Abstract
Induced pluripotent stem (iPS) cells have demonstrated they can undergo self-renewal, attain pluripotency, and differentiate into various types of functional cells. In clinical transplantation of iPS cells, however, a major problem is the prevention of tumorigenesis. We speculated that tumor formation could be inhibited by means of irradiation. Since the main purpose of this study was to explore the prevention of tumor formation in human iPS (hiPS) cells, we tested the effects of irradiation on tumor-associated factors such as radiosensitivity, pluripotency and cell death in hiPS cells. The irradiated hiPS cells showed much higher radiosensitivity, because the survival fraction of hiPS cells irradiated with 2 Gy was < 10%, and there was no change of pluripotency. Irradiation with 2 and 4 Gy caused substantial cell death, which was mostly the result of apoptosis. Irradiation with 2 Gy was detrimental enough to cause loss of proliferation capability and trigger substantial cell death in vitro. The hiPS cells irradiated with 2 Gy were injected into NOG mice (NOD/Shi-scid, IL-2 Rγnull) for the analysis of tumor formation. The group of mice into which hiPS cells irradiated with 2 Gy was transplanted showed significant suppression of tumor formation in comparison with that of the group into which non-irradiated hiPS cells were transplanted. It can be presumed that this diminished rate of tumor formation was due to loss of proliferation and cell death caused by irradiation. Our findings suggest that tumor formation following cell therapy or organ transplantation induced by hiPS cells may be prevented by irradiation.
Keywords: iPS cells, pluripotency, tumorigenesis, irradiation, apoptosis, transplantation
INTRODUCTION
Induced pluripotent stem (iPS) cells were first generated by subjecting mouse fibroblasts to nuclear reprogramming through the retrovirus-mediated transfection of four transcription factors (Oct3/4, Sox2, Klf4 and c-Myc) [1], and human iPS (hiPS) cells were subsequently established by using the same transcription factors [2, 3]. These cells have numerous characteristics in common with embryonic stem (ES) cells, such as the ability to undergo self-renewal and attain pluripotency [1]. This has led to the anticipation of numerous therapeutic applications for hiPS cells without the ethical issues associated with human ES (hES) cells. Many studies recently have suggested that iPS cells can differentiate into various types of functional cells such as cardiomyocytes [4, 5] and neuronal cells [6]. However, a major problem seems to lie in the fact that a small number of differentiated iPS and ES cells remain undifferentiated at transplantation, and these undifferentiated cells can spontaneously differentiate into rapidly proliferating tumors [7–11]. Studies to date have not found effective ways to prevent such tumor formation.
Ionizing radiation, especially in the form of X-rays, is often employed for clinical diagnostic and therapeutic procedures. Moreover, radiation therapy using X-rays is, like surgery and chemotherapy, one of the major therapies for the control of cancer. Previous reports of ours have suggested the biological usefulness of irradiation for the treatment of cancer [12, 13]. Accordingly, we speculated that radiation therapy applied to hiPS cells could overcome the problem of tumorigenesis associated with the applications of hiPS cells in regenerative medicine.
A number of studies have investigated both UV- and γ-irradiated iPS cells and have primarily focused on the DNA damage response such as seen in the cell cycle, p53 signaling, and apoptosis [14–16], but hardly any studies have been reported of tumorigenesis in irradiated iPS cells. Only one study has demonstrated the effect of irradiation on osteogenically differentiated mouse iPS (miPS) cells and assessed tumor formation in vivo [17]. The same study also suggested that the irradiation of iPS cells may make them suitable for regenerative therapy. However, little has been done to estimate the most effective dosage or to research cell death through apoptosis. It is therefore important to start with studies of irradiated hiPS cells and to research the features of hiPS cells following irradiation that may make them suitable for use in regenerative therapy. To this end, the present study was undertaken to investigate the effects of different radiation doses on tumor-associated factors such as radiosensitivity, pluripotency and cell death in undifferentiated hiPS cells. In addition, the effect of radiation on inhibition of tumor formation was assessed in vivo by using hiPS cells subjected to X-ray irradiation.
MATERIALS AND METHODS
hiPS cells culture
The hiPS cell line 201B7 that was generated by using the four transcription factors Oct3/4, Sox2, Klf4 and c-Myc (purchased from the Institute of Physical and Chemical Research, Saitama, Japan) was used in this study. The hiPS cells were grown on Matrigel-coated plates in mTeSR1® medium (Stem Cell Technologies, Vancouver, Canada) at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. The cell medium was changed daily and passaged approximately every 3 to 4 days. For cell counting, hiPS colonies were digested into single cells with StemPro® Accutase® Cell Dissociation Reagent (Invitrogen, San Jose, CA) and counted with a Countess Automated Cell Counter (Invitrogen).
Irradiation technique
The hiPS cells were irradiated at Osaka University Graduate School of Medicine with 4 MV X-rays from a linear accelerator (EXL-6SP; Varian Medical Systems, Palo Alto, CA) and a delivery dose rate of ~1.0 Gy/min.
Colony formation assay
Survival curves were obtained by means of standard colony formation assay. The irradiated hiPS cells were plated onto Matrigel-coated 60 mm-diameter plastic petri-dishes in mTeSR1 with Y-27632 (Wako Pure Chemical Industries, Ltd, Osaka, Japan), aiming for 50–100 colonies per dish. After 10 days of incubation, the cells were fixed with 10% formalin and stained with crystal violet. Colonies with > 50 cells were scored as surviving colonies, and survival fractions (SFs) were calculated and fitted to a linear–quadratic model, which expressed SF as exp(-α × D-β × D2), with D representing the radiation dose.
Immunocytochemistry
The hiPS cells were washed with phosphate buffered saline (PBS), fixed in 1% paraformaldehyde solution for 10 min at room temperature, permeabilized with 0.5% Triton X-100 in PBS, and blocked for 1 h in 10% bovine serum albumin (BSA) in PBS at room temperature. They were then incubated with the primary antibody against Oct3/4 (Abcam plc, Cambridge, UK) at 4 °C overnight, followed by washing with PBS for 10 min and incubation with fluorescein isothiocyanate (FITC)-conjugated secondary antibody and anti-rabbit IgG (GE Healthcare BioSciences, Little Chalfont, UK) for 1 h at room temperature. After mounting in a medium containing DAPI (Invitrogen), the samples were examined with a digital microscope (Biorevo BZ-9000; Keyence, Osaka, Japan).
Extraction of total RNA and reverse transcription PCR
TRizol® reagent was added to the hiPS cells 24 h after irradiation, followed by incubation for 5 min at room temperature, after which 200 μl of chloroform per 1 ml of TRizol® reagent was added. The mixture was then centrifuged for 15 min at 4 °C and the upper aqueous phase was transferred to a fresh tube. RNA from the aqueous phase was precipitated by mixing with isopropanol. Samples were then incubated for 10 min and centrifuged for 10 min at 4 °C, after which the supernatant was removed and the RNA pellet was washed once with 75% ethanol. Next, the pellet was air dried and dissolved in diethyl pyrocarbonate (DPEC)-treated water, and the liquid of 5 μg RNA was reverse transcribed to cDNA. A reverse transcription reaction reagent was made from 5 μl 5 × AMV buffer, 2 μl dNTP (10 mM), 1 μl Oligo dT (0.5 μg/μl), 1 μl R Nasin® (20 u/μl), and 1 μl AMV reverse transcriptase (all from Promega, Madison, WI). Reverse transcription was performed for 1 h at 42 °C and for 10 min at 65 °C. A PCR reaction reagent was made from 5 μl 5 × buffer, 0.5 μl dNTP, 1.5 μl MgCl2, 1 μl primer forward, 1 μl primer reverse, 14.8 μl sterile distilled water (SDW) and 0.2 μl Taq polymerase. The primers are listed in Table 1. The PCR reagent was mixed with 0.5 μl cDNA, and the PCR reaction was performed for 5 min at 94 °C, 30 cycles for 20 s each at 94 °C, at 58 °C and at 72 °C, and for 7 min at 72 °C. PCR products were resolved in 1.5% agarose gel electrophoresis, and ethidium bromide–stained specific bands were visualized under UV light and photographed.
Table 1.
Primer list
| Gene | Primer sequence | Mainly expressing cells |
|---|---|---|
| Oct3/4 | F: 5′-CTTCAGGAGATATGCAAAGCAG-3′ | Pluripotent stem cells |
| R: 5′-CTCTCACTCGGTTCTCGATACT-3′ | ||
| Sox2 | F: 5′-AGTGGAACTTTTGTCGGAGAC-3′ | Pluripotent stem cells |
| R: 5′-GGTATTTATAATCCGGGTGCTC-3′ | ||
| Nanog | F: 5′-GAAACAGAAGACCAGAACTGTG-3′ | Pluripotent stem cells |
| R: 5′-GCTGAGGTATTTCTGTCTCTGA-3′ | ||
| Lin28 | F: 5′-GGAGTATTCTGTATTGGGAGTG-3′ | Pluripotent stem cells |
| R: 5′-ATCTAGACCTCCACAGTTGTAGC-3′ | ||
| Sox17 | F: 5′-GGGATGTCCAAGTAATTTTGG-3′ | Endoderm cells |
| R: 5′-GCCACTTCCCAAGGTGTAAA-3′ | ||
| Foxa2 | F: 5′-TGTGTATTCTGGCTGCAAGG-3′ | Endoderm cells |
| R: 5′-CCCTCCCTCCTTCTTGAAAT-3′ | ||
| Brachury | F: 5′-ACCCAGTTCATAGCGGTGAC-3′ | Mesoderm cells |
| R: 5′-TCTATCCACGTGCCTACAGC-3′ | ||
| Nkx2.5 | F: 5′-GTTGTCCGCCTCTGTCTTCT-3′ | Mesoderm cells |
| R: 5′-TCTATCCACGTGCCTACAGC-3′ | ||
| Nestin | F: 5′-AAGGGAATCTCTTGCCTGCT-3′ | Ectoderm cells |
| R: 5′-CACAAAAGCCAGCATGTCAC-3′ | ||
| β-actin | F: 5′-CTCCTCCCTGGAGAAGAGCTACGA-3′ | |
| R: 5′-ATGATGGAGTTGAAGGTAGTTTCG-3′ |
Real-time PCR
The cDNA (5 μl), diluted by a factor of 20, was added for PCR of the master mix—10 μl THUNDERBIRD® SYBR® green (Toyobo, Tokyo, Japan), 3.8 μl SDW, 0.6 μl primer forward, 0.6 μl primer reverse—to a final volume of 20 μl. Primers are listed in Table 1. The program for the Real-Time Thermocycler Opticon Monitor, Ver. 3.0 (Bio-Rad Laboratories, Hercules, CA) consisted of 5 min at 95 °C, followed by 45 cycles of 10 s at 95 °C, 20 s at 60 °C and 7 s at 95 °C to generate a melting curve. Differences were quantified with the ΔΔCT method, and the expression levels of genes in irradiated cells were compared with those in non-irradiated cells after normalization with β-actin, a housekeeping gene.
Annexin V flow cytometry analysis
The hiPS cells were dissociated by means of Accutase 24 h after irradiation, resuspended in binding buffer and stained with 1 μl of Annexin V-FITC and 7.5 μl of propidium iodide (PI) by using the Annexin V-FITC Apoptosis Detection Kit I (BD Biosciences Pharmingen, San Diego, CA). The cell suspension was then incubated for 15 min at room temperature and analyzed on a FACScan flow cytometer (BD Biosciences, San Jose, CA), and the resultant data were analyzed with CellQuest (BD Biosciences) analysis software.
Terminal transferase dUTP nick end labeling (TUNEL) assay
The TUNEL assay was performed with the aid of the ApopTag Plus Fluorescein In Situ Apoptosis Detection Kit (Millipore, Bedford, MA). The hiPS cells were grown on a Matrigel-coated glass-bottom Lab-Tek® four-well Chamber Slide® (Nalge Nunc, Naperville, IL). Twenty-four hours after irradiation, the cells were fixed with 1% paraformaldehyde for 10 min and washed in two changes of PBS for 5 min per wash. The cells were then fixed with precooled ethanol: acetic acid (2:1) for 5 min at 4 °C and washed in two changes of PBS for 5 min per wash. An equilibration buffer was then applied followed by incubation for at least 10 s. Working Strength TdT Enzyme (Millipore) was added for 1 h at 37 °C, followed by the addition of Working Strength Stop/Wash Buffer (Millipore) for 10 min, after which the cells were washed in three changes of PBS for 1 min per wash. Working Strength Anti-Digoxigenin Conjugate (Millipore) was added for 30 min while avoiding exposure to light, and the cells were then washed in four changes of PBS for 2 min per wash. After the samples had been embedded in mounting medium containing DAPI (Invitrogen), they were examined under a Biorevo BZ-9000 digital microscope (Keyence).
Western blotting
After 24 h of irradiation, lysates were generated with a lysis buffer (50 mM Tris-HCl pH 7.5, 10 mM MgCl2, 0.5 M NaCl, 1% Triton X-100). Samples were prepared in a sample buffer and heated to 95 °C for 5 min, run over 10% polyacrylamide gels and separated. The proteins were then transferred to PVDF membranes (Millipore), which were blocked for 3 h in TBST (Tris buffer solution with 0.2% Triton X) containing 5% skim milk and 5% phosphoBLOCKER (Cell Biolabs, San Diego, CA). Next, the membranes were incubated overnight at 4 °C with a primary antibody against poly (ADP-ribose) polymerase (PARP) and cleaved PARP, then washed in TBST three times and incubated with a secondary antibody, anti-rabbit IgG and anti-mouse IgG conjugated with Streptavidin Horseradish Peroxidase Conjugate (GE Healthcare BioSciences, Little Chalfont, UK) for 1 h. Next, the membranes were washed six times in TBST, and luminescence from the Horseradish Peroxidase was detected on X-ray film (Fuji Photo Film Ltd, Tokyo, Japan) with the aid of ECL Western Blotting Detection Reagents (GE Healthcare BioSciences).
Caspase assay
The hiPS cells were dissociated with Accutase 24 h after irradiation and resuspended in quantities of 1 × 106 cells in 50 μl of Cell Lysis Buffer using the FLICE/Caspase-3 Colorimetric Assay Kit (BioVision, Milpitas, CA). The cell suspension was incubated for 10 min on ice and centrifuged for 1 min (×10 000g). For analysis of caspase activity, 50 μl of reaction buffer was added to 50 μl of the cellular supernatant solution (containing 50 μg of soluble protein) and incubated with 5 μl of caspase-3 substrates in a 96-well plate. After 2 h, caspase-3 activities were assessed with a microplate reader in terms of cell absorbance (measurement wavelength: 415 nm; reference wavelength: 630 nm).
Subcutaneous transplantation of hiPS cells
Animal protocols were approved by the Ethics Review Committee for Animal Experimentation of Osaka University Graduate School of Medicine. All procedures were performed using 7- to 8-week-old female NOG mice (NOD/Shi-scid, IL-2 Rγnull; CLEA, Japan, Inc., Tokyo). Following induction with inhaled sevoflurane (4%), anesthesia was maintained with 2% sevoflurane. Subsequently, three groups received different subcutaneous injections of 1 × 106 non-irradiated hiPS cells suspended in a 50 μl volume of a 1:1 mixture of Matrigel and mTeSR1 medium with Y-27632 (Group 1, n = 10), 1 × 106 hiPS cells irradiated with 2 Gy suspended in the same volume as that of Group 1 (Group 2, n = 10), and 50 μl of PBS as control (Group 3, n = 5) by referring to previous studies [10, 11]. Injections were performed within 2 h of irradiation.
Post mortem immunohistochemical staining
Animals were sacrificed by following protocols approved by the Ethics Review Committee for Animal Experimentation of Osaka University Graduate School of Medicine. Tumors were sectioned for hematoxylin and eosin (H&E) staining, and tumor formations were interpreted by an experienced pathologist.
Statistics
The results were expressed as mean values with standard deviations. The statistical significance was tested by means of Student's t-test. A P-value of < 0.05 was considered to be statistically significant.
RESULTS
Radiosensitivity of hiPS cells
For a better understanding of the radiosensitivity of hiPS cells, they were thoroughly examined 24 h after irradiation with 0.5, 2 and 4 Gy (Fig. 1A). An increase was noted in detachment of iPS cells from the surface of the cell culture dish as a result of irradiation with 2 and 4 Gy. For quantification of the radiosensitivity of hiPS cells, a colony formation assay was performed and survival curves were created (Fig. 1B). Figure 1B shows that <10% of the hiPS cells irradiated with 2 Gy survived, indicating that the radiosensitivity of hiPS cells is much higher than that of cancer cells in our previously reported results of laboratory studies [12, 13].
Fig. 1.
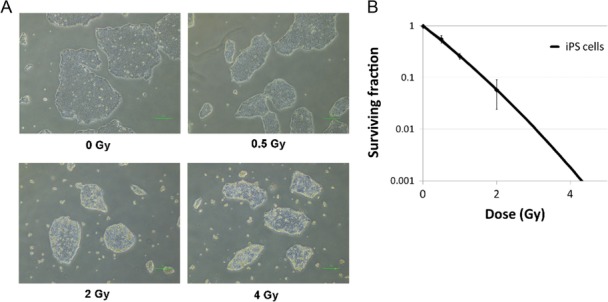
Radiosensitivity of irradiated hiPS cells. (A) Examination of conditions in hiPS cells 24 h after irradiation. Scale bar = 100 μm. (B) Colony formation assay of surviving fractions in irradiated hiPS cells. Results were normalized to non-irradiated cells. Each bar represents mean ± s.d.
The effect of irradiation on pluripotency and differentiation markers in hiPS cells
To determine the effect of irradiation on pluripotency of hiPS cells, the expression of the pluripotency marker Oct3/4 was immunocytochemically investigated 24 h after irradiation with 0.5, 2 and 4 Gy (Fig. 2A). No significant changes in the expression of Oct3/4 were detected for any dose. Additionally, the expression of mRNA levels of the pluripotency markers Oct3/4, Sox2, Nanog and Lin28 were investigated by means of real-time PCR under the same conditions as for the examination of Oct3/4 expression (Fig. 2B). Significantly, the mRNA levels showed that the hiPS cells retained all pluripotency markers after irradiation with 0.5 Gy, but that there was a significant decrease in pluripotency markers Oct3/4, Sox2 and Lin28, but not Nanog, after irradiation with 2 and 4 Gy. Next, to study the effect of irradiation on differentiation in hiPS cells, the expression of the differentiation markers, such as Sox17, Foxa2 (endoderm marker), Brachury, Nkx2.5 (mesoderm marker) and Nestin (ectoderm marker), were investigated by means of reverse transcription PCR 24 h after irradiation (Fig. 2C). Figure 2C shows that the expression of none of the differentiation markers changed in response to any dose, suggesting that the hiPS cells may not have differentiated. We therefore consider that irradiated hiPS cells can maintain their pluripotency and that the expression of mRNA levels of the pluripotency markers can be expected to return over time to the same values as those for non-irradiated hiPS cells, as was previously observed in hES cells [18].
Fig. 2.
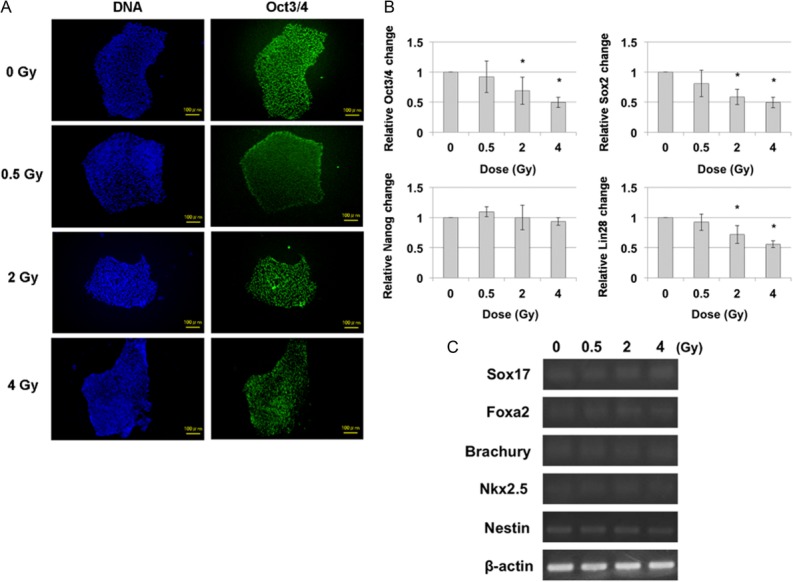
Pluripotency and differentiation marker expressions of hiPS cells 24 h after irradiation. (A) Immunocytochemical analysis of pluripotency marker Oct3/4 in hiPS cells 24 h after irradiation. Green: Oct3/4, Blue: DNA. Scale bar = 100 μm. (B) Real-time-PCR for expression pluripotency markers (Oct3/4, Sox2, Nanog and Lin28) 24 h after irradiation. Each bar represents mean ± s.d. *P < 0.05 (Student's t-test for comparison with non-irradiated cells). (C) Reverse transcription PCR of various differentiation markers of three germ layers. The gene expressions of Sox17, Foxa2 (endoderm marker), Brachury, Nkx2.5 (mesoderm marker) and Nestin (ectoderm marker) were obtained 24 h after irradiation. β-actin served as the loading control.
The effect of irradiation on cell death in hiPS cells
The relative extent of apoptosis and cell death after irradiation was investigated by double staining the hiPS cells with Annexin V (for early apoptosis) and PI (for cell death) and analyzing hiPS cells with flow cytometry (Fig. 3A). The results showed that early apoptosis and cell death (late apoptosis or necrosis) of hiPS cells irradiated with 0.5 Gy increased slightly compared with those of control cells. However, a dramatic increase in cell death was observed after irradiation with 2 and 4 Gy. This confirmed that the majority of hiPS cells had died after irradiation with 2 and 4 Gy, but since it was also necessary to investigate what kind of cell death is important, the TUNEL assay was performed for detection of DNA fragmentation in apoptotic cells (Fig. 3B). The results showed that TUNEL-positive cells were visible mainly in hiPS cells irradiated with 2 and 4 Gy. In addition, western blotting was performed for analysis of PARP and cleaved PARP (Fig. 3C). Irradiation with 2 and 4 Gy reduced the expression levels of PARP and increased those of cleaved PARP more than those of cells irradiated with 0.5 Gy and control cells. Moreover, the caspase-3 assay showed that activities of caspase-3 increased for each dose (Fig. 3D). Thus, it seems safe to presume that cell death of the irradiated hiPS cells was mainly in the form of apoptosis.
Fig. 3.
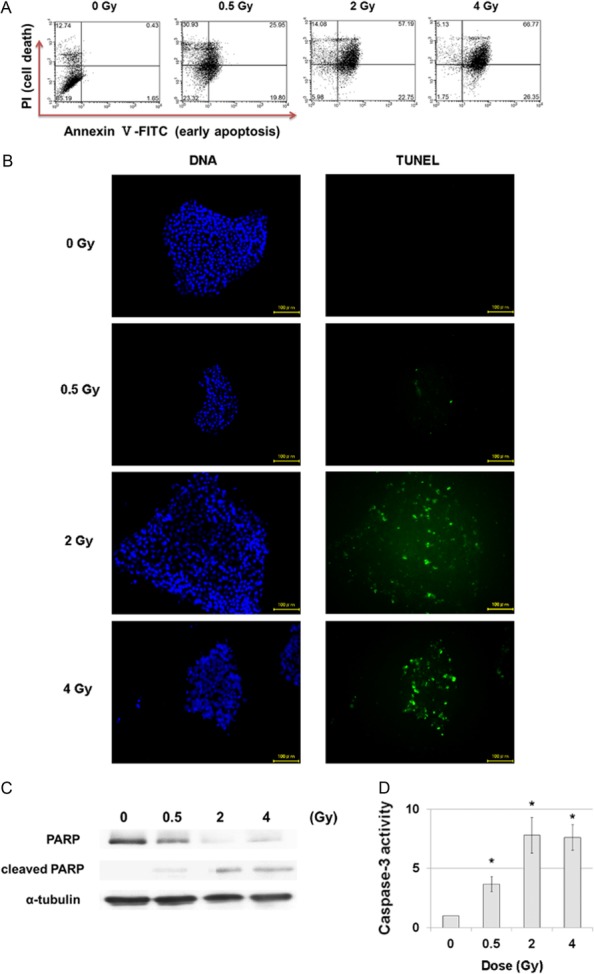
Cell death of hiPS cells 24 h after irradiation. (A) Flow cytometry of Annexin V-FITC and PI double-stained hiPS cells 24 h after irradiation. (B) TUNEL assay of hiPS cells 24 h after irradiation. Green: TUNEL, Blue: DNA. Scale bar = 100 μm. (C) Western blotting for analysis of PARP and cleaved PARP 24 h after irradiation. Levels of α-tubulin were determined as the internal control for protein loading. (D) Caspase assay for analysis of caspase-3 activity 24 h after irradiation. Each bar represents mean ± s.d. *P < 0.05 (Student's t-test, for comparison with non-irradiated cells).
Transplantation of irradiated hiPS cells
The foregoing investigations of irradiated hiPS cells were performed in vitro and showed that hiPS cells were characterized by dramatically high radiosensitivity and significantly increased cell death following irradiation with 2 and 4 Gy. Since the main purpose of this experiment was to study the possibility of prevention of tumor formation in hiPS cells, we decided to use hiPS cells irradiated with 2 Gy in vivo because irradiation with 2 Gy was detrimental enough to trigger cell death in the in vitro studies. Figure 4A illustrates the strategy used for this experiment. Irradiated hiPS cells were injected into NOG mice to determine the effect of irradiation on tumor formation (Fig. 4B). Remarkably, when non-irradiated hiPS cells were transplanted, the rate of tumor formation was 100% (n = 10), but when hiPS cells irradiated with 2 Gy were transplanted, the rate was only 10% (n = 10). It can thus be assumed that this dramatic reduction in the rate of tumor formation was due to the effect of irradiation on cell death. H&E staining was also performed for histological evaluation (Fig. 4C).
Fig. 4.
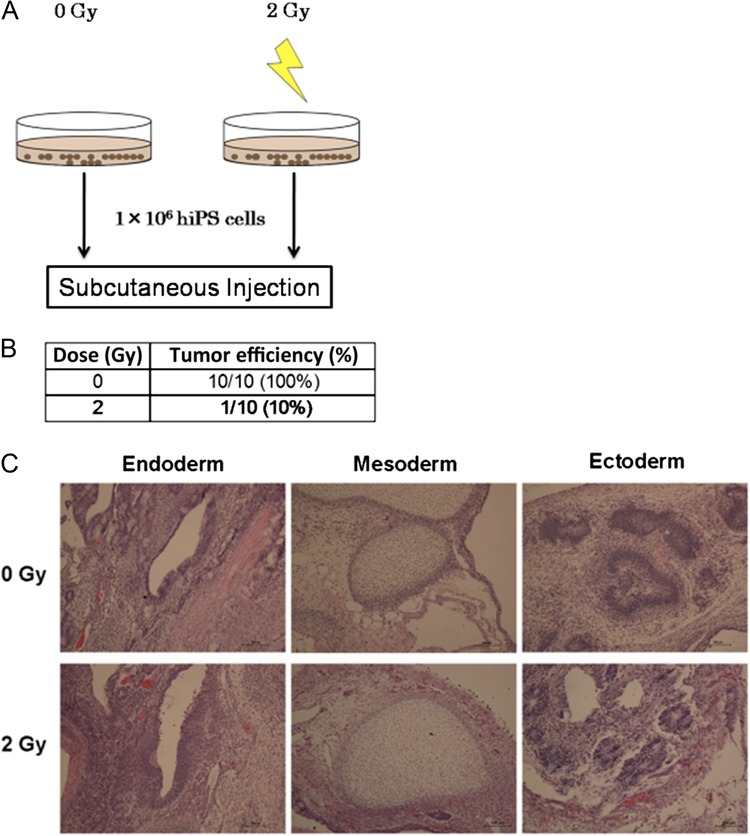
Transplantation of irradiated hiPS cells. (A) Schematic summary of the experimental design. (B) Tabular summary of the effect on tumor formation after subcutaneous transplantation in non-irradiated hiPS cells and hiPS cells irradiated with 2 Gy. (C) H&E staining for histological evaluation of tumors obtained from non-irradiated hiPS cells and hiPS cells irradiated with 2 Gy. Scale bar = 100 μm.
DISCUSSION
In recent years, there have been significant advances in the therapeutic applications of hiPS cells. Many studies have indicated that none of the ethical issues associated with hES cells apply to hiPS cells. For clinical cell transplantation of hiPS cells, however, a major obstacle is the prevention of tumor formation [9–11]. Needless to say, tumor formation associated with transplantation must be avoided at all costs when the procedure is used in humans. To this end, we hypothesized that tumor formation could be prevented by means of irradiation, which is often used for clinical diagnostic and therapeutic procedures. Previous studies of ours have conducted many biological experiments that have shown the effect of irradiation on cancer cells [12, 13], and we used the findings of these studies to perform the trials for prevention of tumor formation reported here. In other studies, similar experiments were used for hES cells and osteogenically differentiated miPS cells [17, 19]. Tumor formation could not be prevented by means of irradiation in hES cells, but in osteogenically differentiated miPS cells it could. However, these studies paid little attention to the tumor-associated factors of radiosensitivity and cell death in either of these cell types. For this reason, we examined the effects of irradiation on such factors as radiosensitivity, pluripotency, and cell death in undifferentiated hiPS cells.
First, the radiosensitivity in hiPS cells was examined in order to determine SFs. Our laboratory reported previously that the SF of HT1080, a human fibrosarcoma cell line, was > 70% after 2 Gy of X-ray irradiation [12]. Some researchers reported that the SF of mES cells irradiated with 2 Gy of X- and γ-rays was ~45% [20, 21], and Hayashi et al. found that the SF of miPS cells irradiated with 2 Gy of X-rays was ~35% [22]. However, the SF of hiPS cells irradiated with 2 Gy in our study was < 10%, suggesting that hiPS cells have much higher radiosensitivity than cancer, mES and miPS cells. In other words, our finding demonstrates that most of the hiPS cells showed a marked loss of proliferation potency after irradiation.
The effect of irradiation on pluripotency markers was investigated 24 h after irradiation. The expression of Oct3/4 showed no significant change in response to an increase in the dose of immunocytochemistry. This finding was the same as that reported elsewhere for DNA damage in response to irradiation with 1 and 2 Gy of γ-rays in hES and hiPS cells [15, 18, 23]. In our study, however, the expressions of mRNA levels of pluripotency markers Oct3/4, Sox2 and Lin28 significantly decreased in response to 2 and 4 Gy of irradiation. To clarify this discrepancy, differentiation markers (endoderm, mesoderm and ectoderm markers) were evaluated 24 h after irradiation and it was found that there were no changes in any of the markers, suggesting that the irradiated hiPS cells had not differentiated. Momcilović et al. reported that the levels of mRNA expressions of pluripotency markers in hES cells decreased 6 h after 2 Gy irradiation and returned to the same values as those of non-irradiated hES cells 24 and 48 h after irradiation [18]. We therefore believe that irradiated hiPS cells continue to remain pluripotent and that the reduction in mRNA levels of pluripotency markers due to their high radiosensitivity is temporary.
The relative extent of apoptosis and cell death in hiPS cells was also studied 24 h after irradiation. Our results showed that irradiation of 2 and 4 Gy caused major cell death in response to an increase in the dose, which is the same result as that reported for irradiated hES cells [19]. Finally, we needed to determine what kind of cell death is important. The results of the TUNEL assay, western blotting and the caspase-3 assay performed to examine apoptosis in irradiated hiPS cells, suggested that the majority of cell death was due to apoptosis.
While this study was helpful for a better understanding of many significant characteristics of irradiated hiPS cells, the main purpose of this experiment was to study the prevention of tumor formation of hiPS cells. It showed that 2 Gy of irradiation of hiPS cells was detrimental enough to cause them to lose their ability to proliferate and engender major cell death. For the in vivo experiment, hiPS cells irradiated with 2 Gy were injected into NOG mice for analysis of the effect of irradiation on tumor formation. Surprisingly, our results demonstrated that hiPS cells irradiated with 2 Gy and transplanted into one group of mice showed a significant reduction in tumor formation in comparison with that in non-irradiated hiPS cells transplanted into another group. This finding indicated that the number of surviving hiPS cells irradiated with 2 Gy was reduced because of apoptosis induced by irradiation and that this reduction led to a reduction in tumor formation. While it is clear that tumor formation prevented in the injected 1 × 106 hiPS cells as a result of irradiation administered with reference to previous protocols [10, 11], possible changes in the number of hiPS cells to be injected need to be investigated in our future studies. Our results are very different from those reported for hES cells by Wilson et al., who found that tumor formation of hES cells irradiated with 4 Gy was almost the same as that of non-irradiated hES cells [19]. We speculate that these differences between hiPS and hES cells can be accounted for by the differences in radiosensitivity between the two types of cells. As mentioned earlier, mES cells are more resistant to irradiation than miPS and hiPS cells [20–22], which probably means that hES cells will also be more resistant to irradiation than hiPS cells. This points to the need for the radiosensitivity of iPS and ES cells to be examined in detail in the very near future. Moreover, Fukawatase et al. reported that there were almost no changes in chromosomal aberrations of hiPS cells irradiated with X-rays compared with those in non-irradiated cells [24]. This report therefore suggested that cell therapy used by irradiation may be safe for clinical application.
In summary, our study is, to the best of our knowledge, the first to demonstrate that tumor formation in hiPS cells can be inhibited by means of irradiation. The hiPS cells irradiated in our experiments have shown much higher radiosensitivity as well as substantial changes in pluripotency markers and significant death and apoptosis after irradiation. These results suggest it may be possible to prevent by means of irradiation tumor formation in hiPS cells used for cell therapy or organ transplantation. However, if this method works only for differentiated hiPS cells, it may be necessary to assess the ratio of partially differentiated hiPS cells by using differentiated factors and to determine what constitutes a safe irradiation dosage. In addition, follow-up studies are needed to examine the possibility of tumor formation over the longer term. Before any definite conclusions can be reached, further studies of the effect of irradiation on hiPS cells are therefore essential.
FUNDING
This work was supported by a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (Grant No. 26290055).
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCES
- 1. Takahashi K, Yamanaka S.. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–76. [DOI] [PubMed] [Google Scholar]
- 2. Takahashi K, Tanabe K, Ohnuki M et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–72. [DOI] [PubMed] [Google Scholar]
- 3. Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007;318:1917–20. [DOI] [PubMed] [Google Scholar]
- 4. Germanguz I, Sedan O, Zeevi-Levin N, et al. Molecular characterization and functional properties of cardiomyocytes derived from human inducible pluripotent stem cells. J Cell Mol Med 2011;15:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kawamura M, Miyagawa S, Miki K, et al. Feasibility, safety, and therapeutic efficacy of human induced pluripotent stem cell-derived cardiomyocyte sheets in a porcine ischemic cardiomyopathy model. Circulation 2012;126:29–37. [DOI] [PubMed] [Google Scholar]
- 6. Molcanyi M, Riess P, Haj-Yascin NN, et al. Developmental potential of the murine embryonic stem cells transplanted into the healthy rat brain—novel insights into tumorigenesis. Cell Physiol Biochem 2009;24:87–94. [DOI] [PubMed] [Google Scholar]
- 7. Przyborski SA. Differentiation of human embryonic stem cells after transplantation in immune-deficient mice. Stem Cells 2005;23:1242–50. [DOI] [PubMed] [Google Scholar]
- 8. Lensch MW, Schlaeger TM, Zon LI, et al. Teratoma formation assays with human embryonic stem cells: a rationale for one type of human–animal chimera. Cell Stem Cell 2007;1:253–8. [DOI] [PubMed] [Google Scholar]
- 9. Miura K, Okada Y, Aoi T, et al. Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol 2009;27:743–5. [DOI] [PubMed] [Google Scholar]
- 10. Müller FJ, Goldmann J, Löser P, et al. A call to standardize teratoma assays used to define human pluripotent cell lines. Cell Stem Cell 2010;6:412–4. [DOI] [PubMed] [Google Scholar]
- 11. Gutierrez-Aranda I, Ramos-Mejia V, Bueno C, et al. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells 2010;28:1568–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ogata T, Teshima T, Kagawa K, et al. Particle irradiation suppresses metastatic potential of cancer cells. Cancer Res 2005;65:113–20. [PubMed] [Google Scholar]
- 13. Akino Y, Teshima T, Kihara A, et al. Carbon-ion beam irradiation effectively suppresses migration and invasion of human non-small-cell lung cancer cells. Int J Radiat Oncol Biol Phys 2009;75:475–81. [DOI] [PubMed] [Google Scholar]
- 14. Zhao Z, Yu R, Yang J, et al. Maxadilan prevents apoptosis in iPS cells and shows no effects on the pluripotent state or karyotype. PLoS One 2012;7:33953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Momcilovic O, Knobloch L, Fornsaglio J, et al. DNA damage responses in human induced pluripotent stem cells and embryonic stem cells. PLoS One 2010;5:13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lan ML, Acharya MM, Tran KK, et al. Characterizing the radioresponse of pluripotent and multipotent human stem cells. PLoS One 2012;7:50048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayashi T, Misawa H, Nakahara H, et al. Transplantation of osteogenically differentiated mouse iPS cells for bone repair. Cell Transplant 2012;21:591–600. [DOI] [PubMed] [Google Scholar]
- 18. Momcilović O, Choi S, Varum S, et al. Ionizing radiation induces ataxia telangiectasia mutated–dependent checkpoint signaling and G2 but not G1 cell cycle arrest in pluripotent human embryonic stem cells. Stem Cells 2009;27:1822–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wilson KD, Sun N, Huang M, et al. Effects of ionizing radiation on self-renewal and pluripotency of human embryonic stem cells. Cancer Res 2010;70:5539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bañuelos CA, Banáth JP, MacPhail SH, et al. Mouse but not human embryonic stem cells are deficient in rejoining of ionizing radiation–induced DNA double-strand breaks. DNA Repair 2008;7:1471–83. [DOI] [PubMed] [Google Scholar]
- 21. Rebuzzini P, Pignalosa D, Mazzini G, et al. Mouse embryonic stem cells that survive γ-rays exposure maintain pluripotent differentiation potential and genome stability. J Cell Physiol 2012;227:1242–9. [DOI] [PubMed] [Google Scholar]
- 22. Hayashi N, Monzen S, Ito K, et al. Effects of ionizing radiation on proliferation and differentiation of mouse induced pluripotent stem cells. J Radiat Res 2012;53:195–201. [DOI] [PubMed] [Google Scholar]
- 23. Sokolov MV, Panyutin IV, Onyshchenko MI, et al. Expression of pluripotency-associated genes in the surviving fraction of cultured human embryonic stem cells is not significantly affected by ionizing radiation. Gene 2010;455:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fukawatase Y, Toyoda M, Okamura K, et al. Ataxia telangiectasia derived iPS cells show preserved x-ray sensitivity and decreased chromosomal instability. Sci Rep 2014;4:5421. [DOI] [PMC free article] [PubMed] [Google Scholar]


