Abstract
Rituximab, an anti-CD20 monoclonal antibody, was originally used to treat B-cell malignancies. Its use has significantly increased in recent years, as it is now also used to treat a variety of autoimmune diseases including rheumatoid arthritis and ANCA-associated vasculitis (AAV). Initial studies suggested that the adverse effects of rituximab were minimal. Though the risk of malignancy with rituximab-based immunosuppressive regimens appears similar to that of the general population, there are now concerns regarding the risk of infectious complications. Rituximab has been associated with serious infections, including Pneumocystis jiroveci pneumonia (PJP) and the reactivation of hepatitis B virus (HBV) and tuberculosis (TB). The risk of infection appears to be the result of a variety of mechanisms, including prolonged B-cell depletion, B-cell–T-cell crosstalk, panhypogammaglobulinaemia, late-onset neutropenia and blunting of the immune response after vaccination. Importantly, the risk of infectious complications is also related to individual patient characteristics and the indication for rituximab. Individualization of treatment is, therefore, crucial. Particular attention should be given to strategies to minimize the risk of infectious complications, including vaccinating against bacterial and viral pathogens, monitoring white cell count and immunoglobulin levels, prophylaxis against PJP and screening for HBV and TB.
Keywords: immunology, immunosuppression, infection, rituximab, vasculitis
Introduction
Rituximab is a chimeric anti-CD20 monoclonal antibody that was the first monoclonal antibody approved by the US Food and Drug Administration (FDA) [1]. Initially approved to treat cancer, specifically B-cell malignancies, rituximab’s use in clinical practice has significantly increased in recent years [1]. It is now used to treat a variety of autoimmune diseases, including rheumatoid arthritis (RA), membranoproliferative glomerulonephritis (MPGN), systemic lupus erythematosus (SLE) and ANCA-associated vasculitis (AAV) [2–4].
As a precursor of antibody-secreting plasma cells, B-cells have an important role in the pathogenesis of antibody-mediated autoimmune disease [5]. They generate cytokines, activate T cells and can act as antigen-presenting cells [5]. The CD20 receptor is found on the cell surface of B-cells at their pre-B-cell stage [6]. It is not expressed on other tissues and does not appear on B-cells after their differentiation into plasma cells [5]. Rituximab targets the CD20 receptor leading to B-cell depletion through complement-mediated cytotoxicity, antibody-dependent cellular cytotoxicity and B-cell apoptosis [6].
Susceptibility to infection with rituximab therapy
Initial studies suggested that the adverse effects of rituximab therapy were minimal and predominantly limited to ‘tolerable’ reactions to the initial infusion [1]. However, we now know that treatment with rituximab is not without risk [7]. Though the risk of malignancy with rituximab-based immunosuppressive regimens appears similar to that of the general population, there are concerns regarding the risk of serious infections [8–10]. In this issue, Trivin et al [11]. discussed the risk of infections associated with rituximab in different autoimmune disorders. This risk appears to be the result of a variety of mechanisms, including prolonged B-cell depletion, B-cell–T-cell crosstalk, panhypogammaglobulinaemia, late-onset neutropenia and blunting of the immune response after vaccination.
B-cell reconstitution is variable after administration of rituximab. However, B-cells usually return to pretreatment levels within 12 months of the initial treatment [9]. Rarely, B-cell depletion can persist beyond this [12, 13]. Despite mature plasma cells lacking the CD20 receptor, panhypogammaglobulinaemia occurs relatively frequently during this period, though severe panhypogammaglobulinaemia associated with infection is less common [5]. Besada et al. [14] published a study in 2013 reporting that 37% of patients receiving maintenance rituximab for granulomatosis with polyangiitis (GPA) had to discontinue rituximab largely due to hypogammaglobulinaemia. Patients that suffered severe infections were noted to have a more profound decline in total immunoglobulins during treatment [14]. Moreover, lower levels of immunoglobulins increased the risk of chronic infection [14]. In 2016, Besada [15] demonstrated that low baseline levels of immunoglobulin G (IgG) were associated with hypogammaglobulinaemia during treatment. These studies highlight the importance of assessing immunoglobulin levels prior to and during treatment with rituximab.
Late-onset neutropenia, defined as neutropenia occurring at least 4 weeks after administration of rituximab, has been reported to occur with variable frequency [16, 17]. Tesfa et al. [16] reported neutropenia in 23% of patients with GPA and 20% of patients with SLE treated with rituximab, whereas it occurred in only 3% of RA patients. Late-onset neutropenia was associated with more severe B-cell depletion and an increased risk of severe infections [16]. A later study published by Salmon et al. [17] reported only 1.3% of patients with RA and 2.3% of patients with other autoimmune disease developed late-onset neutropenia. Salmon et al. [17] also found a much lower rate of infectious complications associated with neutropenia. Thus, late-onset neutropenia after rituximab administration may not be as significant a risk factor for infection as hypogammaglobulinaemia. The mechanism of this neutropenia remains unclear and it may be underdiagnosed, as most patients are asymptomatic.
As mentioned earlier, there is a blunted immune response after vaccination in those that have received rituximab [18, 19]. Bingham et al. [18] demonstrated that patients treated with rituximab have decreased responses to pneumococcal polysaccharide vaccines and to neoantigen vaccines. Furthermore, Heusele et al. [8] reported that pneumococcal vaccination prior to treatment significantly decreased the risk of serious bacterial infections. Accordingly, current guidelines emphasize the importance of patients receiving vaccinations prior to rituximab therapy to minimize the infection risk during their treatment course [20, 21].
Several other factors appear to contribute to the risk of developing severe infections with rituximab treatment. In this issue, Trivin et al. describe diabetes mellitus, azathioprine use, renal impairment and higher total dose of rituximab as being associated with an increased risk of infection during rituximab therapy. Heusele et al. [8] also reported diabetes mellitus and renal impairment as being associated with infectious complications. In addition, they noted that those that suffered serious infections were significantly older and were more likely to have been receiving a prednisolone dose >15 mg/day.
It is important to note that there are patient- and disease-related factors that have an impact on infection risk, in addition to the risk caused by immunosuppression treatment alone. All the following influence the risk of infections associated with rituximab: the presence of underlying malignancy such as a lymphoproliferative disorder, complement dysregulation or leucopenia in SLE, damaged respiratory mucosal barrier in patients with GPA, urinary losses of immunoglobulins in the nephrotic state and even patient age (consider the younger lupus patient versus the older AAV patient). Indeed, when used in patients with RA, rituximab was associated with the same risk of severe infections as that of a placebo, yet the addition of rituximab to chemotherapy in patients with lymphoma increased the risk of fatal infections by 45% [22–25]. These influences make it difficult to attribute the risk of infections entirely to rituximab.
Rituximab therapy and its use in renal disease
Rituximab is increasingly used to treat a wide range of renal diseases, including AAV, idiopathic membranous nephropathy, MPGN, lupus nephritis, minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS) and antibody-mediated renal transplant rejection and as part of desensitizing regimens enabling ABO-/human leucocyte antigen (HLA)-incompatible kidney transplantation.
Cyclophosphamide remains the first-line treatment for life-threatening organ involvement in AAV. Rituximab is indicated in relapsing AAV, in disease resistant to cyclophosphamide or when cyclophosphamide is contraindicated, as is the case with urothelial malignancy or in patients of childbearing age. In the UK, ∼40% of patients with AAV will receive rituximab at some point in their disease course. This is equivalent to ∼800 patients every year. Based on the Rituximab for the Treatment of Wegener's Granulomatosis and Microscopic Polyangiitis (RAVE) trial, it is reasonable to conclude that rituximab is at least as safe as cyclophosphamide in the treatment of AAV and possibly more effective than cyclophosphamide in patients with relapsing disease [4].
Rituximab has been used to treat a variety of other glomerulonephritides. Reports of the adverse event rate following rituximab administration in idiopathic membranous nephropathy, lupus nephritis, MCD and FSGS are all encouraging. Ruggenenti et al. [26] described no treatment-related serious adverse events when treating 100 cases of idiopathic membranous nephropathy with rituximab. Similarly, Cravedi et al. [27] observed no infectious complications after rituximab administration in a smaller cohort of patients with resistant idiopathic membranous nephropathy. Rovin et al. [28] reported an increased frequency of neutropenia and leucopenia in a group of patients with lupus nephritis treated with rituximab. However, overall rates of serious adverse events, including infections, were similar in the rituximab and non-rituximab-treated groups. Terrier et al. [29] described severe infections in 9% of patients treated with rituximab for SLE, mostly occurring in the first 3 months. Finally, a recent systematic review of rituximab therapy for frequently relapsing/steroid-dependent MCD and FSGS also described rituximab as being well tolerated [30]. The infection rate appears to be higher in those with cryoglobulinaemia treated with rituximab. Terrier et al. [29] reported that 26% of those with non-viral cryoglobulinaemia treated with rituximab suffered severe infections. Infections occurred more often in those ≥70 years of age with essential type 2 mixed cryoglobulinaemia and renal impairment (eGFR <60 ml/min/1.73 m2) who were also receiving high-dose steroids. Additional studies are needed to determine rituximab’s safety profile in this patient group.
Schachtner et al. [31] reported that ABO-incompatible kidney transplant recipients with prior treatment with rituximab were more likely to develop cytomegalovirus (CMV) infection, BK virus–associated nephropathy and severe sepsis. Furthermore, ABO-incompatible kidney transplant recipients who had a poor HLA mismatch showed the highest rates of infection. Kamar et al. [32] found that more transplant recipients died as a consequence of an infectious disease when treated with rituximab (9.09% versus 1.55%). The predictive factors for death due to infection included the combined use of rituximab and anti-thymocyte globulin (ATG), age and bacterial and fungal infections [32]. However, Scemla et al. [33] and Kahwaji et al. [34] reported that rituximab treatment did not increase the risk of infections in highly sensitized renal transplant recipients. Further research is required to more clearly establish the infection risk of rituximab use in kidney transplantation.
Significant infections associated with rituximab therapy
Most infections encountered during rituximab treatment are secondary to bacterial pathogens. In this issue, Trivin et al. report that 79% of all infections in a cohort of patients receiving rituximab for glomerular disease were of bacterial origin. Pneumonia was the most commonly reported infection. Heusele et al [8] reported that more than half the patients that suffered a serious bacterial infection in their study had a history of a 48-h hospitalization in the last 90 days. Hospital-acquired infection may thus be an important cause of bacterial infection in this patient population.
Although rituximab predominantly depletes B-cells, rituximab is effective for conditions in which the evidence for the role of B-cells in pathogenesis is limited, including AAV, MCD and FSGS [30, 35]. Indeed, evidence suggests that B-cell–T-cell crosstalk is interrupted by rituximab and thus it can increase the risk of viral and fungal infections. Literature is limited in this regard.
The FDA issued a warning in 2013 that patients receiving treatment with rituximab have an increased risk of hepatitis B virus (HBV) reactivation [36]. They recommended that all patients should be screened for HBV prior to commencing treatment with rituximab [36]. The American Gastroenterological Association subsequently published a review of HBV reactivation with immunosuppressive therapy in 2015 [37]. They concluded that rituximab poses a high risk for HBV reactivation compared with other immunosuppressive agents [37]. However, this opinion was based primarily on reports of HBV reactivation in patients with haematological malignancies who received rituximab-based treatment regimens [37]. The risk of reactivation of HBV has been reported to be lower for patients receiving rituximab treatment for autoimmune conditions than those receiving rituximab for haematological conditions [38]. This may be a result of the differing adjunctive agents used in the management of these diseases.
Jain et al. [39] showed that the use of rituximab for stem cell transplantation was not associated with an increased risk of CMV reactivation. However, in a small cohort of AAV patients in the Rituximab versus Cyclophosphamide in ANCA-associated Vasculitis (RITUXIVAS) trial there were two cases of CMV infections [40]. In a large group of SLE patients, rituximab use was associated with a significantly increased incidence of herpes virus infection compared with placebo [41]. In patients with RA, the risk of fungal infections with rituximab appears to be very low; there were just seven cases reported in a study encompassing a sizeable 14 000 patient-year period [42]. The addition of rituximab to chemotherapy in lymphoma patients significantly increases the risk of Pneumocystis jiroveci pneumonia (PJP) infections [43]. In the RAVE trial, opportunistic infections were not reported in the rituximab arm but three fatal cases were seen in the cyclophosphamide arm [4]. Elsegeiny et al. [44] very elegantly demonstrate the effect of rituximab alone on T lymphocyte cytokines, as well as the role of T-lymphocytes in the development PJP infection. We do not see this effect in the RA population, highlighting that patient and disease factors are important in determining the risk of infection with rituximab treatment. Nonetheless, even in lymphoma patients, PJP prophylaxis is highly effective in preventing infection [43]. As the literature regarding the infection risk of individual rheumatic and renal conditions treated with rituximab is limited, one can postulate that the infection risk for AAV patients is intermediate between that of RA and lymphoma. Although reactivation of tuberculosis (TB) is common with tumour necrosis factor blockade, it is atypical mycobacterial infections that are reported with rituximab [45, 46]. In fact, rituximab has been successfully administered to RA patients with latent TB [45]. Other opportunistic infections such as cryptococcal meningitis are limited to individual case reports [47]. The overall risk of opportunistic infections with rituximab appears to be as low as 0.05 cases/patient-year, at least in the RA population [42].
Progressive multifocal leucoencephalopathy (PML) is a rare demyelinating disease of the human brain that results from lytic infection of oligodendrocytes caused by the reactivation of JC polyomavirus. It presents as progressive motor symptoms, cortical blindness, quadriparesis, coma and death. By the year 2011, the World Health Organization (WHO) collaborating centre for International Drug Monitoring Adverse Events Data Bank retrieved 182 cases of PML associated with the use of monoclonal antibodies, of which 114 cases were associated with the use of rituximab [48]. Although the majority of these cases were seen in haematological malignancies with confounding effects of other immunosuppressive drugs, there were six cases of RA [48]. As these numbers were more frequent than expected, risk mitigation strategies have been proposed. Carson et al. [49] described 57 patients with PML following rituximab treatment for a variety of conditions. The majority of patients had lymphoproliferative disorders. The other conditions included SLE, RA, idiopathic autoimmune pancytopenia and immune thrombocytopenia. They concluded that rituximab administration may increase the risk of developing PML but absolute risks are probably low. Certainly patients need to be warned about this very rare but fatal complication.
Minimizing the risk of infections during rituximab therapy
It is almost 20 years since the introduction of rituximab and undoubtedly the last decade has seen increased use of rituximab for a variety of indications. More information about adverse events is evolving. While it is certain that the use of rituximab will continue to increase, attention must be paid to reduce the risk of complications. We have a better understanding of the use of rituximab and the accompanying infectious risks for malignancies than we do for autoimmune disorders. In conditions such as RA, where the use of rituximab is more common, the evidence base is much stronger than it is for the use of rituximab for rarer conditions like MPGN, AAV and SLE [50]. However, rituximab appears to be much safer than other biologics used in rheumatic disease [51]. It is undeniable that the risk of infections cannot be entirely attributed to rituximab, and disease and patient factors are crucial as can be seen in the RA and lymphoma populations. National registries need to be created to collect information on the infectious complications associated with the use of rituximab in rare conditions. Until this information is available, a pragmatic approach should be taken to minimize the infection risk.
As rituximab starts binding to B-cells within hours of infusion, every opportunity should be taken to vaccinate patients for bacterial and viral antigens, including pneumococcus, influenza A and B and haemophilus influenza B (in combination with meningitis C), at least 4 weeks before the first dose of rituximab. Ideally, patients should also have completed a course of HBV vaccination prior to receiving rituximab, though in practice this can be challenging given the time period required for HBV vaccination. Although response to vaccination can be blunted after rituximab treatment, vaccination still offers partial protection [18]. Therefore, vaccination should be considered even after a patient has received rituximab therapy, although it should be delayed by 5–6 months [18]. Generally speaking, live vaccines such as the shingles vaccine are contraindicated in immunosuppressed patients; accidental administration has been described without adverse effects but cannot be recommended.
Panhypogammaglobulinaemia is a well-recognized effect of rituximab treatment, although the reduction in pathological autoantibodies seems to be far more marked than the decrease in overall antibody levels [52]. Immunoglobulin levels <400 mg/dL are associated with an increased risk of infections; therefore, baseline IgG levels should be checked prior to rituximab treatment [52]. If possible, other treatment strategies should be adopted in patients with hypogammaglobulinaemia. Currently there is no evidence that panhypogammaglobulinaemia is related to cumulative rituximab dose or that it is sustained. Prior exposure to cyclophosphamide may increase the risk of panhypogammaglobulinaemia. Less than 5% of patients may require intravenous immunoglobulin replacement to reduce infection risk [53].
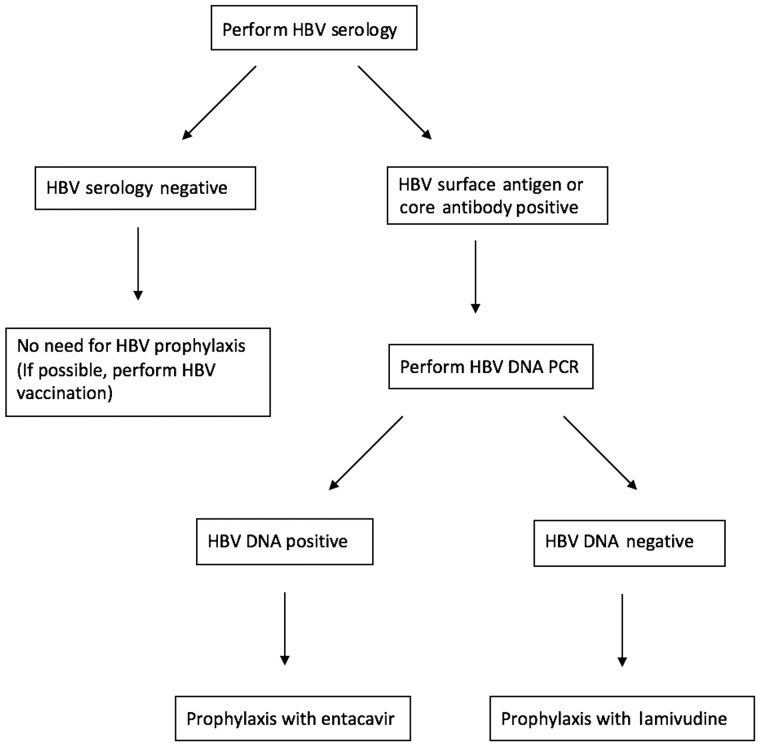
PJP prophylaxis has clearly been shown to be effective in the lymphoma population and is recommended [54]. Patients considered high risk for reactivation of TB can be considered for chemoprophylaxis. Patients should also be screened for HBV [37]. Prophylaxis with lamivudine or entacavir is effective in preventing reactivation [37]. Entacavir is recommended in patients with HBV DNA positivity [37]. Figure 1 illustrates an approach to minimizing HBV reactivation in patients receiving rituximab.
Fig. 1.
Algorithm for minimizing the risk of hepatitis B virus reactivation in patients receiving ritumaxib. PCR, polymerase chain reaction.
The safety of rituximab in children is yet to be established, but several case reports and series have been published [55]. Rituximab is contraindicated during pregnancy and contraception advice is mandatory. Chakravarty et al. [56] reported the outcomes of 231 pregnancies associated with maternal use of rituximab. The results were confounded by the use of other concomitant teratogenic drugs. There were several cases of spontaneous abortions, premature births, stillbirths and infections and there were two cases of congenital malformation.
As evidence suggests that certain risk factors, such as age, comorbidities and prior exposure to and concomitant use of other immunosuppressive drugs, are associated with an increased risk of infections, particular attention should be given to strategies to minimize modifiable risks. However, in some individuals it is not possible to modify risk. Therefore, the decision to treat with rituximab is only made provided the benefits of treatment outweigh these risks. Table 1 summarizes our management strategy for minimizing the risk of infectious complications during rituximab therapy.
Table 1.
Practice points for minimizing the risk of infectious complications during rituximab therapy
| Practice points | |
|---|---|
| 1. | Consider patient-specific risk factors prior to treatment:
|
| 2. | Screen for HBV prior to treatment
|
| 3. | Assess risk of TB reactivation and need for chemoprophylaxis:
|
| 4. | If possible, vaccinate for bacterial and viral pathogens, including:
|
| 5. | Prescribe prophylaxis for Pneumocystis jiroveci pneumonia |
| 6. | Contraceptive advice for both men and women |
| 7. | Monitor full blood count and immunoglobulins prior to and during treatment
|
| 8. | Remain vigilant for signs and symptoms suggestive of infection during treatment course |
Conclusion
Rituximab is highly effective in a variety of autoimmune disorders and has an established role in RA and AAV. However, the adverse effects are far from limited to the initially described ‘tolerable’ infusion reactions [1]. The risk of complications depends upon both the patient characteristics and the indication for rituximab use. Individualization of treatment is therefore important. An unanswered question is whether data published about rituximab can be extrapolated for other, newer B-cell-targeted therapies.
Conflict of interest statement
None declared.
References
- 1. Leget GA, Czuczman MS.. Use of rituximab, the new FDA-approved antibody. Curr Opin Oncol 1998; 10: 548–551 [DOI] [PubMed] [Google Scholar]
- 2. Edwards JC, Cambridge G.. Sustained improvement in rheumatoid arthritis following a protocol designed to deplete B lymphocytes. Rheumatology 2001; 40: 205–211 [DOI] [PubMed] [Google Scholar]
- 3. Turner-Stokes T, Lu TY, Ehrenstein MR. et al. The efficacy of repeated treatment with B-cell depletion therapy in systemic lupus erythematosus: an evaluation. Rheumatology 2011; 50: 1401–1408 [DOI] [PubMed] [Google Scholar]
- 4. Stone JH, Merkel PA, Spiera R. et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363: 221–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kado R, Sanders G, McCune WJ.. Suppression of normal immune responses after treatment with rituximab. Curr Opin Rheumatol 2016; 28: 251–258 [DOI] [PubMed] [Google Scholar]
- 6. Seyfizadeh N, Seyfizadeh N, Hasenkamp J. et al. A molecular perspective on rituximab: a monoclonal antibody for B cell non Hodgkin lymphoma and other affections. Crit Rev Oncol Hematol 2016; 97: 275–290 [DOI] [PubMed] [Google Scholar]
- 7. Hogan J, Avasare R, Radhakrishnan J.. Is newer safer? Adverse events associated with first-line therapies for ANCA-associated vasculitis and lupus nephritis. Clin J Am Soc Nephrol 2014; 9: 1657–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heusele M, Clerson P, Guery B. et al. Risk factors for severe bacterial infections in patients with systemic autoimmune diseases receiving rituximab. Clin Rheumatol 2014; 33: 799–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kelesidis T, Daikos G, Boumpas D. et al. Does rituximab increase the incidence of infectious complications? A narrative review. Int J Infect Dis 2011; 15: e2–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Daalen EE, Rizzo R, Kronbichler A. et al. Effect of rituximab on malignancy risk in patients with ANCA-associated vasculitis. Ann Rheum Dis 2016. (in press) [DOI] [PubMed] [Google Scholar]
- 11. Trivin C, Tran A, Moulin B. et al. Infectious complications of a rituximab-based immunosuppressive regimen in patients with glomerular disease. Clin Kidney J 2017; 10: 461–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu TY, Jonsdottir T, van Vollenhoven RF. et al. Prolonged B-cell depletion following rituximab therapy in systemic lupus erythematosus: a report of two cases. Ann Rheum Dis 2008; 67: 1493–1494 [DOI] [PubMed] [Google Scholar]
- 13. Venhoff N, Effelsberg NM, Salzer U. et al. Impact of rituximab on immunoglobulin concentrations and B cell numbers after cyclophosphamide treatment in patients with ANCA-associated vasculitides. PLoS One 2012; 7: e37626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Besada E, Koldingsnes W, Nossent JC.. Long-term efficacy and safety of pre-emptive maintenance therapy with rituximab in granulomatosis with polyangiitis: results from a single centre. Rheumatology 2013; 52: 2041–2047 [DOI] [PubMed] [Google Scholar]
- 15. Besada E. Low immunoglobulin levels increase the risk of severe hypogammaglobulinemia in granulomatosis with polyangiitis patients receiving rituximab. BMC Musculoskelet Disord 2016; 17: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tesfa D, Ajeganova S, Hagglund H. et al. Late-onset neutropenia following rituximab therapy in rheumatic diseases: association with B lymphocyte depletion and infections. Arthritis Rheum 2011; 63: 2209–2214 [DOI] [PubMed] [Google Scholar]
- 17. Salmon JH, Cacoub P, Combe B. et al. Late-onset neutropenia after treatment with rituximab for rheumatoid arthritis and other autoimmune diseases: data from the autoImmunity and rituximab registry. RMD Open 2015; 1: e000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bingham CO 3rd, Looney RJ, Deodhar A. et al. Immunization responses in rheumatoid arthritis patients treated with rituximab: results from a controlled clinical trial. Arthritis Rheum 2010; 62: 64–74 [DOI] [PubMed] [Google Scholar]
- 19. van der Kolk LE, Baars JW, Prins MH. et al. Rituximab treatment results in impaired secondary humoral immune responsiveness. Blood 2002; 100: 2257–2259 [PubMed] [Google Scholar]
- 20. Smolen JS, Keystone EC, Emery P. et al. Consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis 2007; 66: 143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ntatsaki E, Carruthers D, Chakravarty K. et al. BSR and BHPR guideline for the management of adults with ANCA-associated vasculitis. Rheumatology 2014; 53: 2306–2309 [DOI] [PubMed] [Google Scholar]
- 22. Salliot C, Dougados M, Gossec L.. Risk of serious infections during rituximab, abatacept and anakinra treatments for rheumatoid arthritis: meta-analyses of randomised placebo-controlled trials. Ann Rheum Dis 2009; 68: 25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hua Q, Zhu Y, Liu H.. Severe and fatal adverse events risk associated with rituximab addition to B-cell non-Hodgkin's lymphoma (B-NHL) chemotherapy: a meta-analysis. J Chemother 2015; 27: 365–370 [DOI] [PubMed] [Google Scholar]
- 24. Emery P, Deodhar A, Rigby WF. et al. Efficacy and safety of different doses and retreatment of rituximab: a randomised, placebo-controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (Study Evaluating Rituximab's Efficacy in MTX iNadequate rEsponders (SERENE)). Ann Rheum Dis 2010; 69: 1629–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Emery P, Fleischmann R, Filipowicz-Sosnowska A. et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum 2006; 54: 1390–1400 [DOI] [PubMed] [Google Scholar]
- 26. Ruggenenti P, Cravedi P, Chianca A. et al. Rituximab in idiopathic membranous nephropathy. J Am Soc Nephrol 2012; 23: 1416–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cravedi P, Sghirlanzoni MC, Marasa M. et al. Efficacy and safety of rituximab second-line therapy for membranous nephropathy: a prospective, matched-cohort study. Am J Nephrol 2011; 33: 461–468 [DOI] [PubMed] [Google Scholar]
- 28. Rovin BH, Furie R, Latinis K. et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the Lupus Nephritis Assessment with Rituximab study. Arthritis Rheum 2012; 64: 1215–1226 [DOI] [PubMed] [Google Scholar]
- 29. Terrier B, Launay D, Kaplanski G. et al. Safety and efficacy of rituximab in nonviral cryoglobulinemia vasculitis: data from the French Autoimmunity and Rituximab registry. Arthritis Care Res 2010; 62: 1787–1995 [DOI] [PubMed] [Google Scholar]
- 30. Kronbichler A, Kerschbaum J, Fernandez-Fresnedo G. et al. Rituximab treatment for relapsing minimal change disease and focal segmental glomerulosclerosis: a systematic review. Am J Nephrol 2014; 39: 322–330 [DOI] [PubMed] [Google Scholar]
- 31. Schachtner T, Stein M, Reinke P.. ABO desensitization affects cellular immunity and infection control after renal transplantation. Transpl Int 2015; 28: 1179–1194 [DOI] [PubMed] [Google Scholar]
- 32. Kamar N, Milioto O, Puissant-Lubrano B. et al. Incidence and predictive factors for infectious disease after rituximab therapy in kidney-transplant patients. Am J Transplant 2010; 10: 89–98 [DOI] [PubMed] [Google Scholar]
- 33. Scemla A, Loupy A, Candon S. et al. Incidence of infectious complications in highly sensitized renal transplant recipients treated by rituximab: a case-controlled study. Transplantation 2010; 90: 1180–1184 [DOI] [PubMed] [Google Scholar]
- 34. Kahwaji J, Sinha A, Toyoda M. et al. Infectious complications in kidney-transplant recipients desensitized with rituximab and intravenous immunoglobulin. Clin J Am Soc Nephrol 2011; 6: 2894–2900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shah S, Hruskova Z, Segelmark M. et al. Treatment of severe renal disease in ANCA positive and negative small vessel vasculitis with rituximab. Am J Nephrol 2015; 41: 296–301 [DOI] [PubMed] [Google Scholar]
- 36. Mitka M. FDA: increased HBV reactivation risk with ofatumumab or rituximab. JAMA 2013; 310: 1664. [DOI] [PubMed] [Google Scholar]
- 37. Perrillo RP, Gish R, Falck-Ytter YT.. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015; 148: 221–244 e3 [DOI] [PubMed] [Google Scholar]
- 38. Barone M, Notarnicola A, Lopalco G. et al. Safety of long-term biologic therapy in rheumatologic patients with a previously resolved hepatitis B viral infection. Hepatology 2015; 62: 40–46 [DOI] [PubMed] [Google Scholar]
- 39. Jain T, John J, Kotecha A. et al. Cytomegalovirus infection in autologous stem cell transplant recipients in the era of rituximab. Ann Hematol 2016; 95: 1323–1327 [DOI] [PubMed] [Google Scholar]
- 40. Jones RB, Tervaert JW, Hauser T. et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med 2010; 363: 211–220 [DOI] [PubMed] [Google Scholar]
- 41. Merrill JT, Neuwelt CM, Wallace DJ. et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum 2010; 62: 222–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Vollenhoven RF, Fleischmann RM, Furst DE. et al. Longterm safety of rituximab: final report of the Rheumatoid Arthritis Global Clinical Trial Program over 11 years. J Rheumatol 2015; 42: 1761–1766 [DOI] [PubMed] [Google Scholar]
- 43. Jiang X, Mei X, Feng D. et al. Prophylaxis and treatment of Pneumocystis jiroveci pneumonia in lymphoma patients subjected to rituximab-contained therapy: a systemic review and meta-analysis. PLoS One 2015; 10: e0122171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Elsegeiny W, Eddens T, Chen K. et al. Anti-CD20 antibody therapy and susceptibility to Pneumocystis pneumonia. Infect Immun 2015; 83: 2043–2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen YM, Chen HH, Lai KL. et al. The effects of rituximab therapy on released interferon-γ levels in the QuantiFERON assay among RA patients with different status of Mycobacterium tuberculosis infection. Rheumatology 2013; 52: 697–704 [DOI] [PubMed] [Google Scholar]
- 46. Lutt JR, Pisculli ML, Weinblatt ME. et al. Severe nontuberculous mycobacterial infection in 2 patients receiving rituximab for refractory myositis. J Rheumatol 2008; 35: 1683–1685 [PubMed] [Google Scholar]
- 47. Marchand T, Revest M, Tattevin P. et al. Early cryptococcal meningitis following treatment with rituximab, fludarabine and cyclophosphamide in a patient with chronic lymphocytic leukemia. Leuk Lymphoma 2013; 54: 643–645 [DOI] [PubMed] [Google Scholar]
- 48. Keene DL, Legare C, Taylor E. et al. Monoclonal antibodies and progressive multifocal leukoencephalopathy. Can J Neurol Sci 2011; 38: 565–571 [DOI] [PubMed] [Google Scholar]
- 49. Carson KR, Evens AM, Richey EA. et al. Progressive multifocal leukoencephalopathy after rituximab therapy in HIV-negative patients: a report of 57 cases from the Research on Adverse Drug Events and Reports project. Blood 2009; 113: 4834–4840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Buch MH, Smolen JS, Betteridge N. et al. Updated consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis 2011; 70: 909–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Singh JA, Cameron C, Noorbaloochi S. et al. Risk of serious infection in biological treatment of patients with rheumatoid arthritis: a systematic review and meta-analysis. Lancet 2015; 386: 258–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cortazar FB, Pendergraft WF 3rd, Wenger J. et al. The effect of continuous B cell depletion with rituximab on pathogenic autoantibodies and total IgG levels in ANCA vasculitis. Arthritis Rheumatol 2017; 69: 1045–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Roberts DM, Jones RB, Smith RM. et al. Rituximab-associated hypogammaglobulinemia: incidence, predictors and outcomes in patients with multi-system autoimmune disease. J Autoimmun 2015; 57: 60–65 [DOI] [PubMed] [Google Scholar]
- 54. Maertens J, Cesaro S, Maschmeyer G. et al. ECIL guidelines for preventing pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother 2016; 71: 2397–2404 [DOI] [PubMed] [Google Scholar]
- 55. Niu XL, Hao S, Wang P. et al. Single dose of rituximab in children with steroid-dependent minimal change nephrotic syndrome. Biomed Rep 2016; 5: 237–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chakravarty EF, Murray ER, Kelman A. et al. Pregnancy outcomes after maternal exposure to rituximab. Blood 2011; 117: 1499–1506 [DOI] [PubMed] [Google Scholar]