Abstract
Background
5-HT1B receptor agonists enhance cocaine intake during daily self-administration sessions but decrease cocaine intake when tested after prolonged abstinence. We examined if 5-HT1B receptor agonists produce similar abstinence-dependent effects on methamphetamine intake.
Methods
Male rats were trained to self-administer methamphetamine (0.1 mg/kg, i.v.) on low (fixed ratio 5 and variable ratio 5) and high (progressive ratio) effort schedules of reinforcement until intake was stable. Rats were then tested for the effects of the selective 5-HT1B receptor agonist, CP 94,253 (5.6 or 10 mg/kg), or the less selective but clinically available 5-HT1B/1D receptor agonist, zolmitriptan (10 mg/kg), on methamphetamine self-administration both before and after a 21-day forced abstinence period during which the rats remained in their home cages.
Results
The inverted U-shaped, methamphetamine dose-response function for intake on the fixed ratio 5 schedule was shifted downward by CP 94,253 both before and after abstinence. The CP 94,253-induced decrease in methamphetamine intake was replicated in rats tested on a variable ratio 5 schedule, and the 5-HT1B receptor antagonist SB 224,289 (10 mg/kg) reversed this effect. CP 94,253 also attenuated methamphetamine intake on a progressive ratio schedule both pre- and postabstinence. Similarly, zolmitriptan attenuated methamphetamine intake on a variable ratio 5 schedule both pre- and postabstinence, and the latter effect was sustained after each of 2 more treatments given every 2 to 3 days prior to daily sessions.
Conclusions
Unlike the abstinence-dependent effect of 5-HT1B receptor agonists on cocaine intake reported previously, both CP 94,253 and zolmitriptan decreased methamphetamine intake regardless of abstinence. These findings suggest that 5-HT1B receptor agonists may have clinical efficacy for psychostimulant use disorders.
Keywords: methamphetamine; addiction; CP 94,253; zolmitriptan; rodent
Significance Statement.
Psychostimulant addiction (e.g., cocaine and methamphetamine) remains a prevalent problem in the United States. Prior studies suggest that the serotonin1B receptor (5-HT1BR) modulates cocaine reinforcement and incentive motivation dependent on whether there is a period of abstinence from cocaine in male rats. This study demonstrates that measures of methamphetamine drug taking in rats are attenuated by pretreatment with 5-HT1BR agonists, including the FDA-approved agonist zolmitriptan, regardless of abstinence history. Our findings suggest that 5-HT1BR agonists may be useful for treating psychostimulant use disorders.
Introduction
Psychostimulant addiction remains a prevalent problem worldwide (NDIC, 2011; NIDA, 2015), and yet there are still no FDA-approved, effective pharmacological treatments for psychostimulant use disorders. We and others have suggested that the serotonin1B receptor (5-HT1BR) may be a useful target for medication development for these disorders (Callahan and Cunningham, 1995; Rocha et al., 1997; Miszkiel et al., 2011; Neisewander et al., 2014). Advances in medicinal chemistry have discovered drugs with high selectivity for 5-HT1BRs (Koe et al., 1992; Selkirk et al., 1998; Murray and Rees, 2009; Rodriguez et al., 2014). The agonist 5-propoxy-3-(1,2,3,6-tetrahydro-4-pyridinyl)-1H-pyrrolo[3,2-b] pyridine (CP 94,253) and the antagonist 1’-methyl-5-[[2’-methyl-4’-(5-methyl-1,2,4-oxadiazol-3-yl)biphenyl-4-yl]carbonyl]-2,3,6,7-tetrahydrospiro[furo[2,3-f]indole-3,4’-piperidine (SB 224,289) have high affinities (Ki = 2 and 8.2 nM, respectively) for 5-HT1BRs (Koe et al., 1992; Selkirk et al., 1998). The selectivity profiles of CP 94,253 and SB 224,289 have established these drugs as useful tools for studying the role of 5-HT1BRs in psychostimulant addiction. Another group of 5-HT1BR agonists are tryptamine based, including zolmitriptan (Zomig), which is an FDA-approved drug used to treat migraine headaches. Although zolmitriptan is not as selective for 5-HT1BRs as CP 94,253, it has a high affinity (Ki = 5.01 nM) for 5-HT1BRs.
Initial experiments examining the effects of 5-HT1BR agonists found that these drugs facilitated cocaine self-administration (Parsons et al., 1998). However, we found that the effects of 5-HT1BR agonists vary depending on whether or not animals have undergone abstinence. Specifically, CP 94,253 shifts the cocaine self-administration dose-response curve leftward when given as a pretreatment prior to a daily self-administration session (preabstinence) but produces a downward shift when given as a pretreatment prior to resumption of self-administration after prolonged (i.e., 21 days) abstinence (Pentkowski et al., 2009; Pentkowski et al., 2014). In addition, CP 94,253 pretreatment increases breakpoints and cocaine intake on a progressive ratio (PR) schedule compared with vehicle pretreatment when tested during daily maintenance sessions. In contrast, following a 21-day period of forced abstinence (postabstinence), CP 94,253 decreases cocaine intake and response rates on the PR schedule. Furthermore, CP 94,253, attenuates cocaine-seeking behavior in tests of both cue-induced and cocaine-primed reinstatement following a few weeks of extinction training during which the rats were abstinent (Pentkowski et al., 2009). Cocaine-seeking behavior under these conditions reflects incentive motivational effects produced by the cues and cocaine priming injections (Markou et al., 1993). Therefore, these results suggest that preabstinence administration of 5-HT1BR agonists facilitates the reinforcing and motivational properties of cocaine while postabstinence 5-HT1BR agonists attenuate these effects.
This study examined if CP 94,253 produces a similar abstinence-dependent decrease in methamphetamine intake. First, we examined CP 94,253 pretreatment effects on the methamphetamine self-administration dose-response function using low ratio schedules of reinforcement (i.e., fixed and variable ratio 5; FR5 and VR5). Second, we examined CP 94,253 pretreatment effects on a PR schedule of methamphetamine reinforcement as this more demanding schedule is particularly sensitive to changes in motivation for a drug. Third, we examined if the effects of CP 94,253 pretreatment on methamphetamine intake were 5-HT1BR mediated by administering the 5-HT1BR antagonist SB 224,289 to reverse the agonist effects. Fourth, we examined if CP 94,253 and SB 224,289 affected locomotor activity. Finally, we examined if acute and intermittent repeated treament with zolmitriptan affected methamphetamine intake. Since both methamphetamine and cocaine enhance monoaminergic neurotransmission by an action at monoamine transporters, we hypothesized that CP 94,253 would increase methamphetamine intake preabstinence and decrease methamphetamine intake postabstinence similar to that observed with cocaine intake.
Methods
Animals
Male Sprague Dawley rats (Charles River) weighing 225 to 250 g were single-housed in a climate-controlled environment on a 14:10 reverse light/dark cycle (lights off at 6:00 am). Rats had ad libitum access to food except for initial self-administration training when they were food restricted to 90% of their ad libitum weights. The experiments proceeded in accordance with a protocol approved by the Arizona State University Institutional Animal Care and Use Committee.
Drugs
Methamphetamine hydrochloride (Sigma-Aldrich) was dissolved in bacteriostatic saline (Hospira Inc.) and filtered with 0.2-µm membrane Acrodisc syringe filters (PALL Corporation). CP 94,253 hydrochloride (Tocris Bioscience) was dissolved in saline, and SB 224,289 hydrochloride (Tocris Biosciences) was dissolved in 10% (2-hydroxypropyl)-β-cyclodextrin (Sigma-Aldrich) in saline and sonicated for 2 minutes. Zolmitriptan (Sigma-Aldrich) was dissolved in 10% dimethyl sulfoxide in saline and sonicated for 5 minutes. CP 94,253, SB 224,289, and zolmitriptan were prepared fresh daily. Vehicle refers to the respective solvent. All drug injections, with the exception of self-administered methamphetamine, were injected at a volume of 1 mL/kg body weight.
Surgery
Rats underwent surgery for implantation of chronic indwelling catheters into the jugular vein as detailed previously (Pockros et al., 2011). Rats had 6 to 7 days of recovery before commencing self-administration training. Catheters were flushed daily with 0.1 mL of either timentin (experiment 1; 66.67 mg/mL; GlaxoSmithKline) or cefazolin (experiment 2, 3, 4, and 5; 10 mg/mL; WG Critical Care, LLC) mixed with heparin/saline (70 U/mL; APP Pharmaceuticals). In addition, catheter patency was tested periodically by administering 0.05 mL of methohexital sodium (16.7 mg/mL; Jones Pharma Inc.), a dose that produces brief loss of muscle tone when administered i.v.
Apparatus
The operant conditioning chambers (Med Associates) contained an active and inactive lever, a cue light, and a tone generator as previously described (Pentkowski et al., 2010). All chambers had infusion pumps (Med Associates) that connected to liquid swivels (Instech) fastened to an outlet polyethylene tubing sheltered within a metal leash (PlasticsOne) that attached to the rat’s catheter. All operant conditioning chambers were housed within sound attenuating boxes that contained a ventilation fan.
General Procedures
Experimental sessions commenced at approximately the same time of day, 6 days/week during the rats’ dark cycle. Rats were first trained to self-administer 0.1 mg/kg (i.v.) methamphetamine on an FR1 schedule of reinforcement, and they later progressed to either a FR5, VR5, or PR schedule based on individual performance. In all experiments, schedule advancement during training occurred once rats received at least 10 reinforcers/session for 2 consecutive sessions, and testing commenced once rats reached a stability criterion of <15% variability in the number of infusions obtained across 3 consecutive sessions. The training dose was choosen as it has been shown to be effective in producing methamphetamine acquisition (Kitamura et al., 2006; Clemens et al., 2006; Krasnova et al., 2010). The VR5 schedule was chosen for its tendency to sustain consistent steady response rates (Domjan, 2003). Session length was 2 hours, except for training and testing on the PR schedule for which sessions were 4 hours long to capture break points in most rats. In the PR schedule, the number of active lever responses required to obtain each subsequent infusion increased exponentially (e.g., 1, 2, 4, 6, 9, 12, 15, etc.), identical to the exponential equation from Richardson and Roberts (1996). The PR schedule was chosen because it is progressively more effortful to obtain reinforcement, reflecting in part how motivated a rat is to work for methamphetamine (Markou et al., 1993). Therefore, the pattern of changes across various schedules of reinforcement can help inform how treatments affect behavioral processes. Completion of any of the operant schedules activated the tone and light cue followed 1 second later by an infusion of 0.1 mL methamphetamine delivered across 6 seconds (dose is given in the specific experiments section). The tone, light, and pump were then turned off, and simultaneously a house-light was activated for 20 seconds to signal a timeout period during which additional lever responses were recorded but had no programmed consequences. After the timeout period, the house-light turned off and methamphetamine was available again. Abstinence from methamphetamine occurred for 21 or more consecutive days, during which the rats were maintained in the home cage, handled, and weighed daily for i.v. administration of timentin or cefazolin to maintain catheter patency.
Experiment 1: Effects of CP 94,253 on the Methamphetamine Self-Administration Dose-Response Function Pre- and Postabstinence
The timeline for this experiment is outlined in Figure 1. Beginning 2 days prior to the start of training and continuing throughout the experiment, rats were food restricted to 90% of their initial free-feeding body weight (291 ± 4.6 g). During training, rats progressed from a FR1 to FR5 schedule (~17–20 sessions) after reaching a criterion of ≥10 infusions for 2 consecutive sessions. Once reinforcement rates met the stability criterion on the FR5, the rats commenced training on a within-session dose-response procedure. For these sessions, each methamphetamine dose (0.003, 0.01, 0.03, 0.1, and 0.30 mg/kg, i.v.) was available in ascending order for 30 minutes with a 5-minute time-out period between doses. After again meeting the stability criterion for reinforcement rates across the within-session dose-response training days (20–28 sessions), rats were randomly assigned to receive an injection of either CP 94,253 (5.6 mg/kg, s.c.) or vehicle 15 minutes prior to their daily dose-response session. The dose of CP 94,253 was selected based on our previous research demonstrating that it selectively reduces cocaine intake without affecting sucrose intake (Pentkowski et al., 2009). After this first test session, additional training sessions were given until rats met the stability criterion. Then rats were tested again, receiving the treatment opposite from their first treatment (i.e., animals that received vehicle first, now received CP 94,253 and vice versa). After this test session, rats underwent abstinence for 21 days, during which they remained in their home cages but continued to receive daily i.v. administration of heparin/antibiotic to maintain catheter patency.
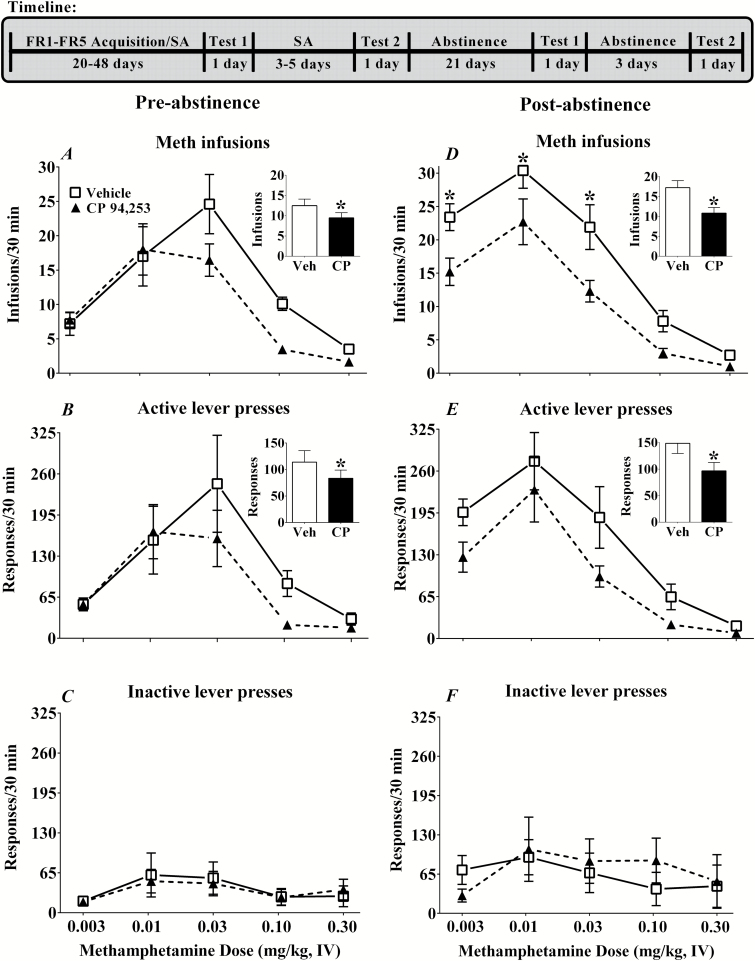
Figure 1.
Effects of the 5-HT1B receptor agonist, CP 94,253 on infusions (A,D), active lever (B,E), and inactive lever (C,F) responses on a FR5 schedule of methamphetamine (0.1 mg/kg, i.v.) reinforcement during pre- and postabstinence tests (left and right panels, respectively). Data are expressed as the mean (± SEM) during the 30-minute test period for each of the methamphetamine doses tested (0.003–0.30 mg/kg, i.v.). Rats (n = 10) were tested twice, receiving pretreatment with vehicle (1 mL/kg, s.c.; open squares) prior to one test and CP 94,253 (5.6 mg/kg, s.c.; filled triangles) prior to the other test, with order of pretreatment counterbalanced. Insets in A-E show a main effect of CP 94,253 averaged across methamphetamine doses. Asterisks (*) represent a difference from vehicle condition (main effect or Tukey’s posthoc test, P<.05).
For the postabstinence tests, rats received CP 94,253 or vehicle, and 15 minutes later, they were given access to methamphetamine using the same within session dose-response procedure as used during preabstinence tests. Rats then remained abstinent in the home cage for 3 days to allow time for CP 94,253 to be eliminated and to reinstate an abstinence period prior to the second test. On the second test day, the rats received the treatment opposite from that given prior to the first postabstinence test. A total of 10 rats ran through the entire experiment and their average final weight was 468 ± 4.94 g.
Experiment 2: Effects of CP 94,253 on Methamphetamine Self-Administration on a Progressive Ratio Schedule Pre- and Postabstinence
The timeline for this experiment is outlined in Figure 2. A new cohort of experimentally naïve rats was food restricted and trained to self-administer methamphetamine, progressing from a FR1 to a VR5 schedule during 2-hour sessions (11–13 sessions) using the same procedure as the previous experiment, with the exception that food restriction was gradually discontinued once rats were on the VR5 schedule. After meeting the stability criterion on the VR5 schedule, rats were trained on the PR schedule of methamphetamine reinforcement during 4-hour sessions until again meeting the stability criterion (10–18 sessions). We capped session length to 4 hours to ensure that CP 94,253 would remain effective throughout the test (Parsons et al., 1998). We also reduced the methamphetamine dose to one-half the training dose (0.05 mg/kg, i.v.) with the intention that more rats would reach break point during the 4-hour session. Breakpoint was defined as the last schedule of reinforcement completed prior to a 1-hour period during which the next required ratio failed to be completed, or 4 hours had elapsed, whichever came first. After rats met the stability criterion on the PR schedule, they were randomly assigned to either a CP 94,253 (10 mg/kg, s.c.; n = 8) or a vehicle group (n = 7), counterbalanced for similar number of total drug infusions during training. The dose of CP 94,253 was higher than the previous experiment to sustain CP 94,253 levels throughout testing, and based on previous research this dose selectively decreases cocaine intake while not affecting sucrose intake (Pentkowski et al., 2009). These groups received their respective treatments 15 minutes before testing on the PR schedule. After testing, both groups of rats were placed into abstinence for 21 days as described in experiment 1. Postabstinence, both groups received their injections, which were identical to those given preabstinence, 15 minutes prior to a test for resumption of methamphetamine self-administration on the PR schedule.
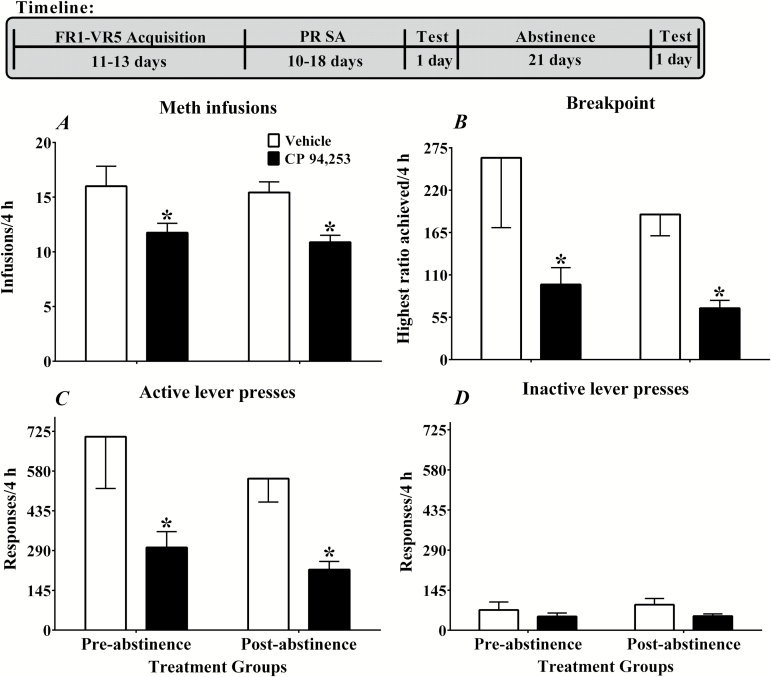
Figure 2.
Effects of the 5-HT1B receptor agonist, CP 94,253, on infusions (A), breakpoints (B), active lever (C), and inactive lever (D) responses under a progressive ratio schedule of methamphetamine (0.05 mg/kg, i.v.) reinforcement during pre- and postabstinence tests. Data are expressed as the mean (± SEM) during 4-hour sessions. Rats were pretreated 15 minutes prior to the start of the test sessions with either vehicle (1 mL/kg, s.c.; white bars; n = 7) or CP 94,253 (10 mg/kg, s.c.; black bars; n = 8). Asterisks (*) represent a difference from vehicle at each time point (Tukey’s posthoc test, P<.05).
Experiment 3: Reversing the Attenuating Effects of CP 94,253 on Methamphetamine Self-Administration with SB 224,289
The timeline for this experiment is outlined in Figure 3. A new cohort of experimentally naïve rats was trained to self-administer methamphetamine, progressing from a FR1 to a VR5 schedule (~12–17 sessions) of methamphetamine (0.1 mg/kg, i.v.) reinforcement. Rats were food restricted only during acquisition of self-administration and all sessions lasted for 2 hours. Once reinforcement rates stabilized under free feeding conditions (~4–12 sessions), rats were randomly assigned to 1 of 2 groups (n = 14 and 17, respectively); Group 1 received an i.p. injection of either vehicle or SB 224,289 (10 mg/kg, i.p.) 30 minutes prior to the first test and the opposite treatment injection 30 minutes prior to the second test (i.e., rats that received vehicle on test 1 received SB 224,289 on test 2 and vice versa). Group 1 also received a vehicle injection 15 minutes prior to both tests. For Group 2, identical procedures were followed except that rats received vehicle followed by CP 94,253 (5.6 mg/kg, s.c.) on one test day and SB 224,289 (10 mg/kg, i.p.) followed by CP 94,253 (5.6 mg/kg, s.c.) on the other test day.
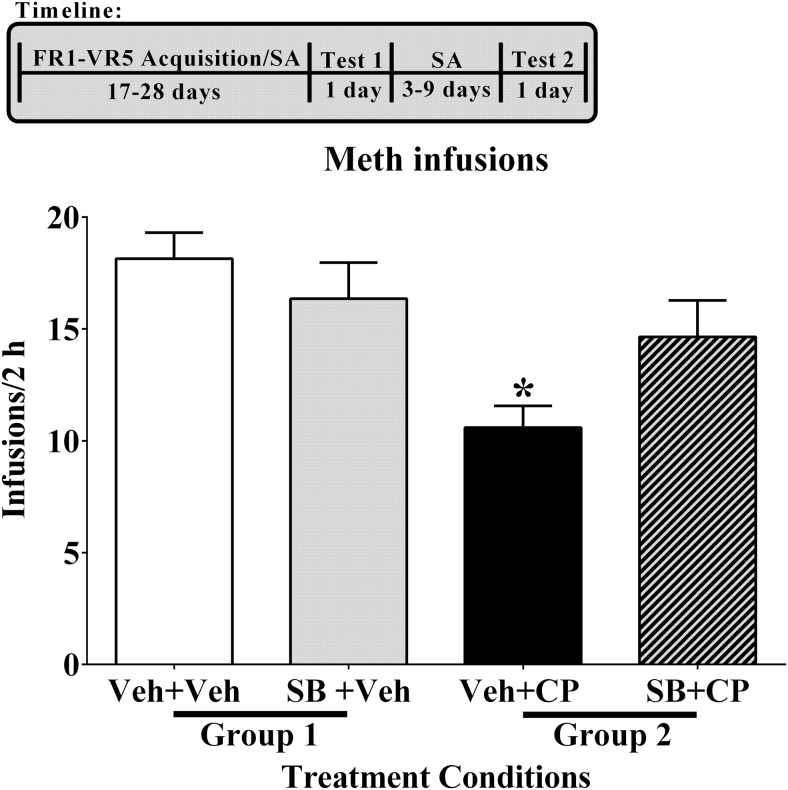
Figure 3.
Reversing the attenuating effects of CP 94,253 on methamphetamine (0.1 mg/kg, i.v.) self-administration with SB 224,289 during tests that occurred preabstinence. On the first test day, rats (group 1: n=14, group 2: n=17) received a pretreatment of either vehicle (Veh, white bar) or SB 224,289 (SB; 10 mg/kg, i.p., gray bar) 30 minutes before the 2-hour session. They then received a treatment of either vehicle (Veh) or CP 94,253 (CP; 5.6 mg/kg, s.c.; black bar) 15 minutes prior to the test session that commenced under a VR5 schedule of reinforcement. Conditions were identical on the second test day for all rats except that the pretreatment given 30 minutes before session start was reversed such that rats that had received vehicle previously were given SB 224,289 and rats that had received SB 224,289 previously were given vehicle. Data are expressed as the mean (+ SEM). Asterisk (*) represents a difference from all other groups (Tukey’s posthoc test, P<.05).
In addition to testing whether the antagonist would reverse the effects of the agonist in this experiment, we also verified that these rats showed a CP 94,253-induced decrease in methamphetamine intake postabstinence (not included on timeline). A subset of rats, randomly selected from both Group 1 and Group 2, underwent 21 days of abstinence as described above. They were then assigned to 2 groups, counterbalanced for the number of infusions obtained during training. One group (n=11) received an injection of CP 94,253 (5.6 mg/kg, s.c.) while the other group (n=11) received an injection of vehicle 15 minutes prior to a test for resumption of methamphetamine self-administration (0.1 mg/kg, i.v.).
Experiment 4: Effects of 5-HT1BR Drugs on Spontaneous Locomotion
The timeline for this experiment is outlined in Figure 4. Rats from experiment 2 were used and they had a history of methamphetamine self-administration (38 sessions) and had undergone an abstinence period (23 days). On abstinence day 24, rats were placed into locomotor test chambers for a 60-minute habituation period. The test chambers (45.72 x 25.4 x 20.32 cm) were similar to the home cages and had a camera mounted above to record horizontal movement with Topscan software (Clever Systems). The rats were then tested twice for locomotor activity with 3 rest days intervening the 2 test days. They remained in their home cages during rest days. Thirty minutes prior to the first test, rats were pretreated with either vehicle or SB 224,289 (10 mg/kg, i.p.) and 30 minutes prior to the second test the rats received the opposite treatment as that given on the first test (i.e., rats that received vehicle on test 1 received SB 224,289 on test 2 and vice versa). They were also randomly assigned to 1 of 2 groups. Group 1 (n=7) received a vehicle injection 15 minutes prior to both tests, and Group 2 (n=7) received CP 94,253 (10 mg/kg, s.c.) 15 minutes prior to both tests. The tests began by placing the rat into the test chamber, and distance traveled was measured for 2 hours.
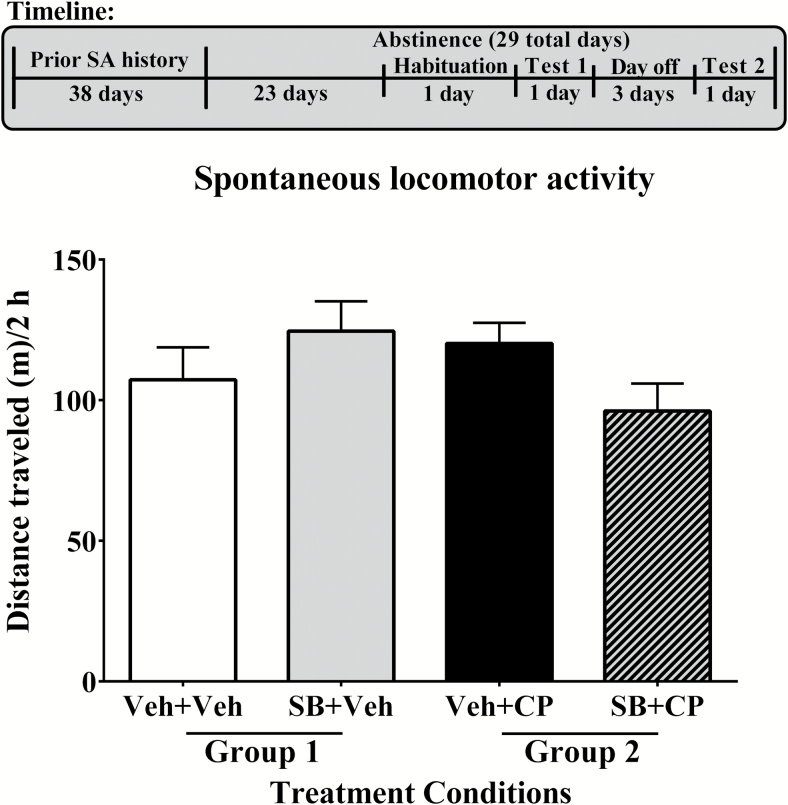
Figure 4.
Effects of 5-HT1B receptor drugs on spontaneous locomotion. Rats were placed on abstinence from methamphetamine for a total of 29 days following acquisition and stabilization on a progressive ratio schedule of reinforcement. Habituation to the locomotor testing chambers occurred on abstinence day 24. On the first test day, rats (group 1: n=7, group 2: n=7) received a pretreatment of either vehicle (Veh, white bar) or SB 224,289 (SB; 10 mg/kg, i.p., gray bar) 30 minutes before commencement of the session. They then received a treatment of either vehicle (Veh) or CP 94,253 (CP; 10 mg/kg, s.c., black bar) 15 minutes prior to the same session. Conditions were identical on the second test day for all rats except that the pretreatment given 30 minutes before session start was reversed such that rats who had received vehicle previously were given SB 224,289 and vice versa. All rats underwent 3 days off between tests 1 and 2 while remaining in abstinence. Data are expressed as the mean (+SEM) distance traveled in meters across the 2-hour session. Pretreatment with 5-HT1B receptor drugs produced no significant differences in spontaneous locomotion.
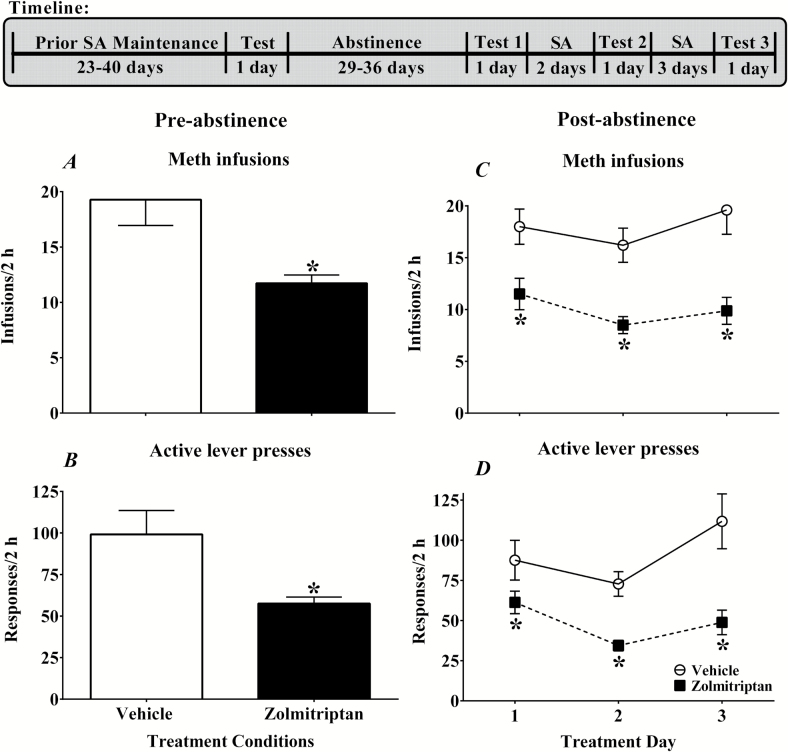
Experiment 5: Effects of Zolmitriptan on Methamphetamine Self-Administration Pre- and Postabstinence
The timeline for this experiment is outlined in Figure 5. Rats that were used in experiment 3 were tested for the effects of zolmitriptan on methamphetamine after achieving stable SA rates on a VR5 schedule (~3–11 sessions) of methamphetamine reinforcement (0.1 mg/kg, i.v.) across 2-hour training sessions. Rats were then randomly assigned to either a zolmitriptan (10 mg/kg, s.c.; n=9) or a vehicle treatment group (n=6), counterbalanced for similar number of total drug infusions. Rats received their assigned treatment 15 minutes prior to the start of a self-administration session. Then rats underwent a period of abstinence for 29 to 36 days followed by a test phase. During the test phase, the rats again received their assigned treatment of either zolmitriptan or vehicle 15 minutes prior to each of 3 self-administration sessions with 2 to 3 self-administration sessions without treatment between each of the treatment test days.
Figure 5.
Effects of zolmitriptan, a 5-HT1B/1D receptor agonist, on infusions (A,C), and active lever (B,C) responses on a VR5 schedule of methamphetamine (0.1 mg/kg, i.v.) reinforcement during pre- and postabstinence tests (left and right panels, respectively). Data are expressed as the mean (±SEM). Rats were pretreated 15 minutes prior to the start of the 2-hour sessions with either vehicle (white bar/open circles; n = 6) or zolmitriptan (10 mg/kg, s.c.; black bar/filled squares; n = 9). Asterisks (*) represent a difference from vehicle condition (t test or ANOVA main effect, P<.05).
Data Analysis
Statistical analyses were conducted with IBM SPSS Statistics v. 23. Descriptive statistics are reported as the mean ± SEM. Self-administration data, including active and inactive lever responses, and infusions obtained were analyzed by either repeated-measures or a mixed-design ANOVA with drug pretreatment(s) and dose of methamphetamine as between-subject or within-subject factors depending on the experimental design. In addition, breakpoint and total distance travelled were analyzed as described above for experiments 2 and 4, respectively. All sources of significant effects were further analyzed by Tukey’s posthoc tests. There was some attrition in each experiment due to catheter failure or failure to acquire SA.
Results
Experiment 1
Methamphetamine produced an inverted U-shaped dose-effect function, and CP 94,253 decreased methamphetamine infusions and active lever responses both pre- and postabstinence (Figure 1). For the preabstinence tests, there were main effects of methamphetamine dose for both infusions [F (4, 36) = 17.67, P < .05] and active lever responses [F (4, 36) = 8.40, P < .05]. Posthoc tests indicated that the 0.01- and 0.03-mg/kg doses produced higher values than the lowest dose (0.003 mg/kg) and the highest dose (0.30 mg/kg) produced lower values than all doses except 0.10 mg/kg (Tukey’s comparisons, P < .05) (Figure 1A,B). There were also main effects of treatment, which indicated that averaged across methamphetamine dose, rats exhibited lower infusion and active lever response rates when pretreated with CP 94,253 than when pretreated with vehicle (Figure 1A,B,insets). There were no significant effects for inactive lever responses during preabstinence tests (Figure 1C).
Analysis of postabstinence infusions showed main effects of treatment [F (1, 9) = 30.74, P < .05] and methamphetamine dose [F (4, 36) = 35.27, P < .05], as well as a treatment by methamphetamine dose interaction [F (4, 36) = 2.87, P < .05]. CP 94,253 pretreatment decreased infusions at the 3 lowest doses of methamphetamine compared with vehicle pretreatment (Tukey’s comparisons, P < .05) (Figure 1D). The analysis of postabstinence active lever responses also revealed main effects for treatment [F (1, 9) = 10.38, P < .05] and methamphetamine dose [F (4, 36) = 22.20, P < .05], but no treatment by methamphetamine dose interaction. The main effect of methamphetamine dose was due to the inverted U-shaped dose-response function similar to preabstinence. The main effect of treatment shows that averaged across methamphetamine doses, rats exhibited lower infusions and active lever response rates when pretreated with CP 94,253 than when pretreated with vehicle (Figure 1D,E, insets). There were no significant effects for inactive lever responses during the postabstinence tests (Figure 1F).
Experiment 2
CP 94,253 decreased methamphetamine infusions, breakpoint, and active lever responses (Figure 2, A-C). Most rats reached breakpoint within the 4-hour test during both the preabstinence (80%) and postabstinence (73%) tests. The analyses showed main effects of pretreatment across pre- and postabstinence tests for infusions [F (1, 13) = 9.86, P < .05], breakpoints [F (1, 13) = 6.46, P < .05], and active lever responses [F (1, 12) = 11.09, P < .05]. In each case, CP 94,253 pretreatment decreased these measures compared with the vehicle pretreatment (Tukey’s comparisons, P < .05). There were no main effects for inactive lever responses (Figure 2D) and no pretreatment by time interaction effects for any of the measures.
Experiment 3
CP 94,253 decreased methamphetamine infusions, and this effect was blocked by SB 224,289 (Figure 3). Data analysis showed a main effect of the first pretreatment (i.e., vehicle vs SB 224,289) [F (1, 29) = 6.86, P < .05] and an interaction between the first and second (vehicle vs CP 94,253) pretreatments [F (1, 29) = 12.52, P < .05]. Posthoc tests showed that pretreatment with vehicle + CP 94,253 decreased methamphetamine infusions compared with all other pretreatment conditions, including SB 224,289 + CP 94,253 (Tukey’s comparisons, P < .05). This finding indicates that administration of the antagonist SB 224,289 blocked the attenuating effect of CP 94,253 on methamphetamine intake, suggesting this effect was mediated by 5-HT1BRs. There were no effects for active or inactive lever responses (data not shown).
The rats in this experiment underwent 21 days of abstinence and were then tested for the effects of CP 94,253 (5.6 mg/kg, s.c.) on resumption of methamphetamine self-administration (0.1 mg/kg, i.v.). Similar to the results from experiment 1, pretreatment with a 5.6-mg/kg dose of CP 94,253 decreased the number of infusions and active lever responses compared with pretreatment with vehicle during the postabstinence tests (data not shown). The mean number of methamphetamine infusions obtained in the vehicle vs CP 94,253 pretreatment groups was 12.40 ± 0.88 and 8.90 ± 0.71, respectively [t (9) = 7.72, P < .05]. The mean number of active lever responses in the vehicle vs CP 94,253 pretreatment groups was 70.30 ± 20.81 and 49.20 ± 17.89, respectively [t (9) = 2.62, P < .05]. There was no group difference for inactive lever responses.
Experiment 4
Neither CP 94,253 or SB 224,289 pretreatment altered locomotor activity (Figure 4). There were no main or interaction effects between treatment and the total distance traveled by rats.
Experiment 5
Acute zolmitriptan treatment decreased methamphetamine infusions and active lever responses during preabstinence tests (Figure 5A,B). Comparisons between vehicle and zolmitriptan showed a difference in infusions [t (10) = 3.50, P < .05] and active lever responses [t (10) = 2.90, P < .05]. There were no effects on inactive lever responses after vehicle or zolmitriptan treatment with means of 39.18 ± 16.37 and 16.55 ± 6.41, respectively.
Zolmitriptan pretreatment given intermittently across 3 postabstinence tests consistently decreased infusions and active lever responses (Figure 5C,D). The ANOVA showed a main effect of treatment group for infusions [F (1, 11) = 21.92, P < .05] but no effect of treatment day or treatment group by treatment day interaction. For active lever responses, there were significant main effects of treatment group [F (1, 10) = 21.17, P < .05] and treatment day [F (2, 20) = 6.08, P < .05], but no treatment group by treatment day interaction. Posthoc tests for treatment day showed that zolmitriptan treatment produced lower active lever response rates on day 2 compared with treatment days 1 and 3. There were no effects on inactive lever responses after vehicle or zolmitriptan treatment with means of 7.89 ± 3.68 and 3.56 ± 0.68, respectively.
Discussion
Unlike the abstinence-dependent modulatory role of 5-HT1BR agonists on cocaine intake that we observed previously (Pentkowski et al., 2012, 2014), this study found that 5-HT1BR agonists attenuated methamphetamine intake when given either pre- or postabstinence. Specifically, a moderate dose of CP 94,253 (5.6 mg/kg, s.c.) decreased methamphetamine intake and active lever response averaged across methamphetamine dose available both when administered during maintenance of daily self-administration sessions and following a period of abstinence (main effect of pretreatment, Figure 1, insets). After abstinence, the effect of CP 94,253 was more pronounced at lower doses of methamphetamine, primarily because intake appeared higher under the vehicle pretreatment condition postabstinence compared with preabstinence. This enhancement of cocaine intake postabstinence is consistent with sensitized cocaine self-administration reported previously (Schenk and Partridge, 1997). Thus, the findings suggest that CP 94,253 attenuates expression of the abstinence-induced, enhanced sensitivity to methamphetamine observed with vehicle pretreatment. CP 94,253 (10 mg/kg, s.c.) also decreased methamphetamine intake (0.05 mg/kg, i.v.) under a PR schedule of reinforcement both pre- and postabstinence (Figure 2), further suggesting attenuation of methamphetamine reinforcing and/or motivational effects. Importantly, administration of the 5-HT1BR antagonist, SB 224,289 (10 mg/kg, i.p.), blocked the attenuating effects of CP 94,253 (5.6 mg/kg, s.c.) on methamphetamine intake in rats tested during maintenance of self-administration on a VR5 schedule (Figure 3), suggesting that the effects of the agonist are mediated by 5-HT1BRs. Finally, we report that zolmitriptan (10 mg/kg, s.c.) also attenuated methamphetamine intake on a VR5 schedule. Zolmitriptan treatment given acutely during maintenance, as well as given intermittently following abstinence, decreased methamphetamine intake and active lever responses (Figure 5).
There are a number of possible reasons for the 5-HT1BR agonist-induced decreases in methamphetamine self-administration, including an effect on methamphetamine reinforcement value and/or incentive motivation, an effect on anxiety, or an effect on motor capability. The decrease in methamphetamine intake is unlikely due to impairments in motor capability, as treatment with the 5-HT1BR ligands did not alter spontaneous locomotion (Figure 4) or inactive lever responses at the doses used in the present study. Furthermore, previous research from our laboratory has shown that CP 94,253 (0.3–10.0 mg/kg, s.c.) has no effect on sucrose reinforcement (Pentkowski et al., 2009). Although the antagonist SB 224,289 decreases cocaine-induced locomotion in drug naïve rats (Hoplight et al., 2005), it has no effect on locomotion in rats with a history of cocaine self-administration (Pentkowski et al., 2009, 2014). Thus, it seems unlikely that CP 94,253 or SB 224,289 altered methamphetamine intake by impairing motor capability.
We cannot rule out the possibility that CP 94,253 may have influenced methamphetamine intake nonspecifically by increasing anxiety-like behaviors rather than attenuating reinforcement per se. Indeed, previous studies have found that either 5-HT1BR agonists or antagonists can increase baseline and cocaine-induced anxiety-like behaviors (Lin and Parsons, 2002; Hoplight et al., 2005; Pentkowski et al., 2009). It is important to note that the rats in these previous studies were naïve to the experimental procedures used to assess anxiety-like behaviors, which would likely maximize any potential drug effect. In contrast, rats tested for CP 94,253 effects on methamphetamine self-administration in the present study were well habituated to the testing environment, which would likely minimize potential anxiogenic effects. Furthermore, animals with increased exposure to stress, such as foot-shock, often increase rather than decrease drug intake (Goeders and Guerin, 1994; Ahmed and Koob, 1997; Piazza and Le Moal, 1998; Logrip et al., 2012), mitigating the idea that CP 94,253 may have induced anxiety-like states that interfered with responding. Finally, it seems that potential anxiogenic effects of CP 94,253 on reinforcement would manifest as a decrease in both sucrose and psychostimulant intake, yet CP 94,253 has been shown to selectively decrease cocaine intake (Pentkowski et al., 2009).
We suggest that the most likely explanation for the agonist-induced decreases in methamphetamine intake in the present study is that 5-HT1BRs modulate psychostimulant reinforcement and/or incentive motivation (Pentkowski et al., 2012, 2014). The decrease of methamphetamine intake in response to 5-HT1BR stimulation following abstinence may result from an attenuation of the expression of enhanced sensitivity to the reinforcing effects of methamphetamine. This explanation is consistent with our previous findings that 5-HT1BR agonists or 5-HT1BR overexpression by viral gene transfer attenuate cocaine self-administration following a period of abstinence, as well as reduce cocaine-seeking behavior (Pentkowski et al., 2009, 2012, 2014). Furthermore, these agonist effects are reversed by 5-HT1BR antagonists, including SB 224,289, supporting a 5-HT1BR mechanism.
We were surprised that CP 94,253 attenuated methamphetamine intake prior to any abstinence given that this same treatment enhances cocaine intake prior to abstinence (Pentkowski et al., 2009, 2014). However, others have shown that 5-HT1BR agonists attenuate d-amphetamine intake without prolonged abstinence, as well as attenuate d-amphetamine-induced responding for conditioned reward (Fletcher and Korth, 1999; Fletcher et al., 2002; Miszkiel et al., 2012; Miszkiel and Przegalinski, 2013). Therefore, the 5-HT1BR agonist enhancement of cocaine intake prior to abstinence may be due to differences in the pharmacological actions of cocaine vs amphetamines. While both amphetamines and cocaine inhibit and downregulate 5-HT, dopamine, and norepinephrine transporters (Azzaro and Rutledge, 1973; Ritz, Cone, and Kuhar, 1990), they interact differently with the transporters. Amphetamines, including methamphetamine, not only inhibit monoamine transport, but also redistribute intracellular monoamines by acting at the vesicular monoamine transporter causing release of monoamines into the cytosol while at the same time reversing monoamine transport across the plasma membrane, resulting in monoamine release (Sulzer et al., 1995; Sager and Torres, 2011; Panenka et al., 2013). Cocaine and amphetamines also interact at different sites on the dopamine transporter and produce differential effects on the releasable vesicular pool and on regulation of vesicular monoamine transporter (Thomsen et al., 2009). The latter effects may result in differences in the releasable pool of dopamine after cocaine vs methamphetamine following acute or subchronic administration (Brown et al., 2001).
Although the specific mechanisms responsible for the attenuating effect of 5-HT1BR agonists on cocaine and methamphetamine self-administration are unclear, we hypothesize that such mechanisms may involve a dysregulation of 5-HT1BR functions (Neisewander et al., 2014). 5-HT1BRs are widely distributed in the brain (Bruinvels et al., 1994; Lanfumey and Hamon, 2004; Varnas et al., 2005; Clark et al., 2006) and are expressed as either 5-HT terminal autoreceptors or heteroreceptors on terminals of non-5-HTergic cells. In both cases, these receptors negatively couple to adenylyl cyclase activity via G-proteins and function to inhibit neurotransmitter release (Hen 1992; Sari, 2004; McDevitt and Neumaier, 2011; Barnes and Neumaier, 2011). Several manipulations in the mesolimbic system have provided evidence for a modulatory role of 5-HT1BRs in psychostimulant addiction; specifically, overexpression of 5-HT1BRs in the nucleus accumbens of rats facilitates the rewarding and reinforcing effects of cocaine (Neumaier et al., 2002; Pentkowski et al., 2012). Furthermore, local activation of 5-HT1BRs in the ventral tegmental area alters cocaine-induced increases in dopamine levels in the nucleus accumbens and cocaine-induced decreases in gamma-aminobutyric acid levels (Parsons et al., 1999; O’Dell and Parsons, 2004). Similarily, activation of 5-HT1BRs in the nucleus accumbens dose-dependently decreases the rewarding and reinforcing effects of amphetamine (Fletcher and Korth, 1999; Fletcher, 2002).
The present findings suggest that 5-HT1BRs are potential targets for developing pharmacotherapies for psychostimulant addiction. Here we show that the clinically available anti-migraine medication zolmitriptan, which is a 5-HT1B/1DR agonist, attenuated methamphetamine intake. The attenuation of methamphetamine intake was likely mediated, at least in part, by stimulation of 5-HT1BRs, although it is possible that 5-HT1DRs may have also contributed to the attenuation effect. Zolmitriptan, unlike CP 94,253, has a higher affinity for 5-HT1DRs (Ki = 0.63 nM) than for 5-HT1BRs (Ki = 5.01 nM; Murray, et al., 2011). CP 94,253 also has affinity for 5-HT1DRs (Ki = 49 nM; Koe et al., 1992), and therefore 5-HT1DRs may also contribute to its effects on methamphetamine self-administration. The effects of zolmitriptan on methamphetamine self-administration were not likely due to a decrease in general activity, as we did not observe any differences in inactive lever responses in our treatment groups. Furthermore, previous research found that zolmitriptan (1–30 mg/kg, i.p.) attenuates alcohol-induced aggression in mice but has no effect on locomotion (de Almeida et al., 2001).
In conclusion, this study provides evidence that the selective 5-HT1BR agonist, CP 94,253, attenuates methamphetamine self-administration pre- and postabstinence under several schedules of reinforcement and in an antagonist-reversible manner. These findings build upon previous research demonstrating similar effects of 5-HT1BR agonists on d-amphetamine self-administration (Fletcher and Korth, 1999; Fletcher et al., 2002; Miszkiel et al., 2012; Miszkiel and Przegalinski, 2013), and together the effect of the agonists on self-administration of amphetamines contrasts with the enhanced cocaine self-administration that has been observed prior to any abstinence (Pentkowski et al., 2009, 2014). These results suggest that 5-HT1BR agonists may differentially modulate cocaine and methamphetamine self-administration initially, but that after a period of abstinence, the agonists inhibit the reinforcing effects of both psychostimulants. The latter findings suggest that 5-HT1BR agonists may have potential therapeutic effects for psychostimulant abuse. In addition, we have provided evidence that the FDA-approved 5-HT1D/1BR agonist, zolmitriptan, also attenuates methamphetamine self-administration both pre- and postabstinence. Our findings suggest that 5-HT1BR agonists warrant further investigation as putative treatments of psychostimulant use disorders. Important future directions include determining the neural circuitry involved in the agonist effects, whether the effects are also observed in female rats, and whether the effects are observed in rats given more extensive access to the stimulants and more extensive abstinence.
Statement of Interest
None.
Acknowledgments
We thank Nathan Pentkowski for his input on this study and Juliette Venault, Allegra Campagna, Katelin Ennis, Thomas Benson, Jennifer Taylor, and JP Bonadonna for their technical assistance.
This work was supported by the National Institute on Drug Abuse (DA011064 to J.L.N. and DA025606 to M.F.O.), and the National Institutes of General Medical Sciences for ASU Post-baccalaureate Research Education Program for Biomedical Research and Initiative to Maximize Student Development (GM071798 and GM099650 to R.G.).
References
- Ahmed SH, Koob GF (1997) Cocaine- but not food-seeking behavior is reinstated by stress after extinction. Pharmacol 132:289–295. [DOI] [PubMed] [Google Scholar]
- Azzaro AJ, Rutledge CO (1973) Selectivity of release of norepinephrine, dopamine, and 5-hydroxytryptamine by amphetamine in various regions of rat brain. Biochem Pharmacol 22:2801–2813. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Neumaier JF (2011) Neuronal 5-HT receptors and SERT. Tocris Bioscience Review 1–16. [Google Scholar]
- Brown JM, Hanson GR, Fleckenstein AE (2001) Regulation of the vesicular monoamine transporter-2: a novel mechanism for cocaine and other psychostimulants. J Pharm Exp Ther 296:762–767 [PubMed] [Google Scholar]
- Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, Hoyer D, Palacios JM (1994) Localization of 5-HT1B, 5-HT1Da, 5-HT1E, and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacol 33:367–386. [DOI] [PubMed] [Google Scholar]
- Callahan PM, Cunningham KA (1995) Modulation of the discriminative stimulus properties of cocaine by 5-HT1B and 5-HT2C receptors. J Pharm Exp Ther 274: 1414–1424. [PubMed] [Google Scholar]
- Clark MS, McDevitt RA, Neaumaier JF (2006) Quantitative mapping of tryptophan hydroxlase-2, 5-HT1A, 5-HT1B, and serotonin transporter expression across the anteroposterior axis of the rat dorsal and median raphe nuclei. J Comp Neurol 498:611–623. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Cornish JL, Hunt GE, McGregor IS (2006) Intravenous methamphetamine self-administration in rats: effects of intravenous or intraperitoneal MDMA co-administration. Pharmacol Biochem Behav 85:454–463 [DOI] [PubMed] [Google Scholar]
- de Almeida RM, Nikulina EM, Faccidomo S, Fish EW, Miczek KA (2001) Zolmitriptan-a 5-HT1B/D agonist, alcohol, and aggression in mice. Psychopharmacology (Berl) 157:131–141. [DOI] [PubMed] [Google Scholar]
- Domjan M. (2003) The essentials of learning and conditioning. Belmont, CA: Wadsworth/Thomson Learning:123 [Google Scholar]
- Fletcher PJ, Azampanah A, Korth KM (2002) Activation of 5-HT1B receptors in the nucleus accumbens reduces self-administration of amphetamine on a progresive ratio schedule. Pharmacol Biochem Behav 71:717–725. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Korth KM (1999) Activation of 5-HT1B receptors in the nucleus accumbens reduces amphetamine-induced enhancement of responding for conditioned reward. Psychopharmacology (Berl) 142:165–174. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Guerin GF (1994) Non-contingent electric footshock facilitates the acquisition of intravenous cocaine self-administration in rats. Psychopharmacology (Berl) 114(1):63–70. [DOI] [PubMed] [Google Scholar]
- Hen R. (1992) Of mice and flies: commonalities among 5-HT receptors. Trends Pharmacol Sci 13:160–165. [DOI] [PubMed] [Google Scholar]
- Hoplight BJ, Vincow ES, Neumaier JF (2005) The effects of SB 224289 on anxiety and cocaine-related behaviors in a novel object task. Physiol Behav 84:707–714. [DOI] [PubMed] [Google Scholar]
- Kitamura O, Wee S, Specio SE, Koob GF, Pulvirenti L (2006) Escalation of methamphetamine self-administration in rats: a dose-effect function. Psychopharmacology 186:48–53 [DOI] [PubMed] [Google Scholar]
- Koe KB, Nielsen JA, Macor JE, Heym J (1992) Biochemical and behavioral studies of the 5-HT1B receptor agonist, CP-94,253. Drug Develop Res 26(3):241–250. [Google Scholar]
- Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, Warner JE, Goldberg SR, Cadet JL (2010) Methampetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLos One 5:e8790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfumey L, Hamon M (2004) 5-HT1 receptors. CNS Neurol Disord Drug Targets 3:1–10. [DOI] [PubMed] [Google Scholar]
- Lin D, Parsons LH (2002) Anxiogenic-like effect of serotonin1B receptor stimulation in the rat elevated plus-maze. Pharmacol Biochem Behav 74:581–587. [DOI] [PubMed] [Google Scholar]
- Logrip ML, Zorrilla EP, Koob GF (2012) Stress modulation of drug self-administration: implications for addiction comorbidity with post-traumatic stress disorder. Neuropharmacol 62:552–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Weiss F, Gold LH, Caine SB, Schulteis G, Koob GF (1993) Animal models of drug craving. Psychopharmacology (Berl) 112:163–182. [DOI] [PubMed] [Google Scholar]
- McDevitt RA, Neumaier JF (2011) Regulation of dorsal raphe nuclues function by serotonin autoreceptors: a behavioral perspective. J Chem Neuroanat 41:234–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miszkiel J, Adamczyk P, Filip M, Przegalinski E (2012) The effect of serotonin 5-HT1B receptor ligands on amphetamine self-administration in rats. Eur J Pharmacol 677:111–115. [DOI] [PubMed] [Google Scholar]
- Miszkiel J, Filip M, Przegalinski E (2011) Role of serotonin (5-HT)1B receptors in psychostimulant addiction. Pharmacol Rep 63:310–1315. [DOI] [PubMed] [Google Scholar]
- Miszkiel J, Przegalinski E (2013) Effects of serotonin (5-HT)1B receptor ligands on amphetamine-seeking behavior in rats. Pharmacol Rep 65:813–822. [DOI] [PubMed] [Google Scholar]
- Murray CW, Rees DC (2009) The rise of fragment-based drug discovery. Nat Chem 1:187–192. [DOI] [PubMed] [Google Scholar]
- Murray KC, Stephens MJ, Rank M, D’Amico J, Gorassini MA, Bennett DJ (2011). Polysynaptic excitatory postsynaptic potentials that trigger spasms after spinal cord injury in rats are inhibited by 5-HT1B and 5-HT1F receptors. J Neurophysiology 106:925–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Drug Intelligence Center (2011) The Economic Impact of Illicit Drug Use on American Society. Washington, DC: Department of Justice. [Google Scholar]
- National Instiute on Drug Abuse (2015) Trends and Statistics. National Institute on Drug Abuse; http://www.drugabuse.gov/related-topics/trends-statistics Accessed November 24, 2015. [Google Scholar]
- Neisewander JL, Cheung TH, Pentkowski NS (2014) Dopamine D3 and 5-HT1B receptor dysregulation as a result of psychostimulant intake and forced abstinence: implications for medications development. Neuropharmacology 76:301–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumaier JF, Vincow ES, Arvanitogiannis A, Wise RA, Carlezon WA Jr (2002) Elevated expression of 5-HT1B receptors in nucleus accumbens efferents sensitizes animals to cocaine. J Neurosci 22:10856–10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Parsons LH (2004) Serotonin1B receptors in the ventral tegmental area modulate cocaine-induced increases in nuclues accumbens dopamine levels. J Pharm Exp Ther 311:711–719. [DOI] [PubMed] [Google Scholar]
- Panenka WJ, Procyshyn RM, Lecomte T, MacEwan GW, Flynn SW, Honer WG, Barr AM (2013) Methamphetamine use: a comprehensive review of molecular, preclinical and clinical findings. Drug Alcohol Depend 129:167–179. [DOI] [PubMed] [Google Scholar]
- Parsons LH, Weiss F, Koob GF (1998) Serotonin1B receptor stimulation enhances cocaine reinforcement. J Neurosci 18:10078–10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parson LH, Koob GF, Weiss F (1999) RU 24969, a 5-HT1B/1A receptor agonist, potentiates cocaine-induced increases in nucleus accumbens dopamine. Synapse 32:132–135. [DOI] [PubMed] [Google Scholar]
- Pentkowski NS, Acosta JI, Browning JR, Hamilton EC, Neisewander JL (2009) Stimulation of 5-HT 1B receptors enhances cocaine reinforcement yet reduces cocaine-seeking behavior. Addict Bio 14:419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentkowski NS, Duke FD, Weber SM, Pockros LA, Teer AP, Hamilton EC, Thiel KJ, Neisewander JL (2010) Stimulation of medial prefrontal cortex serotonin 2C (5-HT2C) receptors attenuates cocaine-seeking behavior. Neuropsychopharmacology 35:2037–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentkowski NS, Cheung TH, Toy WA, Adams MD, Neumaier JF, Neisewander JL (2012) Protracted withdrawal from cocaine self-administration flips the switch on 5-HT1B receptor modulation of cocaine abuse-related behaviors. Biol Psychiatry 72:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentkowski NS, Harder BG, Brunwasser SJ, Bastle RM, Peartree NA, Yanamandra K, Adams MD, Der-Ghazarian T, Neisewander JL (2014) Pharmacological evidence for an abstinence-induced swtich in 5-HT1B receptor modulation of cocaine self-administration and cocaine-seeking behavior. ACS Chem Neurosci 5:168–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Le Moal M (1998) The role of stress in drug self-administration. Trends Pharmacol Sci 19:67–74. [DOI] [PubMed] [Google Scholar]
- Pockros LA, Pentkowski NS, Swinford SE, Neisewander JL (2011) Blockade of 5-HT2A receptors in the medial prefrontal cortex attenuates reinstatement of cue-elicited cocaine seeking behavior in rats. Psychopharmacology (Berl) 213:307–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Cone EJ, Kuhar MJ (1990) Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure-activity study. Life Sci 46:635–645. [DOI] [PubMed] [Google Scholar]
- Rocha BA, Ator R, Emmett-Oglesby MW, Hen R (1997) Intravenous cocaine self-administration in mice lacking 5-HT1B receptors. Pharmacol Biochem Behav 57:407–412. [DOI] [PubMed] [Google Scholar]
- Rodriguez D, Brea J, Loza MI, Carlsson J (2014) Structure-based discovery of selective serotonin 5-HT1B receptor ligands. Structure 22:1140–1151. [DOI] [PubMed] [Google Scholar]
- Sager JJ, Torres GE (2011) Proteins interacting with monoamine transporters: current state and future challenges. Biochemistry 50:7295–7310. [DOI] [PubMed] [Google Scholar]
- Sari Y. (2004) Serotonin1B receptors: from protein to physiological function and behavior. Neurosci Biobehav Rev 28:565–582. [DOI] [PubMed] [Google Scholar]
- Selkirk JV, Scott C, Ho M, Burton MJ, Watson J, Gaster LM, Collin L, Jones BJ, Middlemiss DN, Price GW (1998) SB-224289-a novel selective (human) 5-HT1B receptor antagonist with negative intrinsic activity. Br J Pharmacol 125:202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, and Ewing A (1995) Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. Journal Neurosci 15:4102–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, and Partridge B (1997) Sensitization and tolerance in psychostimulant self-administration. Pharmacol Biochem Behav 57:543–550. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Han DD, Gu HH, Caine SB (2009) Lack of cocaine self-administration in mice expressing a cocaine-insenstive dopamine transporter. J Pharm Exp Ther 331:204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varnas K, Hurd YL, Hall H (2005) Regional expression of 5-HT1B receptor mRNA in the human brain. Synpase 56:21–28. [DOI] [PubMed] [Google Scholar]