Abstract
We sought to investigate the long-term outcomes after radical prostatectomy (RP) and external-beam radiation therapy (EBRT) for the treatment of localized prostate cancer in Japanese patients. RP and radiation therapy are curative treatments for localized prostate cancer. However, there is controversy around which treatment is superior in Japanese patients. The aim of our retrospective study was to compare the long-term clinical outcomes of each treatment. We retrospectively evaluated the overall survival (OS), cancer-specific survival (CSS) and biochemical failure–free survival (BFS) for patients who had been diagnosed with localized prostate cancer and treated with RP (n = 248) or conventional 2D or 3D-CRT EBRT (n = 182) between 1995 and 2009. The median OS was superior in the RP group compared with that in EBRT group (P < 0.001), although CSS was comparable for both treatment groups; BFS was superior for the EBRT group compared with that for the RP group (P = 0.04). Univariate analysis identified a prostate-specific antigen count (PSA)of ≥20 vs <20 mg/ml, clinical T-stage of the tumor and Gleason score as predictors for CSS. However, multivariate analysis did not identify a factor for CSS. Subgroup analysis was also performed based on clinical T stage, PSA and Gleason score, but there was no difference in each subgroup between RP and EBRT. Both treatments provided satisfactory clinical outcomes in terms of disease control in localized prostate cancer.
Keywords: external-beam radiation therapy, localized prostate cancer, overall survival, radical prostatectomy, retrospective study
INTRODUCTION
The prevalence of prostate cancer (PCa) in Japan has increased following the introduction of serum prostate-specific antigen (PSA) measurement, with most patients being diagnosed with localized disease. Despite early identification, the survival rate for PCa has not improved in Japan [1]. PCa is the sixth leading cause of cancer death in Japanese men, with an estimated annual incidence of 51 534, representing 11.8% of all cancers [1].
Common approaches for the treatment of localized PCa include active surveillance, radical prostatectomy (RP), radiotherapy, and hormonal therapy. Currently, both RP and radiotherapy are considered to be the curative treatments for localized PCa. However, the relative treatment efficacy of prostatectomy and radiotherapy for PCa remains controversial. Randomized clinical trials (RCTs) that have been conducted to compare treatment outcomes for prostatectomy and radiotherapy have closed early due to poor recruitment or have been underpowered [2–4]. Observational studies have provided evidence of better survival outcomes with surgery, compared with radiotherapy, with surgery being particularly beneficial for younger men and patients with less health comorbidities and with an intermediate- or high-risk localized PCa [5–7]. Although there has been significant advancement in radiation therapy in recent years, Merino et al. reported a better prognosis for patients treated with surgery compared with those treated with intensity-modulated radiation therapy [8]. In Asian populations, Kim et al. reported comparable outcomes for surgery and radiation therapy [9]. In fact, a very small RCT conducted in Japan to compare outcomes of surgery and radiotherapy concluded that neither surgery nor radiotherapy demonstrated favorable long-term outcomes [4]. Yamamoto et al. reported the same results in their observational study of patients with locally advanced PCa [10]. Thus, there is no clear evidence to inform clinical decisions regarding adequate treatment for localized PCa in Japanese patients. There is also a need for a long period of post-treatment observation to provide a comprehensive evaluation of the effectiveness of surgical and radiotherapy treatment for PCa. Therefore, the aim of our study was to evaluate the effectiveness of RP and curative radiotherapy treatment for localized PCa in Japanese patients in terms of long-term, cause-specific survival (CSS) and overall survival (OS).
MATERIALS AND METHODS
This is a retrospective cohort study of patients with a clinically localized PCa who underwent RP or curative external-beam radiation therapy (EBRT) at Osaka City University Hospital or Bell Land General Hospital between 1995 and 2009. Permission to access the database for review of the medical records of these patients was approved by the local research ethics committee at Osaka City University.
Following an explanation of therapeutic alternatives to the patient, treatment selection between RP and EBRT was decided by the attending physician. Of 430 eligible patients, RP was performed in 248 and EBRT in 182. RP was performed using a standard open surgical approach, including lymphadenectomy, for all patients. Patients receiving EBRT were treated at 2 Gy per fraction, using a linear accelerator to provide 2D or 3D conformal radiation, with a median dose of 66 Gy (range 60–74 Gy). Pelvic lymph nodes were included in the radiation field, with 40–54 Gy.
Patients in the RP group who had been treated with salvage androgen deprivation therapy (ADT) were included to minimize the possibility of selection bias in the present study. However, patients in the EBRT group with adjuvant ADT were also included. Since adjuvant ADT following EBRT improves local cancer control and survival in patients with localized PCa [11, 12], patients in the EBRT group treated with adjuvant ADT were included in the analysis.
Staging and risk groups
Each patient's age, initial PSA levels at diagnosis and Gleason score were recorded. All patients underwent a digital rectal examination (DRE), bone scan and computed tomography (CT) scan of the lungs, abdomen and pelvis prior to treatment. Clinical T stage was determined according to DRE or magnetic resonance imaging (MRI). Patients were stratified according to the D'Amico classification [13].
Follow-up
For the RP group, biochemical failure was defined as three consecutive PSA levels of ≥0.2 ng/ml. For the radiotherapy group, biochemical failure was defined in accordance with the Phoenix consensus as a rise in PSA of ≥2 ng/ml above the lower PSA limit reached during radiotherapy [14].
Statistical analysis
Differences in clinicopathological variables between the two groups were analyzed by Chi-squared analysis. The Kaplan–Meier method was used to analyze the actuarial survival rates, with between-group differences in survival curves evaluated using log rank tests. Cox proportional stepwise multivariate analysis was used to evaluate the association between clinicopathological variables and CSS. The following variables were evaluated as predictors: treatment type, RP vs EBRT; age, <70 vs ≥70 years; PSA level, <10 vs ≥10 ng/ml, and <20 vs ≥20 ng/ml; clinical T-stage, <T2 vs ≥T3; and Gleason score, <8 vs ≥8. All P-values were two-sided, and a value of <0.05 was considered to be statistically significant. Subgroup analysis was also performed. We analyzed the actuarial survival rates, with between-treatment group by clinical T stage (≥T3 or ≤T2), PSA (≥20 or <20), Gleason score (≥8 or ≤7). Statistical analyses were performed using Microsoft Excel®.
RESULTS
Patient characteristics
Relevant characteristics of the patient study group are listed in Table 1. Follow-up duration was longer for the RP group (median, 106 months) compared with the EBRT group (median, 77 months; P < 0.001). Patients were younger in the RP group (median, 68 years) compared with in the EBRT group (median, 72 years; P < 0.001). Initial PSA was lower in the RP group (median, 11 ng/ml) compared with in the EBRT group (median, 15.5 ng/ml; P < 0.001). Based on the D'Amico classification of risk factors, the RP group had a higher proportion of patients classified in the high-risk group, and there was a higher proportion in the EBRT group classified in the intermediate-risk group.
Table 1.
Relevant characteristics of the patient study group
| RP | EBRT | P value | |
|---|---|---|---|
| No. of patients | 248 | 182 | |
| Follow-up duration (months) | |||
| Median (range) | 106 (5–216) | 77 (1–197) | <0.001 |
| Age (years) | |||
| Median (range) | 68 (50–77) | 72 (56–85) | <0.001 |
| Initial PSA (ng/ml) | |||
| Median (range) | 11.0 (1.4–59.6) | 15.5 (0.6–300) | <0.001 |
| Clinical risk group | |||
| Low (%) | 28 (11.3) | 22 (12.1) | 0.014 |
| Intermediate (%) | 37 (14.9) | 47 (25.8) | |
| High (%) | 183 (73.8) | 113 (62.1) | |
| Gleason score | |||
| <8 | 164 (66.1) | 106 (58.2) | 0.084 |
| ≥8 | 83 (33.5) | 76 (37.4) | |
| unknown | 1 (4.0) | 0 (0) | |
| Clinical T stage | |||
| 1 | 26 (10.5) | 48 (26.4) | 0.063 |
| 2 | 157 (63.3) | 75 (41.2) | |
| 3 | 63 (25.4) | 53 (29.1) | |
| unknown | 2 (0.8) | 6 (3.3) | |
In the EBRT group, 71 patients (39%) received adjuvant ADT treatment for 2 years after radiotherapy: 4 patients classified in the low-risk group, 13 in the intermediate-risk group and 54 in the high-risk group. Patients in the RP group received salvage ADT treatment (83 patients), radiotherapy (44 patients) and chemotherapy (6 patients). Patients progressing to biochemical failure in the EBRT group received additional ADT treatment (30 patients) and chemotherapy (5 patients).
Treatment outcomes
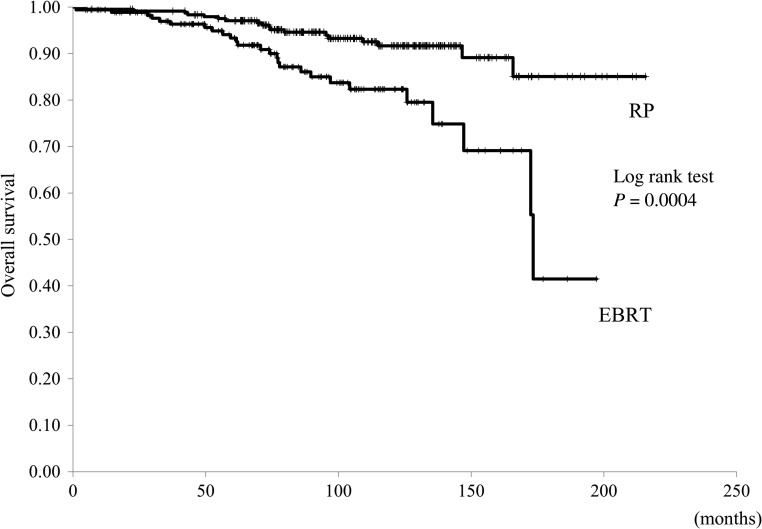
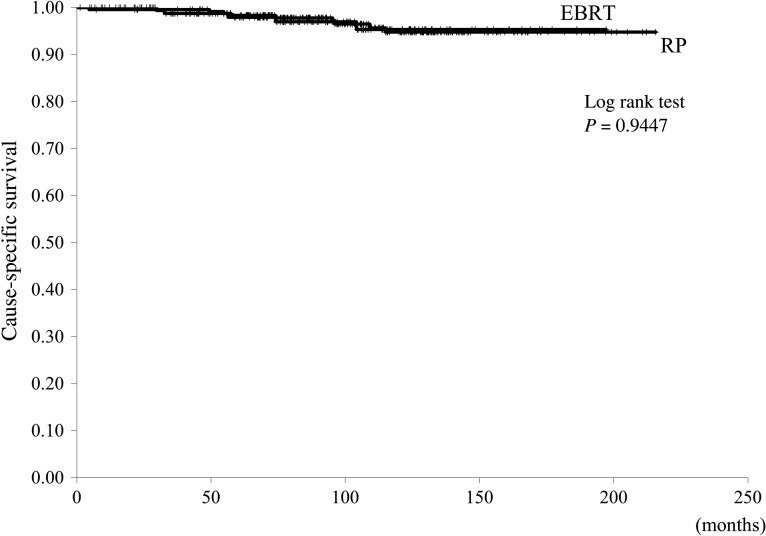
Median OS was superior for patients in the RP group compared with those in the EBRT group: the end-point cut-off of survival was not reached in the RP group, compared with 173 months for the EBRT group (P < 0.001, Fig. 1). However, CSS was not different between the two groups (P = 0.94, Fig. 2). Between-group differences in OS were also identified when patients within each group were stratified into low- and high-risk classifications. For the low-risk patients, end-point cut-off of survival was not reached in the RP group, compared with 161 months for the EBRT group (P = 0.006). For the high-risk patients, the end-point cut-off of survival was not reached in either group (P = 0.05). Similarly, there were no between-group differences in survival for patients in the intermediate-risk category, and the end-point cut-off of survival was not reached in the RP group, compared with 173 months for the EBRT group (P = 0.07). Additionally, CSS was comparable for the RP and EBRT groups, regardless of risk classification.
Fig. 1.
The overall survival curves in the radical prostatectomy (RP) and external-beam radiation therapy (EBRT) groups.
Fig. 2.
The cause-specific survival curves in the radical prostatectomy (RP) and external-beam radiation therapy (EBRT) groups.
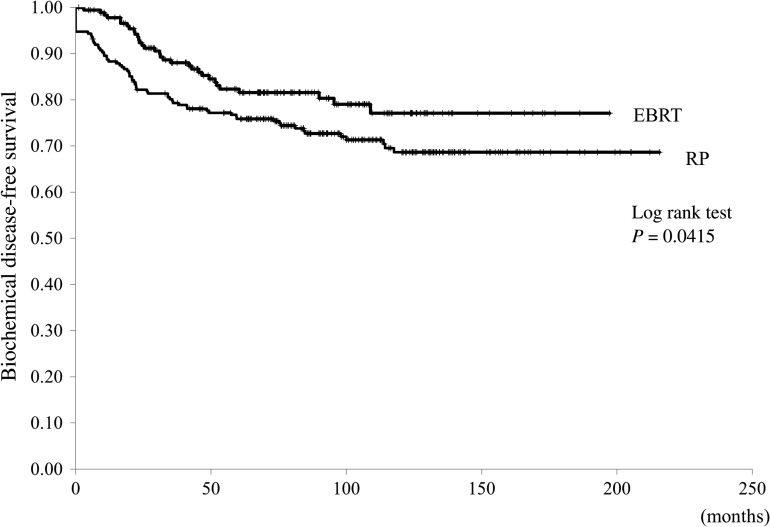
BFS was superior for the EBRT group, compared with the RP group (P = 0.04, Fig. 3). There were no effects of risk classification on BFS in either group, although there was a tendency to longer BFS in patients with a higher risk classification in the EBRT, compared with in the RP group (P = 0.08).
Fig. 3.
The biochemical disease-free survival curves in the radical prostatectomy (RP) and external-beam radiation therapy (EBRT) groups.
The results of the Cox proportional stepwise multivariate analysis of the association between the six clinicopathological variables on CSS are reported in Table 2. Univariate analysis identified PSA ≥20 vs <20 mg/ml, clinical T-stage of the tumor and Gleason score as predictors for CSS. However multivariate analysis did not identify a factor for CSS.
Table 2.
Results of the Cox proportional stepwise multivariate analysis of the association between the six clinicopathological variables and cause-specific survival
| Comparison | Unadjusted | Adjusted | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |||
| Treatment methods | ||||||||
| EBRT vs RP | 1.039 | 0.347 | 3.111 | 0.944 | ||||
| Age (years) | ||||||||
| ≥70 vs <70 | 0.920 | 0.318 | 2.656 | 0.878 | ||||
| PSA (ng/ml) | ||||||||
| ≥10 vs <10 | 4.430 | 0.991 | 19.795 | 0.051 | ||||
| PSA (ng/ml) | ||||||||
| ≥20 vs <20 | 2.970 | 1.031 | 8.561 | 0.044 | 1.203 | 0.355 | 4.082 | 0.767 |
| T stage | ||||||||
| ≥T3 vs ≤T2 | 3.674 | 1.275 | 10.593 | 0.016 | 2.134 | 0.699 | 6.516 | 0.183 |
| Gleason score | ||||||||
| ≥8 vs ≤7 | 2.990 | 1.002 | 8.923 | 0.049 | 1.777 | 0.557 | 5.668 | 0.331 |
Subgroup analysis was also performed by clinical T stage, PSA and Gleason score to compare the mortality rate between RP and EBRT groups, but there was no difference in each subgroup between RP and EBRT (Table 3).
Table 3.
Subgroup analysis of 15-year survival rate in RP and EBRT group
| Category | 15-year survival rate (%) | P value | |
|---|---|---|---|
| RP | EBRT | ||
| T stage | |||
| ≥T3 | 86.6 | 94.9 | 0.321 |
| ≤T2 | 97.5 | 95.4 | 0.332 |
| PSA ≥ 20 | |||
| ≥20 | 89.2 | 91.4 | 0.975 |
| <20 | 96.6 | 98.5 | 0.557 |
| Gleason score | |||
| ≥8 | 89.2 | 96.2 | 0.305 |
| ≤7 | 98.0 | 95.0 | 0.180 |
DISCUSSION
In our retrospective cohort study on the clinical effectiveness of RP and EBRT, we found CSS of patients with localized PCa to be comparable for the two treatment methods, although RP was associated with better OS, compared with EBRT. In contrast, EBRT was superior to RP in terms of BFS.
In Japan, the mortality rate due to PCa has increased in spite of the increased availability of several treatment methods [1]. Of the available treatment options, surgery, radiotherapy, and hormonal therapy are commonly prescribed for the treatment of localized PCa. However, evidence based on prospective studies is not currently available to inform treatment decisions and to confirm the clinical effectiveness of the different treatment methods. We identified one RCT comparing RP and radiotherapy, reporting comparable OS, CSS and BFS for both treatment methods at 10 years, concluding that both treatments provided favorable long-term outcomes [4]. However, this RCT included hormonal therapy in all patients, and the number of registered cases was limited. To the best of our knowledge, a comparison of RP and EBRT, including long-term follow-up, has not been conducted in Japan. Our study provides retrospective evidence of an overall better survival with RP, compared with EBRT, based on the long-term clinical data for 430 patients. We do acknowledge that younger age and lower initial PSA levels in the RP group could be contributing factors to the better OS rate. The possible importance of these factors to OS is highlighted when we consider that the CSS rate was comparable for the two groups.
The natural progression of PCa is usually very slow and, therefore, it is important to implement a cancer treatment approach that avoids PCa-related death. In our institution, surgery is recommended for patients with a life expectancy of longer than 10 years and a Gleason score higher than 4+3. Brachytherapy or active surveillance is available for patients with a Gleason score less than 3+4 and PSA levels lower than 10 ng/ml. The patients are treated by EBRT and/or hormonal therapy based on complications and performance status. A randomized Phase III trial of the clinical benefits of adjuvant ADT was conducted by the Radiation Therapy Oncology Group in patients with locally advanced PCa receiving EBRT [15, 16]. Evidence from this trial indicated that adjuvant hormonal therapy following EBRT was effective in improving local control of the disease and providing freedom from disease progression. Furthermore, the European Organization for Research and Treatment of Cancer conducted a trial to determine whether short-term ADT would achieve a comparable OS rate to that obtained with long-term ADT [12]. Based on their evidence, this group recommended the use of radiotherapy plus long-term ADT for men with locally advanced PCa. Recently, Bolla et al. also reported ADT, given for 3 years after external irradiation, improved the 10-year rate of disease-free survival and OS in patients with PCa at high metastatic risk [17]. In accordance with these results, it is recommended that patients with PCa in the high-risk category receive adjuvant hormonal therapy for 2 years post-irradiation [11, 12]. Use of this recommended adjuvant treatment might have led to similar CSS rates among both treatment groups in our study. Control for adjuvant therapy should be further considered in future studies.
In terms of BFS, it was superior for the EBRT group, compared with the RP group. We did apply the recommended average dose of radiation of 66 Gy provided by conventional irradiation techniques or 3D-CRT. Long-term, randomized, radiotherapy dose trials indicated BFS to be superior in patients who received 78 Gy, compared with patients receiving 70 Gy, with evidence of low-dose radiation therapy being inferior to surgical treatment in Western countries [18–20]. Kupelian et al. reported BFS to be inferior for EBRT doses of <72 Gy compared with doses of ≥72 Gy in the treatment of localized PCa [19]. In Japan, Yamamoto et al. reported that both RP and EBRT provided similar results for the treatment of cT3 PCa [10]. In our study, patients received a median irradiation dose of 60 Gy, with evidence that CSS at this dosage was not inferior to survival rates after surgical treatment. In Asian countries, Kim et al. retrospectively compared treatment outcomes for RP and EBRT in the treatment of localized PCa, reporting 8-year BFS rates of 44% for RP and 72% for EBRT groups (P < 0.001), although OS was inferior for the EBRT group, compared with the RP group [9]. Based on their evidence, Kim et al. concluded that the outcomes for EBRT were not inferior to those for RP. It is important to note that Kim et al. used either 3D-CRT or IMRT for radiation therapy, with an average radiation dose of 76 Gy. Therefore, our study provides important evidence regarding the effectiveness of low-dose radiation therapy in patients with localized PCa.
A number of important outcomes of surgical treatment and radiation therapy could not be evaluated due to the retrospective nature of our study, including quality of life (which has been investigated in previous research) [21, 22]. In these studies, quality of life was found to be comparable for both RP and EBRT treatment, with all aspects of quality of life being well-maintained with the exception of sexual function. Furthermore, quality of life did not influence BFS.
This study is a retrospective study and thus has certain limitations. Especially, it is not to be denied that problems concerning the difference of biochemical failure definition between RP and EBRT remain unsettled. However, these definitions have been confirmed at the RTOG-ASTRO Phoenix Consensus Conference [14].
In conclusion, both RP and EBTR provided satisfactory results in terms of disease control in patients with localized PCa. Furthermore, radiation therapy was an effective treatment for localized PCa, even at a low radiation dose. Based on our evidence, we recommend that clinical decisions regarding treatment should be made on the basis of patient-specific criteria and preferences.
REFERENCES
- 1. Matsuda A, Matsuda T, Shibata A, et al. Cancer incidence and incidence rates in Japan in 2008: a study of 25 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol 2014;44:388–96. [DOI] [PubMed] [Google Scholar]
- 2. Penson DF. An update on randomized clinical trials in localized and locoregional prostate cancer. Urol Oncol 2005;23:280–8. [DOI] [PubMed] [Google Scholar]
- 3. Paulson DF, Lin GH, Hinshaw W, et al. Radical surgery versus radiotherapy for adenocarcinoma of the prostate. J Urol 1982;128:502–4. [DOI] [PubMed] [Google Scholar]
- 4. Akakura K, Suzuki H, Ichikawa T, et al. A randomized trial comparing radical prostatectomy plus endocrine therapy versus external beam radiotherapy plus endocrine therapy for locally advanced prostate cancer: results at median follow-up of 102 months. Jpn J Clin Oncol 2006;36:789–93. [DOI] [PubMed] [Google Scholar]
- 5. Sooriakumaran P, Nyberg T, Akre O, et al. Comparative effectiveness of radical prostatectomy and radiotherapy in prostate cancer: observational study of mortality outcomes. BMJ 2014;348:g1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Abdollah F, Schmitges J, Sun M, et al. Comparison of mortality outcomes after radical prostatectomy versus radiotherapy in patients with localized prostate cancer: a population-based analysis. Int J Urol 2012;19:836–44. [DOI] [PubMed] [Google Scholar]
- 7. Sun M, Sammon JD, Becker A, et al. Radical prostatectomy vs radiotherapy vs observation among older patients with clinically localized prostate cancer: a comparative effectiveness evaluation. BJU Int 2014;113:200–8. [DOI] [PubMed] [Google Scholar]
- 8. Merino T, San Francisco IF, Rojas PA, et al. Intensity-modulated radiotherapy versus radical prostatectomy in patients with localized prostate cancer: long-term follow-up. BMC Cancer 2013;13:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kim YJ, Cho KH, Pyo HR, et al. Radical prostatectomy versus external beam radiotherapy for localized prostate cancer: comparison of treatment outcomes. Strahlenther Onkol 2015;191:321–9. [DOI] [PubMed] [Google Scholar]
- 10. Yamamoto S, Kawakami S, Yonese J, et al. Long-term oncological outcome in men with T3 prostate cancer: radical prostatectomy versus external-beam radiation therapy at a single institution. Int J Clin Oncol 2014;19:1085–91. [DOI] [PubMed] [Google Scholar]
- 11. Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med 1997;337:295–300. [DOI] [PubMed] [Google Scholar]
- 12. Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med 2009;360:2516–27. [DOI] [PubMed] [Google Scholar]
- 13. D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998;280:969–74. [DOI] [PubMed] [Google Scholar]
- 14. Roach M III, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006;65:965–74. [DOI] [PubMed] [Google Scholar]
- 15. Pilepich MV, Caplan R, Byhardt RW, et al. Phase III trial of androgen suppression using goserelin in unfavorable-prognosis carcinoma of the prostate treated with definitive radiotherapy: report of Radiation Therapy Oncology Group Protocol 85–31. J Clin Oncol 1997;15:1013–21. [DOI] [PubMed] [Google Scholar]
- 16. Lawton CA, Winter K, Murray K, et al. Updated results of the phase III Radiation Therapy Oncology Group (RTOG) trial 85–31 evaluating the potential benefit of androgen suppression following standard radiation therapy for unfavorable prognosis carcinoma of the prostate. Int J Radiat Oncol Biol Phys 2001;49:937–46. [DOI] [PubMed] [Google Scholar]
- 17. Bolla M, Van Tienhoven G, Warde P, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol 2010;11:1066–73. [DOI] [PubMed] [Google Scholar]
- 18. Kuban DA, Tucker SL, Dong L, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 2008;70:67–74. [DOI] [PubMed] [Google Scholar]
- 19. Kupelian PA, Elshaikh M, Reddy CA, et al. Comparison of the efficacy of local therapies for localized prostate cancer in the prostate-specific antigen era: a large single-institution experience with radical prostatectomy and external-beam radiotherapy. J Clin Oncol 2002;20:3376–85. [DOI] [PubMed] [Google Scholar]
- 20. Dearnaley DP, Sydes MR, Graham JD, et al. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol 2007;8:475–87. [DOI] [PubMed] [Google Scholar]
- 21. Takizawa I, Hara N, Nishiyama T, et al. Oncological results, functional outcomes and health-related quality-of-life in men who received a radical prostatectomy or external beam radiation therapy for localized prostate cancer: a study on long-term patient outcome with risk stratification. Asian J Androl 2009;11: 283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Namiki S, Ishidoya S, Kawamura S, et al. Quality of life among elderly men treated for prostate cancer with either radical prostatectomy or external beam radiation therapy. J Cancer Res Clin Oncol 2010;136:379–86. [DOI] [PMC free article] [PubMed] [Google Scholar]