Abstract
Epstein-Barr virus (EBV) DNA has been recognized as a promising tumor marker for nasopharyngeal carcinoma (NPC). This study aims to demonstrate the prevalence of plasma EBV DNA and its temporal correlation with treatment outcomes in the modern era. A total of 204 patients with Stage I–IVB NPC treated with intensity-modulated radiotherapy (IMRT) were enrolled. Quantitative plasma EBV DNA measurement was performed before treatment (pre-IMRT), on the fifth week of radiation (mid-IMRT), at 3 months after radiation (post-IMRT), then every 6 months until disease relapse. Progression-free survival (PFS) and overall survival (OS) were analyzed using the Kaplan–Meier method. Plasma EBV DNA was detected in 110 patients (53.9%), with a median pre-IMRT EBV DNA level of 8005 copies/ml. Significant correlation was noted between pre-IMRT EBV DNA level and disease stage, but not between pre-IMRT EBV DNA level and World Health Organization classification. With a median follow-up time of 35.1 months, the 3-year PFS and OS rates were higher in the group with undetectable pre-IMRT EBV DNA level compared with in the group in which it was detectable. When classified according to disease stage and pre-IMRT EBV DNA, patients with early disease and detectable pre-IMRT EBV DNA experienced poorer survival than those with locally advanced disease and undetectable pre-IMRT EBV DNA. According to the dynamic changes in EBV DNA level between pre-IMRT and mid/post IMRT, survival was significantly higher in patients who achieved an undetectable level following treatment. On multivariate analysis, post-IMRT EBV DNA level was the strongest predictor of all treatment outcomes (P < 0.001). Our study demonstrated the clinical significance of the plasma EBV DNA level at specific time points, as well as of the dynamic changes in the EBV DNA level. Disappearance of plasma EBV DNA after treatment was associated with better survival.
Keywords: EBV DNA, nasopharyngeal carcinoma, intensity-modulated radiotherapy, IMRT, predictor, dynamic change
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is an endemic head and neck malignancy in the Asian population. In Thailand, the annual age standardized rate is ~2.8 and ~0.9 per 100 000 in males and females, respectively. [1, 2] In addition to genetic and environmental factors, Epstein-Barr virus (EBV) infection has been strongly associated with the etiology of NPC [3–7]. Studies have demonstrated that when EBV DNA is detectable in tumor tissue, it is also detectable in patients’ serum/plasma. Thus the serum/plasma detection of EBV DNA indicates the presence of tumor EBV DNA [8, 9].
The quantitative measurement of EBV DNA in plasma using real-time quantitative polymerase chain reaction (RTQ-PCR) has illustrated a correlation between EBV DNA concentration and disease stage; thus, it can be implied that the level of circulating EBV DNA may reflect the tumor burden [10–15]. In addition, many studies have explored the value of determining the plasma EBV DNA level for a range of clinical applications: as a promising marker for tumor detection, for disease monitoring and for prognosis for NPC [11, 13, 14, 16–21].
However, these studies have involved different treatments, including a variety of radiotherapy techniques, radiation dose and schedule, and chemotherapy regimens. Hence, the treatment outcomes may differ from those achieved with treatments currently practised. Additionally, the data regarding temporal changes in plasma EBV DNA level in NPC patients is limited. The goal of this study was to determine the prevalence of plasma EBV DNA detection among NPC patients and its temporal correlation with treatment outcomes in the modern treatment era. Furthermore, we aimed to investigate the prognostic value of EBV DNA level for risk stratification and survival outcomes.
MATERIALS AND METHODS
Patients and treatments
Data was obtained from the medical records of Stage I–IVB NPC patients. The inclusion criteria included treatment with definitive radiotherapy (RT) utilizing intensity-modulated radiotherapy (IMRT) with or without chemotherapy and plasma EBV DNA evaluation before, during and after treatment. Those with recurrent disease were excluded. Demographic data (including age, sex and Karnofsky Performance Status) were recorded. The histopathological subtype and tumor staging were reclassified using recent World Health Organization (WHO) classification [22] and the American Joint Committee on Cancer Staging 2010, 7th edition, respectively. Pre-treatment evaluations and scheduled follow-up were performed according to institutional guidelines. Plasma EBV DNA was tested before starting treatment (pre-IMRT), on the fifth week of radiation (mid-IMRT), at 3 months after the radiation (post-IMRT), then every 6 months afterward.
Treatment
Computed tomography simulation using a long thermoplastic mask was performed in all patients. Magnetic Resonance Imaging (MRI) fusion or simulation was optional. Radiation Therapy Oncology Group guidelines were applied for target delineation. High-risk planning target volume (PTV-HR) was defined as the primary tumor and pathologic lymph node with an appropriate margin, and low-risk PTV (PTV-LR) was defined as PTV-HR plus the elective lymph node region. The treatment consisted of definitive RT with a total dose of 70–74 Gy and 50–56 Gy in 33–35 fractions to the PTV-HR and PTV-LR, respectively, in combination with or without concurrent chemotherapy, depending on clinical scenarios. Various kinds of concurrent chemotherapy regimens included weekly cisplatin (n = 169) or carboplatin (n = 8) and triweekly cisplatin (n = 12) or carboplatin (n = 8). Adjuvant cisplatin or carboplatin with 5-fluorouracil was administered in 190 patients (93.1%), with a median of three cycles.
Quantitative measurement of plasma EBV DNA level
DNA extraction from plasma was performed using the QIAmp DNA Blood Mini Kit (Qiagen, Germany). Plasma DNA samples were quantified for EBV DNA using a RTQ-PCR system targeting the BamHI-W fragment region of the EBV genome and 2X TaqMan reagent (Roche). Amplification was carried out using a LightCycler® 2.0 Real-Time PCR system (Roche Applied Science). A plasma EBV DNA concentration of <600 copies/ml was defined as an undetectable level in our institution.
Evaluation of the plasma EBV DNA level at various time points
The plasma EBV DNA level was recorded according to the time of treatment as divided into four phases as follows: pre-IMRT, mid-IMRT, post-IMRT, and plasma EBV DNA level during follow-up or at the time of disease relapse (relapse-EBV DNA).
Dynamic changes in the plasma EBV DNA level were classified into four categories: (i) undetectable pre-IMRT EBV DNA followed by undetectable mid-/post-IMRT EBV DNA (Upre–Umid/post); (ii) undetectable pre-IMRT EBV DNA followed by detectable mid-/post-IMRT EBV DNA (Upre–Dmid/post); (iii) detectable pre-IMRT EBV DNA followed by undetectable mid-/post-IMRT EBV DNA (Dpre–Umid/post); and (iv) detectable pre-IMRT EBV DNA and persistently detectable mid-/post-IMRT EBV DNA (Dpre–Dmid/post).
Statistical analyses
Distant metastasis-free survival (DMFS), progression-free survival (PFS) and overall survival (OS) were analyzed using the Kaplan–Meier method and the log-rank test. A univariate logistic regression model was used to examine the association between clinical factors, EBV status and survival outcomes. A multivariate Cox regression model and the Chi-square test were used to determine the correlation between plasma EBV DNA and treatment outcomes. Factors with a P-value of ≤0.25 in the univariate analysis were entered into the multivariate Cox regression model. All tests were two-sided, and a P-value of <0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS statistics (version 22.0, SPSS Inc., Chicago, Ill).
RESULTS
Between March 2010 and September 2015, a total of 204 patients met the inclusion criteria. The median age was 49 years (range, 11–78 years) and 73.5% were male. Approximately 82% of the patients had non-keratinizing undifferentiated squamous cell carcinoma subtypes. There were 110 patients (53.9%) who had an initially detectable plasma EBV DNA level, with a median baseline EBV DNA level of 8005 copies/ml (range, 817–281 000). Patient and tumor characteristics are shown in Table 1.
Table 1.
Patient demographic and baseline characteristics
| Characteristics | All (n = 204) | Initial plasma EBV DNA status | ||
|---|---|---|---|---|
| Undetectable (n = 94) | Detectable (n = 110) | P-value | ||
| Age, median (range), years | 49 (11–78) | 47 (19–78) | 51 (11–75) | 0.484 |
| Sex | 0.874 | |||
| Male | 150 (73.5%) | 70 (74.5%) | 80 (72.7%) | |
| Female | 54 (26.5%) | 24 (25.5%) | 30 (27.3%) | |
| Karnofsky performance status | 0.211 | |||
| ≥90 | 202 (99%) | 92 (97.9%) | 110 (100%) | |
| <90 | 2 (1%) | 2 (2.1%) | ||
| Histology (WHO classification) | 0.536 | |||
| Type I (keratinizing SCCAa) | 3 (1.5%) | 1 (1.1%) | 2 (1.8%) | |
| Type IIA (NKa, differentiated SCCAa) | 21 (10.3%) | 9 (9.6%) | 12 (10.9%) | |
| Type IIB (NKa, undifferentiated SCCAa) | 178 (87.2%) | 82 (87.2%) | 96 (87.3%) | |
| Type III (basaloid SCCAa) or other | 2 (1%) | 2 (2.1%) | ||
| T stage | 0.007 | |||
| 1–2 | 118 (57.8%) | 64 (68.1%) | 54 (49.1%) | |
| 3–4 | 86 (42.2%) | 30 (31.9%) | 56 (50.9%) | |
| N stage | 0.051 | |||
| 0–1 | 64 (31.4%) | 36 (38.3%) | 28 (25.5%) | |
| 2–3 | 140 (80.6%) | 58 (61.7%) | 82 (74.5%) | |
| AJCCa stage grouping | 0.036 | |||
| I | 2 (1%) | 2 (2.1%) | ||
| II | 31 (15.2%) | 21 (22.3%) | 10 (9.1%) | |
| III | 112 (54.9%) | 47 (50%) | 65 (59.1%) | |
| IVA | 38 (18.6%) | 15 (16%) | 23 (20.9%) | |
| IVB | 21 (10.3%) | 9 (9.6%) | 12 (10.9%) | |
aFisher's exact test; NK = non-keratinizing, SCCA = squamous cell carcinoma, AJCC = American Joint Committee on Cancer.
Plasma EBV DNA level correlated with disease and treatment outcomes
The median values of pre-IMRT EBV DNA levels were 2755 copies/ml (range, 2350–3160), 10 455 copies/ml (range, 2100–281 000) and 8005 copies/ml (range, 817–281 000) for WHO Type I, IIA and IIB, respectively (P-value = 0.019).
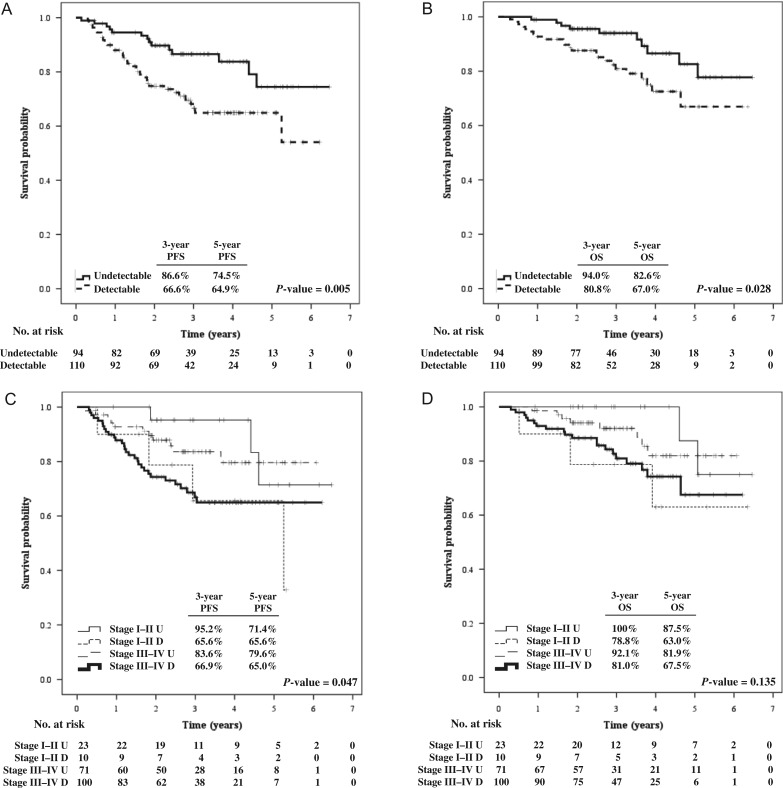
A total of 33 patients experienced disease failure: 10 patients (10.6%) in the undetectable pre-IMRT EBV DNA group and 23 patients (20.9%) in the group in which pre-IMRT EBV DNA was detectable (P = 0.057). With a median follow-up time of 35.1 months (range, 1.6–77.4 months), the 3-year PFS and OS rates in the undetectable group were 86.6% and 94%, while they were 66.6% and 80.8% in the detectable group, respectively (P-value = 0.005 for PFS and 0.028 for OS). The corresponding estimated 5-year PFS and OS rates were 74.5% and 82.6% versus 64.9% and 67%, as demonstrated in Fig. 1A and 1B. Similar significant findings were demonstrated in the temporal changes in plasma EBV DNA level, with the strongest association shown in the post-IMRT EBV DNA. The estimated 3- and 5-year OS rates in patients with undetectable post-IMRT EBV DNA were 89.7% and 76.1%, but only 18.8% and 0% in the group with detectable post-IMRT EBV DNA (P-value < 0.001).
Fig. 1.
Progression-free survival (A) and overall survival (B) according to pre-treatment plasma EBV DNA level and progression-free survival (C) and overall survival (D) according to staging and pre-treatment plasma EBV DNA level. PFS = progression-free survival; OS = overall survival, U = undetectable, D = detectable.
We classified patients according to stage and pre-IMRT EBV DNA status into four different groups: early stage with undetectable (Stage I–II U) and detectable pre-IMRT EBV DNA (Stage I–II D), and locally advanced stage with undetectable (Stage III–IV U) and detectable pre-IMRT EBV DNA (Stage III–IV D). We found a significant difference in PFS (P = 0.047), but not in OS (P = 0.135), as shown in Fig. 1C and 1D.
Prognosis based on dynamic changes in plasma EBV DNA level
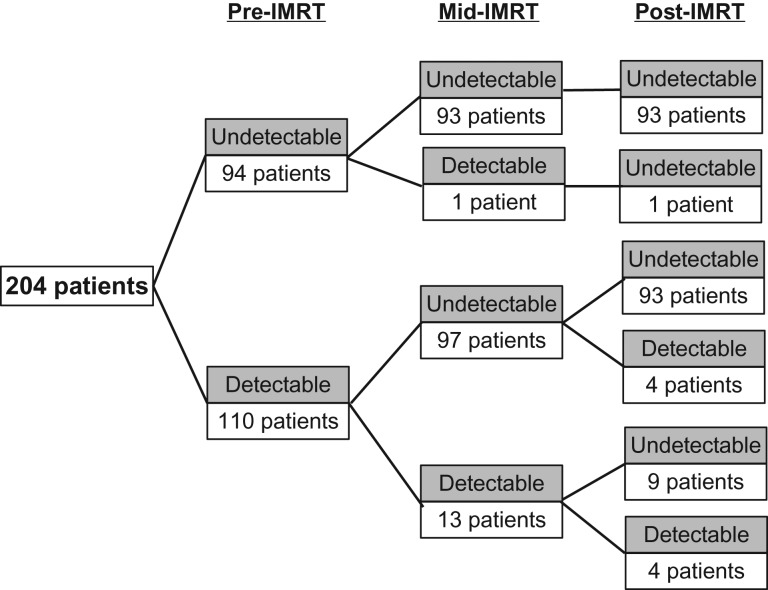
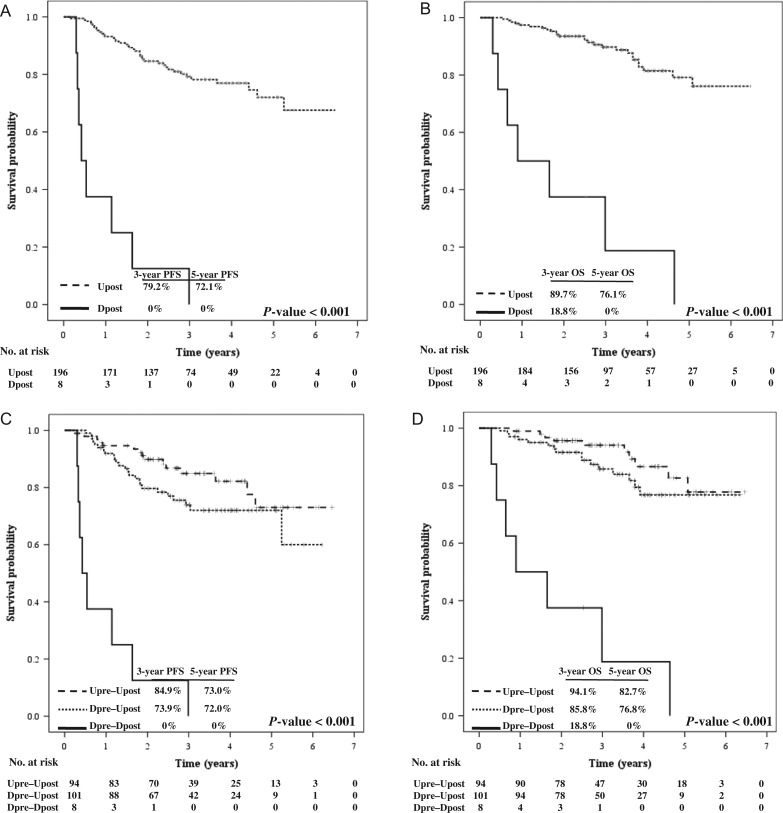
Figure 2 demonstrates the dynamic changes in the plasma EBV DNA level in 204 patients. The majority of patients achieved undetectable mid- and post-IMRT EBV DNA after treatment. Persistently detectable mid-IMRT EBV DNA (Dpre–Dmid) was found in 13 patients (11.8% of the detectable pre-IMRT EBV DNA group). Nine of them achieved an undetectable level at 3 months after treatment, and all were alive at the time of analysis. The other four patients who had persistently detectable post-IMRT EBV DNA (Dpre–Dmid–Dpost) died at the median time of 6.4 months (range, 3.6–30.3 months). Four patients had the reverse pattern (Dpre–Umid–Dpost): three of them developed distant metastasis at 4.3, 6.4 and 19.5 months, the other died from non-cancer cause at 35.7 months after treatment. The 3-year PFS rates were 36.1%, 70.5% and 86.5% in Dpre–Dmid, Dpre–Umid and Upre–Umid (P-value < 0.001), corresponding with 3-year OS rates of 76.9%, 81.3% and 93.9%, respectively (P-value = 0.061). According to post-IMRT EBV DNA status, the 3-year PFS rates were 73.9% and 84.9% in Dpre–Upost and Upre–Upost, respectively (P-value < 0.001). The corresponding 3-year OS rates were 85.8% and 94.1%, respectively (P-value < 0001). Survival according to the dynamic changes in post-IMRT EBV DNA level is illustrated in Fig. 3. Relapse-EBV DNA level was elevated in 23 of 28 evaluable patients, with a median level of 13 300 copies/ml (range, 1310–144 000).
Fig. 2.
Dynamic changes in plasma EBV DNA level in 204 nasopharyngeal carcinoma patients.
Fig. 3.
PFS (A) and OS (B) according to plasma EBV DNA level post-treatment, and PFS (C) and OS (D) according to dynamic change in plasma EBV DNA level post-treatment. PFS = progression-free survival; OS = overall survival, U = undetectable, D = detectable; Upre = EBV DNA undetectable pre-treatment, Upost = EBV DNA undetectable post-treatment, Dpre = EBV DNA undetectable pre-treatment, Dpost = EBV DNA undetectable post-treatment.
Univariate and multivariate analysis
Binary logistic regression model analysis revealed that T-stage and detectable plasma EBV DNA at any time point were significant prognostic factors for distant failure in univariate analysis. Advanced T-stage (T3–4) was the worse prognostic factor for distant metastasis and PFS. Detectable post-IMRT EBV DNA was the significant predictor of distant metastasis, PFS and OS (P-value < 0.001). Advanced age was a poor prognostic indicator for both survival outcomes (Table 2).
Table 2.
Univariate and multivariate analysis of variables correlated with distant metastasis, progression-free survival and overall survival
| Distant metastasis | Progression-free survival | Overall survival | ||||
|---|---|---|---|---|---|---|
| Characteristics | UVA | MVA | UVA | MVA | UVA | MVA |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Age (continuous value) | 1.013 (0.979–1.049) | 1.034 (1.007–1.061) | 1.036 (1.008–1.065) | 1.058 (1.026–1.091) | 1.074 (1.040–1.109) | |
| Sex (female) | 1.119 (0.473–2.648) | 1.935 (0.906–4.136) | 1.486 (0.679–3.254) | 1.645 (0.679–3.986) | ||
| WHO, IIB VS others | 0.559 (0.211–1.476) | 0.740 (0.332–1.649) | 1.013 (0.356–2.886) | |||
| T stage, T1–T2 vs T3–T4 | 0.237 (0.104–0.544) | 0.537 (0.348–0.828) | 0.671 (0.462–0.824) | 0.415 (0.223–0.773) | 0.680 (0.482–0.960) | 0.764 (0.534–1.091) |
| N stage, N0–N2 vs N3 | 0.748 (0.316–1.769) | 0.917 (0.672–1.253) | 1.045 (0.727–1.502) | |||
| Detectable pre-IMRT EBV DNAa | 2.683 (1.134–6.348) | 1.538 (0.611–3.876) | 2.357 (1.263–4.397) | 1.445 (0.736–2.836) | 2.256 (1.070–4.753) | 1.450 (0.644–3.265) |
| Detectable mid-IMRT EBV DNAa | 4.338 (1.641–11.472) | 1.657 (0.571–4.803) | 3.717 (1.658–8.333) | 1.457 (0.597–3.554) | 2.660 (0.927–7.632) | |
| Detectable post-IMRT EBV DNAa | 20.806 (8.258–52.420) | 14.331 (5.124–40.078) | 16.011 (1.309–35.071) | 12.167 (5.133–28.840) | 13.109 (5.631–30.519) | 16.956 (6.511–44.157) |
UVA = univariate analysis, MVA = multivariate analysis, HR = hazard ratio, CI = confidence interval, WHO = World Health Organization classification, pre-IMRT EBV DNA = plasma EBV DNA level before treatment, mid-IMRT EBV DNA = plasma EBV DNA level on the fifth week of radiation, post-IMRT EBV DNA = plasma EBV DNA level at 3 months after completion of treatment. aDetectable versus undetectable plasma EBV DNA.
DISCUSSION
We have clearly demonstrated the significance of the plasma EBV DNA level as a useful tumor marker in NPC patients, for disease monitoring and for prognosis. We used pre- and post-treatment plasma EBV DNA levels, and the dynamic changes in the level during and after treatment, for these purposes. We also performed risk stratification at initial presentation by considering pre-treatment plasma EBV DNA in conjunction with disease staging. This strategy could lead to appropriate treatment selection in an individual patient according to initial risk of tumor recurrence and response to treatment.
Plasma EBV DNA level has been demonstrated as a useful tool as a reliable tumor marker for NPC owing to its high sensitivity and specificity [7, 11, 19, 21, 23, 24]. Using the RTQ-PCR commercial kit, we found that the prevalence of a detectable plasma EBV DNA level in our NPC patients was 53.9%, with the median EBV DNA level being 8005 copies/ml. Previous studies have revealed high detection rates, varying from 70–96%, with the median EBV DNA concentration ranging from 573–21 058 copies/ml [14, 15, 20, 25, 26]. The relatively low detection rate in our study might be due to different PCR techniques and the low sensitivity of our test, which specified >600 copies/ml of EBV DNA concentration as determining detectable status.
According to recent meta-analysis proposing a systematic risk stratification model using plasma EBV DNA level at different time points [26], we classified our patients based on disease staging and pre-treatment plasma EBV DNA level and found that early stage NPC patients with undetectable pre-EBV had the best prognosis, while those with locally advanced disease and detectable pre-EBV had the worst survival. Surprisingly, patients with early disease but detectable pre-EBV experienced poorer PFS and OS than those with locally advanced disease but undetectable pre-EBV. This indicated the significance of pre-treatment EBV DNA level combined with disease stage in terms of prognostication, and may be justification for future treatment based on initial disease presentation; however, further validation in larger prospective studies is required.
A study of 107 NPC patients revealed that detectable mid-IMRT EBV DNA, defined as the plasma EBV DNA level at completion of 4 weeks of chemoradiation, was associated with an unfavorable treatment response and reduced OS [25]. We similarly discovered worse outcomes in the persistent mid-IMRT EBV DNA group. Notably, there was one patient whose mid-IMRT EBV DNA was detectable, despite undetectable EBV DNA at the beginning; plasma EBV DNA later became undetectable, and the patient remained in disease remission for 22.6 months (the time of last follow-up). A falsely positive result is one plausible explanation concerning this patient's data.
Moreover, the present study demonstrated the clinical significance of dynamic changes in plasma EBV DNA level along the treatment course. We revealed that disappearance of plasma EBV DNA, both mid- and post-IMRT EBV DNA levels, was associated with better survival and reflected a favorable treatment response. However, when post-IMRT EBV DNA level was incorporated into the multivariate analysis, mid-IMRT EBV DNA level failed to show its significance because of the greater predictive value of post-IMRT EBV DNA for DMFS, PFS and OS. This emphasized the importance of post-treatment plasma EBV clearance in association with clinical remission in patients who have initially detectable EBV DNA [20, 27]. Thus, attempts to modify patient treatment according to dynamic changes in plasma EBV DNA and tumor response might be made. For example, adjuvant chemotherapy might be offered or intensified in high-risk patients whose post-IMRT EBV DNA has sustained a detectable level. On the other hand, low-risk patients who had initially undetectable pre-IMRT EBV DNA and a favorable treatment response might not gain any benefit from adjuvant treatment; as a result, side effects from chemotherapy could be avoided in these patients. However, further studies on individualized treatment in a large prospective randomized study are awaited. An ongoing randomized study (NCT02135042) in NPC patients is investigating individualized treatment based on post-treatment EBV DNA status, defined as the plasma EBV DNA level at 1 week after chemoradiation.
The limitations of our study are its retrospective nature and short follow-up time. However, the advantages of this study are the modern IMRT technique uniformly performed and the use of a commercial kit for RTQ-PCR (available in many hospitals, offsetting the relatively low sensitivity test).
CONCLUSION
The current study has confirmed the significance of plasma EBV DNA as a promising tumor marker for detection, monitoring and prognosis in NPC patients. We reported the detection rate of plasma EBV DNA in Thai NPC patients. The pre-, mid- and post-treatment plasma EBV DNA levels, as well as the dynamic changes, were observed to be significant predictors of treatment outcomes. As a result, measurement of the plasma EBV DNA level is to be encouraged in routine clinical practice. Personalized treatments based on risk stratification and treatment response are awaited in the future trial.
CONFLICT OF INTEREST
The authors have declared that there are no conflicts of interest.
FUNDING
This work was supported by the Ratchadapiseksompotch Fund, Faculty of Medicine, Chulalongkorn University (grant number RA8/54).
ACKNOWLEDGEMENTS
Results from this study were presented orally at the first meeting of the Federation of Asian Organizations for Radiation Oncology, 25–27 November, 2016, Kyoto, Japan.
REFERENCES
- 1. Curado MP, Edwards B, Shin HR, et al. Nasopharyngeal Carcinoma. Geneva: WHO Press,2007. [Google Scholar]
- 2. National Cancer Institute Thailand; In Imsamran W, Chaiwerawattana A, Wiangnon S, et al.. Cancer in Thailand. Vol. VIII, 2010–2012 Bangkok: New Thammada Press,2015;18–20. [Google Scholar]
- 3. Hildesheim A, Levine PH. Etiology of nasopharyngeal carcinoma: a review Epidemiol Rev 1993;15:466–85. [DOI] [PubMed] [Google Scholar]
- 4. Vasef MA, Ferlito A, Weiss LM. Nasopharyngeal carcinoma, with emphasis on its relationship to Epstein-Barr virus. Ann Otol Rhinol Laryngol 1997;106:348–56. [DOI] [PubMed] [Google Scholar]
- 5. Young LS, Dawson CW, Clark D, et al.. Epstein-Barr virus gene expression in nasopharyngeal carcinoma. J Gen Virol 1998;69:1051–65. [DOI] [PubMed] [Google Scholar]
- 6. Niemhom S, Kitazawa S, Murao S, et al. Co-expression of p53 and bcl-2 may correlate to the presence of Epstein-Barr virus genome and the expression of proliferating cell nuclear antigen in nasopharyngeal carcinoma. Cancer Lett 2000;160:199–208. [DOI] [PubMed] [Google Scholar]
- 7. Dolcetti R, Menezes J.. Epstein-Barr virus and undifferentiated nasopharyngeal carcinoma: new immunobiological and molecular insights on a long-standing etiopathogenic association. Adv Cancer Res 2003;87:127–57. [DOI] [PubMed] [Google Scholar]
- 8. Chang YS, Tyan YS, Liu ST, et al. Detection of Epstein-Barr virus DNA sequences in nasopharyngeal carcinoma cells by enzymatic DNA amplification. J Clin Microbiol 1990;28:2398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mutirangura A, Pornthanakasem W, Theamboonlers A, et al. Epstein-Barr viral DNA in serum of patients with nasopharyngeal carcinoma. Clin Cancer Res 1998;4:665–9. [PubMed] [Google Scholar]
- 10. Lo YM, Chan LY, Chan AT, et al. Quantitative and temporal correlation between circulating cell-free Epstein-Barr virus DNA and tumor recurrence in nasopharyngeal carcinoma. Cancer Res 1999;59:5452–5. [PubMed] [Google Scholar]
- 11. Chan KC, Lo YM. Circulating EBV DNA as a tumor marker for nasopharyngeal carcinoma. Semin Cancer Biol 2002;12:489–96. [DOI] [PubMed] [Google Scholar]
- 12. Leung SF, Zee B, Ma BB, et al. Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol 2006;24:5414–8. [DOI] [PubMed] [Google Scholar]
- 13. Shotelersuk K, Khorprasert C, Sakdikul S, et al. Epstein-Barr Virus DNA in serum/plasma as a tumor marker for nasopharyngeal cancer. Clin Cancer Res 2006;6:1046–51. [PubMed] [Google Scholar]
- 14. Chai SJ, Pua KC, Saleh A, et al. Clinical significance of plasma Epstein-Barr Virus DNA loads in a large cohort of Malaysian patients with nasopharyngeal carcinoma. J Clin Virol 2012;55:34–9. [DOI] [PubMed] [Google Scholar]
- 15. Lo YM, Chan LY, Lo KW, et al. Quantitative analysis cell-free Epstein-Barr virus DNA in plasma of patients with nasopharyngeal carcinoma. Cancer Res 1999;59:1188–91. [PubMed] [Google Scholar]
- 16. Lo YM, Chan AT, Chan LY, et al. Molecular prognostication of nasopharyngeal carcinoma by quantitative analysis of circulating Epstein-Barr virus DNA. Cancer Res 2000;60:6878–81. [PubMed] [Google Scholar]
- 17. Lo YM. Prognostic implication of pretreatment plasma/serum concentration of Epstein-Barr virus DNA in nasopharyngeal carcinoma. Biomed Pharmacother 2001;55:362–5. [DOI] [PubMed] [Google Scholar]
- 18. Leung SF, Chan AT, Zee B, et al. Pretherapy quantitative measurement of circulating Epstein-Barr virus DNA is predictive of posttherapy distant failure in patients with early-stage nasopharyngeal carcinoma of undifferentiated type. Cancer 2003;98:288–91. [DOI] [PubMed] [Google Scholar]
- 19. Tan EL, Looi LM, Sam CK. Evaluation of plasma Epstein-Barr virus DNA load as a prognostic marker for nasopharyngeal carcinoma. Singapore Med J 2006;47:803–7. [PubMed] [Google Scholar]
- 20. Lin JC, Wang WY, Liang WM, et al. Long-term prognostic effects of plasma EBV DNA by minor groove binder-probe real-time quantitative PCR on nasopharyngeal carcinoma patients receiving concurrent chemoradiotherapy. Int J Radiat Oncol Biol Phys 2007;68:1342–8. [DOI] [PubMed] [Google Scholar]
- 21. Han BL, Xu XY, Zhang CZ, et al. Systematic review on Epstein-Barr virus (EBV) DNA in diagnosis of nasopharyngeal carcinoma in Asian populations. Asian Pac J Cancer Prev 2012;13:2577–81. [DOI] [PubMed] [Google Scholar]
- 22. Thompson LD. Update on nasopharyngeal carcinoma. Head Neck Pathol 2007;1:81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wei W, Huang Z, Li S, et al. Pretreatment Epstein-Barr virus DNA load and cumulative cisplatin dose intensity affect long-term outcome of nasopharyngeal carcinoma treated with concurrent chemotherapy: experience of an institute in an endemic area. Oncol Res Treat 2014;37:88–95. [DOI] [PubMed] [Google Scholar]
- 24. Yip TT, Ngan RK, Fong AH, et al. Application of circulating plasma/serum EBV DNA in the clinical management of nasopharyngeal carcinoma. Oral Oncol 2014;50:527–38. [DOI] [PubMed] [Google Scholar]
- 25. Leung SF, Chan KC, Ma BB, et al. Plasma Epstein-Barr viral DNA load at midpoint of radiotherapy course predicts outcome in advanced-stage nasopharyngeal carcinoma. Ann Oncol 2014;25:1204–8. [DOI] [PubMed] [Google Scholar]
- 26. Zhang W, Chen Y, Chen L, et al. The clinical utility of plasma Epstein-Barr virus DNA assays in nasopharyngeal carcinoma: the dawn of a new era?: a systematic review and meta-analysis of 7836 cases. Medicine (Baltimore) 2015;94:e845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shao JY, Li YH, Gao HY, et al. Comparison of plasma Epstein-Barr virus (EBV) DNA levels and serum EBV immunoglobulin A/virus capsid antigen antibody titers in patients with nasopharyngeal carcinoma. Cancer 2004;100:1162–70. [DOI] [PubMed] [Google Scholar]