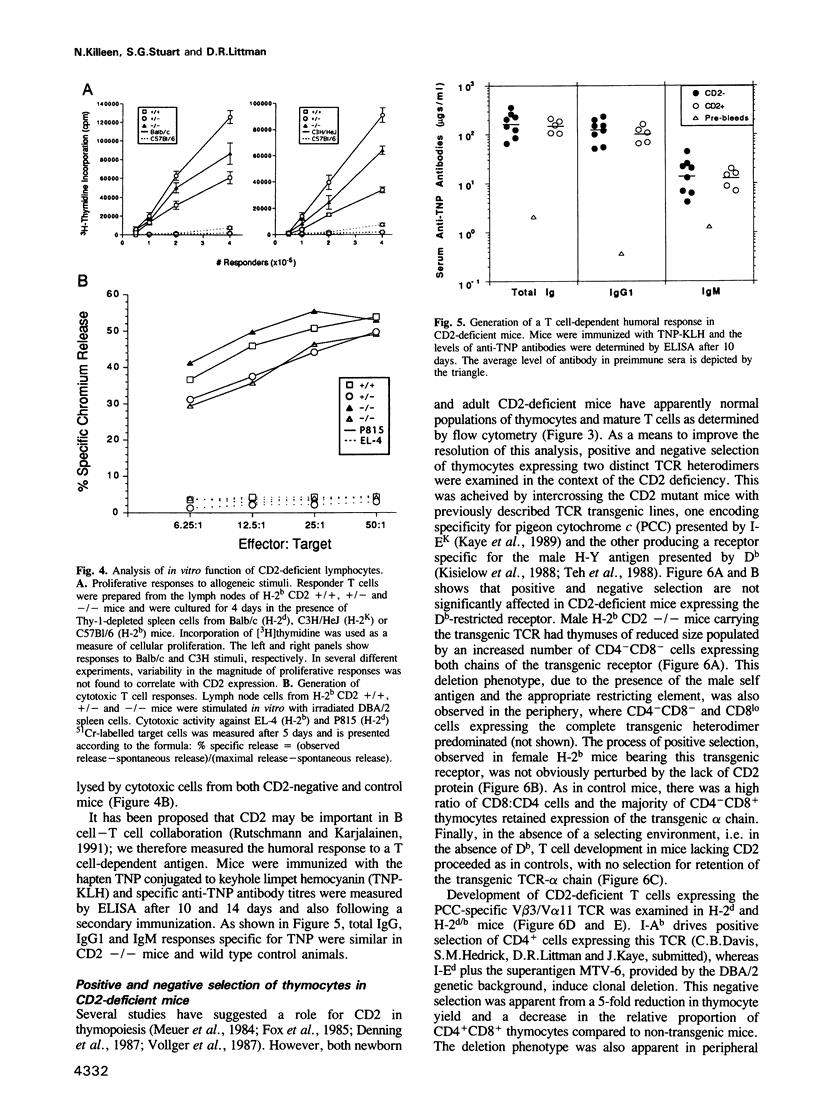
- Van Wauwe J., Goossens J., Decock W., Kung P., Goldstein G. Suppression of human T-cell mitogenesis and E-rosette formation by the monoclonal antibody OKT11A. Immunology. 1981 Dec;44(4):865–871. [PMC free article] [PubMed] [Google Scholar]
- Van de Velde H., von Hoegen I., Luo W., Parnes J. R., Thielemans K. The B-cell surface protein CD72/Lyb-2 is the ligand for CD5. Nature. 1991 Jun 20;351(6328):662–665. doi: 10.1038/351662a0. [DOI] [PubMed] [Google Scholar]
- Vollger L. W., Tuck D. T., Springer T. A., Haynes B. F., Singer K. H. Thymocyte binding to human thymic epithelial cells is inhibited by monoclonal antibodies to CD-2 and LFA-3 antigens. J Immunol. 1987 Jan 15;138(2):358–363. [PubMed] [Google Scholar]
- Wegener A. M., Letourneur F., Hoeveler A., Brocker T., Luton F., Malissen B. The T cell receptor/CD3 complex is composed of at least two autonomous transduction modules. Cell. 1992 Jan 10;68(1):83–95. doi: 10.1016/0092-8674(92)90208-t. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Beyers A. D. T-cell receptors. At grips with interactions. Nature. 1992 Apr 30;356(6372):746–747. doi: 10.1038/356746a0. [DOI] [PubMed] [Google Scholar]
- Yagita H., Asakawa J., Tansyo S., Nakamura T., Habu S., Okumura K. Expression and function of CD2 during murine thymocyte ontogeny. Eur J Immunol. 1989 Dec;19(12):2211–2217. doi: 10.1002/eji.1830191206. [DOI] [PubMed] [Google Scholar]