Abstract
Background
Chronic kidney disease (CKD) is associated with abnormal lipid profiles and altered high-density lipoprotein (HDL) particle size patterns. Lower levels of the larger, cardioprotective HDL particles found in CKD may play a role in the increased risk for cardiovascular disease in these patients. The current study was designed to assess the effects of short-term moderate-intensity aerobic exercise training on the HDL particle pattern and overall lipid profiles in stage 3 CKD patients.
Methods
Forty-six men and women with stage 3 CKD were randomized to either exercise (EX, n = 25) or control (CON, n = 21). Those in the EX group completed 16 weeks of supervised moderate-intensity aerobic exercise three times per week. Serum total cholesterol, HDL cholesterol (HDL-C), triglycerides (TGs), low-density lipoprotein cholesterol (LDL-C), HDL particle size, estimated glomerular filtration rate (eGFR), body composition and peak oxygen uptake (VO2peak) were assessed at baseline and week 16.
Results
The rate of compliance in the EX group was 97 ± 7.2%. No change was observed in eGFR over time in either group. There was an 8.2% improvement in VO2peak in the EX group (P = 0.05), while VO2peak decreased in the CON group. HDL-C, TGs, HDL particle size and body composition remained unchanged in both groups. A trend was found for lower total cholesterol (TC) (P = 0.051) and LDL-C (P = 0.07) in the CON group.
Conclusion
Our findings indicate that a short-term aerobic exercise training intervention in stage 3 CKD patients does not induce changes in HDL particle size or favorable lipid profile modifications.
Keywords: aerobic exercise, chronic kidney disease, HDL particles, lipids
Introduction
Chronic kidney disease (CKD) has been identified as a worldwide public health problem [1]. In the USA, it is estimated that >20 million people currently have CKD, based on the classification determined by the National Kidney Foundation [2]. CKD is associated with a high risk of developing cardiovascular disease (CVD) [3], such that the prevalence of CVD in patients with CKD is significantly greater than the prevalence of CVD in the general population [4]. Mortality due to CVD in the early stages of CKD is more common than progression to end-stage renal disease (ESRD) [5]. Therefore, there are a greater number of CKD patients in stages 1–4 and the overall burden of CVD in the early stages of CKD is presumed to be far greater than in ESRD [6, 7]. Accordingly, management of CVD risk factors has been identified as a vital aspect of treatment of CKD [8]. Among other risk factors for CVD, dyslipidemia has been consistently observed at higher rates in CKD patients [2, 3, 6, 9].
Dyslipidemia is commonly identifiable in the early stages of CKD and frequently accompanies the progression of renal failure [10, 11]. Abnormal lipid profiles associated with CKD are primarily reflected in elevated triglyceride (TG) and decreased high-density lipoprotein cholesterol (HDL-C) levels, while low-density lipoprotein cholesterol (LDL-C) levels are usually normal [12, 13]. In addition to disturbed lipid profiles, components of lipid particles and subclasses of lipid particles are also altered in CKD. CKD is associated with low levels of the primary protein components of HDL, apolipoprotein AI and AII, and decreased production of HDL particles [14]. HDL particles, which function in reverse cholesterol transport and other cardioprotective roles, are known to be dysfunctional in CKD [15, 16]. Larger HDL particles are thought to be most cardioprotective; their large size is considered evidence of a higher capacity for reverse cholesterol transport, and larger HDL particles have a longer life span as compared with smaller HDL particles [17, 18]. Patients with CKD typically display lower amounts of larger HDL particles and higher amounts of medium and small HDL particles. Al-Shahrouri et al. [19] found a significant inverse relationship between estimated glomerular filtration rate (eGFR) and medium HDL particle concentration, and Jenkins [20] reported a significant positive relationship between albumin excretion rate and small HDL particle concentration.
Aerobic exercise training has been associated with positive effects on lipid profiles in the general population, most notably in TG and HDL levels [21–25]. The results of a meta-analysis of studies involving exercise interventions of moderate to high intensity three to five times per week for >12 weeks indicated a general increase in HDL-C, decreases in TG and LDL-C concentrations and no change in total cholesterol [26]. Kraus et al. [27] noted a significant improvement in lipoprotein profiles of subjects who exercised regularly over a period of 6 months, including increased HDL-C and decreased very-low-density lipoproteins and TGs. According to a recent meta-analysis by Sarzynski et al. [28], regular exercise imparts several beneficial effects on lipid particle subclasses, including significant increases in large HDL particles and significant decreases in medium HDL particles.
Strategies to reduce risk factors for CVD in the general population have been proposed as applicable to reduce CVD risk in CKD patients [8]. Possible implications of exercise training in CKD are of interest, given the positive effects of exercise in the general population on the aspects of lipid profiles disrupted in CKD. However, much less is known about the effects of exercise training on the lipid profiles of patients with CKD. Contrary to the majority of findings in the general population, Eidemak et al. [29] reported a significant increase in the total cholesterol of CKD patients after an aerobic exercise training intervention. Similarly, in a study on the effects of exercise training in stage 2–4 CKD patients, our group [30] observed a significant increase in total cholesterol and LDL-C in the exercise training group. Results of other studies have indicated no change in blood lipids of CKD patients following exercise intervention programs [31, 32]. However, Toyama et al. [33] found that a 12-week exercise program resulted in a significant increase in average HDL-C in CKD patients with CVD. In assessing the outcomes of cardiac rehabilitation aerobic exercise programs, Venkataraman et al. [34] observed significant improvements in the lipid profiles of patients with both CVD and CKD; however, these patients still had lower average HDL-C compared with patients without CKD [34]. Notably, none of these studies examined responses of HDL particle patterns to exercise. The particle pattern can provide important information about atherogenicity that cannot be determined through serum lipid concentration alone. Whether the HDL particle pattern responds to exercise in CKD patients similar to those without CKD is not known. Therefore, the purpose of this article is to present a secondary analysis of data from a study assessing the HDL particle pattern and overall lipid responses to a short-term moderate-intensity aerobic exercise training intervention in stage 3 CKD patients. We hypothesized that the 16-week exercise training program would lead to improvements in lipid profiles, specifically through increased HDL number and particle size.
Materials and methods
Detailed descriptions of the materials and methods have been published previously [35, 36]. All procedures were approved by the Institutional Review Board (IRB) at Springfield College and all patients provided written informed consent prior to participating. A total of 1116 patients were assessed for eligibility to participate.
Participants
Eligibility criteria included men and women 35–70 years of age, presence of CKD stage 3 (eGFR 30–59 mL/min/1.73 m2) with either hypertension or diabetes as the primary cause. Potential participants were identified from the database of patients at a private nephrology practice. Patients who were currently engaging in a structured exercise program, who had been diagnosed with atrial fibrillation, who were current cigarette smokers or who had any absolute contraindication to exercise as defined by the American College of Sports Medicine [37] were excluded. Of all patients screened, 981 were excluded and 86 declined to participate, leaving 51 eligible patients.
Procedures
All baseline measurements were completed prior to randomization. A demographics questionnaire was completed to assess medication use. Height was determined using a portable stadiometer and body weight and composition were determined using the Tanita BC-418 Segmental Body Composition Analyzer/Scale (Tanita, Tokyo, Japan), which correlates strongly (r ≥ 0.95, P < 0.001) with both whole-body and regional composition values obtained using dual-energy X-ray absorptiometry [38]. Fasting blood samples were collected via antecubital venipuncture into serum separator tubes. After clotting and centrifugation, one blood sample was sent to a certified medical laboratory (Quest Diagnostics, Springfield, MA, USA) for standard lipid analyses and serum from an additional tube was stored in an individual aliquot at −80°C for HDL particle analyses. Also, a graded exercise test was performed to determine peak oxygen uptake (VO2peak) as a measure of fitness and as a means for prescribing intensity for exercise training. During the subsequent 2 weeks, participants were interviewed via telephone, unannounced, on two separate occasions by a registered dietitian with specialization in nephrology, to collect a 24-h dietary recall. Participants were then randomized to either the exercise (EX, n = 28) or control (CON, n = 23) group.
Participants randomized to the EX group completed three supervised aerobic exercise sessions per week for a total of 16 weeks. Aerobic exercise was completed at 50–60% of VO2peak, with a progressive duration that was initially set at 15–30 min but gradually increased to a total of 55 min per session for most participants. If a session was missed, participants were allowed to make up that session within the subsequent 2 weeks. Participants randomized to the CON group were directed to follow the instructions of their physician, but not to start a formal exercise program. After 16 weeks, data collection procedures used at baseline were repeated. For those assigned to the EX group, anthropometrics and blood were determined a minimum of 48 h following the final exercise session.
Biochemical analyses
Serum total cholesterol, HDL-C and triacylglycerol content were determined using enzymatic methods. LDL-C was determined using the Friedewald method [39]. HDL particle size was determined via high-resolution polyacrylamide gel electrophoresis using the Lipoprint system (Quantimetrix, Manhattan Beach, CA, USA). A total of three bands of HDL were quantitatively evaluated using computer software (National Institutes of Health imaging software utilizing the Lipoprint HDL macro). The software allows for the identification of each subclass according to their relative mobility, with smaller particles migrating further. The area under the curve was calculated for each fraction, allowing for determination of the relative percentage of HDL in each band as well as mean and peak diameter.
Dietary analyses
Dietary analyses were completed using Food Processor Nutrition Analysis Software version 11.0.2 (ESHA Research, Salem, OR, USA). Data from the two baseline recalls and from the two post intervention recalls were averaged to arrive at the baseline and post intervention values.
Statistical analyses
All lipid measures were analyzed using analysis of covariance (ANCOVA), with baseline assessments used as covariates and treatment and control groups compared at 16 weeks. All variables were screened for violations of assumptions for ANCOVA analysis and were deemed acceptable for analysis.
Compliance with ethical standards
Ethical approval
All procedures performed in studies involving human participants were in accordance with the Springfield College IRB and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Results
Of the 51 participants initially randomized, 46 completed the entire intervention (EX = 25, CON = 21). Only data from participants who completed the intervention were included in the analyses. Analyses were conducted on all nonmissing data; if a participant missed an assessment, they were excluded from that particular analysis. Baseline characteristics of participants in each group have previously been published [35] and indicated no difference between groups in any variable measured (P > 0.05), including age, height, weight, gender and disease status. The eGFR was unchanged over time in both groups. Compliance in the EX group was outstanding, with 97 ± 7.2% of scheduled training sessions completed. Anthropometrics, fitness and nutrition results are presented in Table 1. Dietary analysis measures remained unchanged in both groups. There were no significant time or treatment effects on body mass index or body composition. Fitness, as assessed by VO2peak, improved by ∼8.2% in the EX group (P = 0.05), but decreased by ∼3% in the control group.
Table 1.
eGFR, hs-CRP, anthropometrics, fitness, and nutrition
| Baseline |
Week 16 |
|||
|---|---|---|---|---|
| Variable | EX | CON | EX | CON |
| eGFR (mL/min/1.73 m2) | 47.0 ± 12.0 | 48.3 ± 12.7 | 52.4 ± 19.1 | 50.1 ± 16.2 |
| hs CRP (mg/L) | 6.0 ± 3.7 | 5.6 ± 2.96 | 5.9 ± 4.5 | 5.4 ± 3.3 |
| Body mass index (kg/m2) | 34.9 ± 8.0 | 36.5 ± 8.9 | 34.5 ± 7.8 | 36.2 ± 8.9 |
| Body fat (%) | 35.9 ± 9.5 | 37.4 ± 8.6 | 34.7 ± 9.7 | 36.2 ± 8.9 |
| Fat-free mass (lbs) | 140.1 ± 33.3 | 142.7 ± 39.9 | 145.6 ± 32.9 | 142.1 ± 41.5 |
| VO2peak (mL/kg/min) | 19.6 ± 6.7 | 18.0 ± 6.0 | 21.2 ± 7.7a | 17.5 ± 5.7 |
| Total Kcal | 2149 ± 722 | 2077 ± 569 | 2201 ± 850 | 1985 ± 412 |
| Carbohydrate (kcal) | 1167.9 ± 386.1 | 913.7 ± 212.5 | 1134.4 ± 567.7 | 878.9 ± 156.9 |
| Fat (kcal) | 719.9 ± 281.0 | 751.5 ± 296.7 | 712.0 ± 264.8 | 692.8 ± 232.8 |
| Protein (kcal) | 344.9 ± 97.7 | 373.7 ± 127.9 | 348.0 ± 95.2 | 349.67 ± 85.93 |
| Sodium (mg) | 3686.1 ± 1084.3 | 3782.8 ± 1718.9 | 3872.9 ± 1125.4 | 3777.7 ± 1321.4 |
| Phosphorous (mg) | 727.88 ± 321.71 | 700.6 ± 404.8 | 708.7 ± 364.4 | 780.9 ± 327.8 |
Values are mean ± standard deviation.
Indicates significant difference between EX and CON groups at week 16 after controlling for age and baseline values.
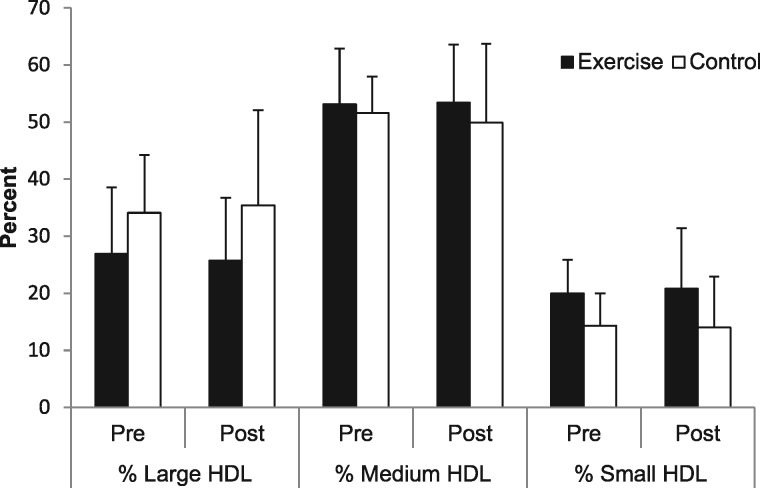
Table 2 displays the serum lipid responses to the intervention. No significant time or treatment effects were found for any lipid variable, although a trend for lower total cholesterol (P = 0.051) and LDL-C (P = 0.07) in the CON group was observed. Findings for HDL remained nonsignificant with gender as a covariate (P = 0.65). Figure 1 displays the responses of HDL particles to the exercise intervention. No significant group or time effects were found for large, medium or small HDL particle concentration. Table 3 includes the number of participants in each group classified according to the level of urine protein at baseline. Due to missing values, data from 42 of 46 participants were included in the analysis of proteinuria, assessed through 24-h urine protein. High-sensitivity C-reactive protein (hs-CRP) values were previously published and indicated no difference between groups at baseline and no time or treatment effects (P = 0.9) [35].
Table 2.
Serum lipid responses to 16 weeks of aerobic exercise in stage 3 CKD patients
| EX |
CON |
|||||
|---|---|---|---|---|---|---|
| Lipid variable, mmol/L (mg/dL) | Baseline | 16 weeks | Baseline | 16 weeks | Adjusted mean difference (95% CI)a | P-valueb |
| Total cholesterol | 4.95 ± 1.18 | 4.89 ± 1.01 | 4.49 ± 0.91 | 4.12 ± 0.92 | 0.44 (−0.001, 0.88) | 0.051 |
| (191.43 ± 41.54) | (189.04 ± 38.91) | (173.59 ± 35.31) | (159.35 ± 35.73) | [16.92 (−0.05, 33.89)] | ||
| HDL | 1.18 ± 0.38 | 1.15 ± 0.39 | 1.34 ± 0.57 | 1.26 ± 0.67 | 0.04 (−0.14, 0.22) | 0.64 |
| (45.74 ± 14.51) | (44.61 ± 14.29) | (51.94 ± 21.99) | (48.82 ± 25.77) | [1.62 (−5.36, 8.60)] | ||
| LDL | 2.93 ± 0.94 | 2.98 ± 0.99 | 2.51 ± 0.57 | 2.27 ± 0.79 | 0.45 (−0.04, 0.94) | 0.07 |
| (113.26 ± 36.27) | (115.13 ± 38.36) | (97.23 ± 31.42) | (87.70 ± 30.60) | [17.38 (−1.51, 36.28)] | ||
| TGs | 1.83 ± 0.81 | 1.83 ± 0.93 | 1.30 ± 0.68 | 1.41 ± 0.80 | 0.003 (−0.45, 0.46) | 0.99 |
| (162.22 ± 71.91) | (161.69 ± 81.96) | (114.76 ± 59.80) | (125.11 ± 70.65) | [0.29 (−40.15, 40.74)] | ||
Values are mean ± standard deviation.
95% confidence interval (CI) for the difference between adjusted means of EX and CON at 16 weeks, controlling for baseline assessment.
P-value for difference between EX and CON at 16 weeks analyzed by a one-way ANCOVA.
Fig. 1.
Response of HDL particle size to 16 weeks of aerobic exercise in stage 3 CKD patients.
Table 3.
Number of subjects at baseline with associated level of proteinuria
| PER (mg/24 h) | EX | CON |
|---|---|---|
| <150 (normal to mildly increased) | 15 | 11 |
| 150–500 (moderately increased) | 6 | 3 |
| >500 (severely increased) | 1 | 6 |
Data are missing for three participants in the EX group and one in the CON group. PER categories are based on KDIGO 2012 clinical practice guideline [58].
PER, protein excretion rate.
Through the course of the study, a total of five participants (three EX, two CON) had changes (additions or deletions) in medications that impact lipids. When removing these participants from statistical analyses, the differences between groups in total cholesterol at week 16 reached significance [EX 5.04 ± 1.07 mmol/L (194.8 ± 41.3 mg/dL), CON 4.09 ± 0.95 mmol/L (158.3 ± 36.6 mg/dL); P = 0.03], but no other significant differences were found in any other lipid variable.
Discussion
The purpose of the present secondary analysis was to examine the HDL particle and overall lipid responses to 16 weeks of aerobic exercise training in patients with stage 3 CKD. The primary finding was that HDL particle size was not changed by short-term moderate-intensity regular aerobic exercise. We also observed a trend for control subjects to have improvements in total cholesterol and LDL-C that was not observed for the EX group.
HDL particle pattern has been shown to improve after the adoption of regular exercise in the general population [28]. Nascent HDL particles are formed in circulation through interactions with apolipoprotein AI and AII. These nascent particles obtain apolipoproteins C and E, forming lipid-poor, small HDL particles. The small HDL particles bind the adenosine triphosphate–binding cassette transporter type I to retrieve free cholesterol from extrahepatic tissue and esterify that cholesterol via the action of lecithin–cholesterol acyltransferase (LCAT), sending the cholesterol ester to the center of the HDL particle, effectively increasing the size of the particle [40]. As the particle increases in size, it becomes a cholesterol-rich, large HDL particle. These particles then interact with the liver to release the lipid contents, reducing the size of the HDL particle. The particles then dissociate from the liver to return to extrahepatic tissue for the removal of additional cholesterol [12]. Several studies have shown the influence of exercise on this process and on the ultimate outcome of HDL-C [28, 41].
Sarzynski et al. [28] conducted a meta-analysis to examine the effects of regular exercise on lipid subclasses. The analyses included 10 exercise interventions from six total cohorts with a total of 1555 men and women, and all analyses for lipoprotein subclasses were done by nuclear magnetic resonance spectroscopy. The exercise protocol varied between the cohorts. Modes employed included cycle, treadmill, elliptical, stair, ski and rowing. The frequency in most groups was three to four times per week and exercise intensity ranged from 50 to 80% of VO2peak. Trial duration ranged from 20 to 35 weeks. After adjustment for age, sex, race, baseline BMI and trait values, regular exercise induced a significant increase in the concentration of large HDL particles and a significant decrease in the concentration of medium HDL particles. HDL-C was also significantly increased.
Changes in HDL particles appear to happen early after the adoption of an exercise program. Dutheil et al. [41] examined the lipoprotein subfraction response to an exercise program in 78 men and women with metabolic syndrome using the Lipoprint system. A group of age-matched healthy adults were also recruited as controls. At baseline, the group with metabolic syndrome had significantly lower amounts of large and medium HDL particles and significantly higher amounts of small HDL particles. Participants in the metabolic syndrome group underwent a 3-week residential treatment program that included energy restriction of 500 kcal/day and 15–20 h of exercise (both aerobic and resistance) per week. After the 3-week period, large HDL particles increased significantly while medium and small HDL particles decreased significantly. Although we did not employ energy restriction, our intervention lasted for 16 weeks, so it is expected that the exposure to exercise was sufficient to impose alterations in lipids based on findings of previous studies [42, 43]. Although no differences in nutrient intake were observed, our relatively small sample size limited the ability to fully understand the possible impact of caloric intake on the lack of HDL particle changes.
In contrast to the reports outlined above, we found that HDL size does not appear to be impacted by the adoption of an aerobic exercise program in patients with CKD. Furthermore, we again found that HDL-C was not impacted by adoption of an aerobic exercise program, as has been found by other groups examining patients with CKD [31, 32]. Together, this information suggests that in patients with CKD, HDL may be resistant to the beneficial effects of exercise. HDL particles have been reported to be dysfunctional in CKD, characterized by a reduced capacity for antioxidant and anti-inflammatory properties, and to be less effective at reverse cholesterol transport [44]. Central to HDL dysfunction is thought to be a deficiency in the primary apolipoproteins, AI and AII. The plasma concentrations of apolipoproteins AI and AII are significantly reduced in CKD [45, 46], and work in animal models of CKD suggests this may be caused by their reduced gene expression in the liver [46]. Gene expression of apolipoprotein AI, along with HDL-C, was shown to increase following 12 weeks of exercise training in previously sedentary women [47]. However, other similar exercise interventions have not significantly impacted apolipoprotein AI [48], and apolipoprotein AI levels were found to not differ between age-matched master athletes, recreational athletes and sedentary controls [49]. Whether apolipoprotein AI levels are altered by exercise in CKD is unknown, but further investigation is warranted given that levels are compromised in the CKD population and given that exercise may improve apolipoprotein AI concentration. Inflammation is also associated with the pathogenic lipid metabolism observed in CKD patients [50]. In the current study, systemic inflammation assessed through plasma hs-CRP was not different between groups, suggesting that differences in inflammation did not impact the effects observed. However, due to the negative effect of inflammation on apolipoprotein AI and HDL, the absence of a decrease in inflammation with exercise training in the current study may have been influential in the lack of change in HDL with exercise.
Another factor potentially impacting HDL particle size is the amount and activity of LCAT. The plasma concentration and activity of LCAT is significantly reduced in CKD [12] and ESRD [44] and the gene expression of LCAT was significantly downregulated in an animal model of CKD [51]. Exercise has been shown to increase LCAT activity in animal models [52], and Lehmann et al. [53] saw a 32% increase in LCAT activity after a 3-month exercise program in adults with type 2 diabetes. However, not all exercise interventions [54] or cross-sectional comparisons [55] have found LCAT content or activity to be impacted by exercise.
The pathogenic nature of lipid profiles in patients with CKD appear to be magnified in patients with accompanying proteinuria [56], with evidence of higher LDL and TG concentrations as well as lower HDL concentrations when proteinuria is present [57]. A total of seven subjects in the EX group and nine subjects in the CON group had urine protein levels >150 mg/24 h, indicating proteinuria (Table 3). Of those, six in the CON group and one in the EX group were classified as ‘severely increased’ according to the Kidney Disease: Improving Global Outcomes Clinical Practice Guidelines [58]. It is not known whether the level of proteinuria affects the lipid response to exercise training. Additionally, reductions in plasma albumin with CKD are associated with lower levels of HDL-C as well as impaired reverse cholesterol transport function of HDL [59, 60]. Plasma albumin was not measured in the current study. A change in albumin from baseline to 16 weeks may have a considerable impact on HDL-C; thus, without this information the results of the current study should be interpreted with caution.
HDL particles are also affected by alterations in the metabolism of TG-rich lipoproteins. In CKD, HDL particles are known to have altered concentrations of TG as compared with healthy controls [12], which are known to reduce HDL particle size. Adoption of an aerobic exercise program has been shown to reduce TGs in numerous populations [61–63], and these improvements are often accompanied by improvements in HDL particle patterns [28]. However, in the current study, and as we have previously reported [30], the adoption of an aerobic exercise program did not significantly reduce TGs, and others have reported similar findings [29, 31, 32]. Perhaps the inability of chronic aerobic exercise to reduce TGs is the root of the resistance of HDL particles to beneficial modification. Also involved in the remodeling of HDL particles is cholesterol ester transfer protein (CETP); however, disruption in CETP is most strongly associated with ESRD and dialysis, whereas our participants were all predialysis. Although measurement of CETP activity may have been useful, we do not expect that it would have been significantly reduced in our sample.
The current intervention employed a moderate-intensity aerobic program, as did many of the other interventions in CKD [29, 31, 33]. Although exercise intensity may be a well-known factor in determining outcomes such as fitness, findings regarding lipids and lipoprotein subfractions are inconsistent. Fisher et al. [64] found that high-intensity interval training improved fitness (VO2peak) better than a moderate-intensity exercise program (11 versus 3% improvement) in young overweight or obese adults. Despite the better improvements in fitness, improvements in lipids and medium HDL particles were similar between groups. Liou et al. [65] conducted a meta-analysis of exercise interventions employing various intensities in patients with coronary artery disease. High-intensity interval training was found to improve mean VO2peak more than moderate-intensity exercise, but no significant differences were found in response of TGs or HDL-C. Other studies have found greater improvements in lipids in higher-intensity aerobic exercise [66] and with higher-versus medium-intensity resistance exercise [67]. Data from cross-sectional studies have provided evidence for a dose–response relationship between improvements in HDL-C and exercise volume [68]. An important consideration is weight loss, which is a confounding factor in exercise studies and is known to impact lipid responses independent of exercise [69]. Whether the response of lipids and lipoprotein subfractions would differ based on exercise intensity in CKD is currently unknown.
Patients in the current study had a mean BMI > 30, indicating obesity. Results of studies involving exercise with obese individuals indicate varying effects on blood lipid parameters. In a meta-analysis of randomized controlled trials investigating responses to aerobic exercise in obese and overweight individuals, reductions in both TGs and HDL-C were found [70]. This is in contrast to findings in a normal weight population in which reductions in TGs and increases in HDL-C have been found with aerobic exercise [24]. The obesity of participants in the current study should be considered when interpreting the results, as it may be an influential factor in the response of HDL-C to the prescribed exercise.
Patients with CKD often take a number of prescription drugs, some of which can affect lipids and lipid metabolism. Our participants in both groups were taking an average of nine prescription medications before and after the treatment. In total, four participants in the EX group and three participants in the CON group had changes in the number of medications they were taking. Of the medication changes in the EX group, only two of the drugs were statins, and these were removed from the participant’s treatment. Four other drugs added or deleted in the EX group are known to impact lipids or lipid metabolism, but were not drugs specifically for cholesterol management. As such, the pharmaceutical regimen in our patients was very stable and unlikely to have had a substantial impact on our findings.
Strengths of the current investigation include the outstanding compliance to the supervised exercise program, so we are very confident that the exercise exposure was completed as prescribed. Nutritional intake was also unchanged, increasing confidence that we were examining the effects of changes in exercise and not diet or a combination of diet and exercise. Furthermore, body weight remained stable, strengthening our ability to attribute effects of the adoption of aerobic exercise without the confounding effects of weight loss. Limitations of the current study include the relatively small sample size and that we did not measure apolipoprotein AI concentration or LCAT activity, which are important regulators of HDL particle size. Information about apolipoprotein AI and LCAT responses to exercise in CKD may provide important information about why patients with CKD may not respond to exercise, with respect to HDL particle size, as compared with the general population. Future studies should consider examining HDL kinetics and how the additional antiatherogenic properties of HDL respond to exercise.
Funding
This study was funded by the National Institutes of Health (1R15HL096097-01).
Conflict of interest statement
None declared.
References
- 1. Levey AS, Eckardt K, Tsukamoto Y. et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2005; 67: 2089–2100 [DOI] [PubMed] [Google Scholar]
- 2. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis 2002; 39(Suppl 1): S1–S266 [PubMed] [Google Scholar]
- 3. Sarnak MJ, Levey AS, Schoolwerth AC. et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation 2003; 108: 2154–2169 [DOI] [PubMed] [Google Scholar]
- 4. 2013 USRDS annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. 2014; 63: e1–e478 [Google Scholar]
- 5. Shulman NB, Ford CE, Hall WD. et al. Prognostic value of serum creatinine and effect of treatment of hypertension on renal function. Results from the hypertension detection and follow-up program. The Hypertension Detection and Follow-up Program Cooperative Group. Hypertension 1989; 13(5 Suppl): I80–I93 [DOI] [PubMed] [Google Scholar]
- 6. Foley RN, Parfrey PS, Sarnak MJ.. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998; 32(5 Suppl 3): S112–S119 [DOI] [PubMed] [Google Scholar]
- 7. Coresh J, Selvin E, Stevens LA. et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298: 2038–2047 [DOI] [PubMed] [Google Scholar]
- 8. Sarnak MJ, Levey AS.. Cardiovascular disease and chronic renal disease: a new paradigm. Am J Kidney Dis 2000; 35(4 Suppl 1): S117–S131 [DOI] [PubMed] [Google Scholar]
- 9. Townsend RR, Wimmer NJ, Chirinos JA. et al. Aortic PWV in chronic kidney disease: a CRIC ancillary study. Am J Hypertens 2010; 23: 282–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cramp DG. Plasma lipid alterations in patients with chronic renal disease. Crit Rev Clin Lab Sci 1982; 17: 77–101 [DOI] [PubMed] [Google Scholar]
- 11. Attman PO, Alaurpovic P.. Lipid abnormalities in chronic renal insufficiency. Kidney Int 1991; 39: S16–S23 [PubMed] [Google Scholar]
- 12. Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol 2006; 290: F262–F272 [DOI] [PubMed] [Google Scholar]
- 13. Vaziri ND, Navab M, Fogelman AM.. HDL metabolism and activity in chronic kidney disease. Nat Rev Nephrol 2010; 6: 287–296 [DOI] [PubMed] [Google Scholar]
- 14. Weiner DE, Sarnak MJ.. Managing dyslipidemia in chronic kidney disease. J Gen Intern Med 2004; 19: 1045–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kwan BH, Kronenberg F, Beddhu S. et al. Lipoprotein metabolism and lipid management in chronic kidney disease. J Am Soc Nephrol 2007; 18: 1246–1261 [DOI] [PubMed] [Google Scholar]
- 16. Koch M, Kutkuhn B, Trenkwalder E. et al. Apolipoprotein B, fibrinogen, HDL cholesterol, and apolipoprotein(a) phenotypes predict coronary artery disease in hemodialysis patients. J Am Soc Nephrol 1997; 8: 1889–1898 [DOI] [PubMed] [Google Scholar]
- 17. Otvos JD, Collins D, Freedman DS. et al. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation 2006; 113: 1556–1563 [DOI] [PubMed] [Google Scholar]
- 18. Guey LT, Pullinger CR, Ishida BY. et al. Relation of increased prebeta-1 high-density lipoprotein levels to risk of coronary heart disease. Am J Cardiol 2001; 108: 360–366 [DOI] [PubMed] [Google Scholar]
- 19. Al-Shahrouri H, Ramirez P, Fanti P. et al. NMR identifies atherogenic lipoprotein abnormalities in early diabetic nephropathy that are unrecognized by conventional analysis. Clin Nephrol 2010; 73: 180–189 [DOI] [PubMed] [Google Scholar]
- 20. Jenkins A, Lyons T, Zheng D. et al. Lipoproteins in the DCCT/EDIC cohort: associations with diabetic nephropathy. Kidney Int 2003; 64: 817–828 [DOI] [PubMed] [Google Scholar]
- 21. Earnest CP, Artero EG, Sui X. et al. Maximal estimated cardiorespiratory fitness, cardiometabolic risk factors, and metabolic syndrome in the aerobics center longitudinal study. Mayo Clin Proc 2013; 88: 259–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ferguson M, Alderson N, Trost S. et al. Effects of four different single exercise sessions on lipids, lipoproteins, and lipoprotein lipase. J Appl Physiol 1998; 85: 1169–1174 [DOI] [PubMed] [Google Scholar]
- 23. Mann S, Beedie C, Jimenez A.. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med 2014; 44: 211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leon A, Sanchez O.. Response of blood lipids to exercise training alone or combined with dietary intervention. Med Sci Sports Exerc 2001; 33(6 Suppl): S502–S515 [DOI] [PubMed] [Google Scholar]
- 25. Ruppar TM, Conn VS, Chase JD. et al. Lipid outcomes from supervised exercise interventions in healthy adults. Am J Health Behav 2014; 38: 823–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pedersen BK, Saltin B.. Evidence for prescribing exercise as therapy in chronic disease. Scand J Med Sci Sports 2006; 16(Suppl 1): 3- 63 [DOI] [PubMed] [Google Scholar]
- 27. Kraus W, Houmard J, Duscha B. et al. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med 2002; 347: 1483–1492 [DOI] [PubMed] [Google Scholar]
- 28. Sarzynski M, Burton J, Rankinen T. et al. The effects of exercise on the lipoprotein subclass profile: a meta-analysis of 10 interventions. Atherosclerosis 2015; 243: 364–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eidemak I, Haaber A, Feldt-Rasmussen B. et al. Exercise training and the progression of chronic renal failure. Nephron 1997; 75: 36–40 [DOI] [PubMed] [Google Scholar]
- 30. Headley S, Germain M, Milch D. et al. Exercise training improves HR responses and VO2peak in predialysis kidney patients. Med Sci Sports Exerc 2012; 44: 2392–2399 [DOI] [PubMed] [Google Scholar]
- 31. Boyce ML, Robergs RA, Avasthi PS. et al. Exercise training by individuals with predialysis renal failure: cardiorespiratory endurance, hypertension, and renal function. Am J Kidney Dis 1997; 30: 180–192 [DOI] [PubMed] [Google Scholar]
- 32. Pechter Ü, Ots M, Mesikepp S. et al. Beneficial effects of water-based exercise in patients with chronic kidney disease. Int J Rehabil Res 2003; 26: 153–156 [DOI] [PubMed] [Google Scholar]
- 33. Toyama K, Sugiyama S, Oka H. et al. Exercise therapy correlates with improving renal function through modifying lipid metabolism in patients with cardiovascular disease and chronic kidney disease. J Cardiol 2010; 56: 142–146 [DOI] [PubMed] [Google Scholar]
- 34. Venkataraman R, Sanderson B, Bittner V.. Outcomes in patients with chronic kidney disease undergoing cardiac rehabilitation. Am Heart J 2005; 150: 1140–1146 [DOI] [PubMed] [Google Scholar]
- 35. Headley S, Germain M, Wood R. et al. Short-term aerobic exercise and vascular function in CKD stage 3: a randomized controlled trial. Am J Kidney Dis 2014; 64: 222–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Headley S, Germain M, Wood R. et al. The blood pressure response to acute and chronic exercise in chronic kidney disease. Nephrology 2017; 7: 22; 72–78 [DOI] [PubMed] [Google Scholar]
- 37. American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 9th edn Philadelphia: Lippincott Williams & Wilkins, 2014 [Google Scholar]
- 38. Pietrobelli A, Rubiano F, St.-Onge M. et al. New bioimpedance analysis system: improved phenotyping with whole-body analysis. Eur J Clin Nutr 2004; 58: 1479–1484 [DOI] [PubMed] [Google Scholar]
- 39. Friedewald WT, Levy RI, Fredrickson DS.. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502 [PubMed] [Google Scholar]
- 40. Brunham LR, Hayden MR.. Human genetics of HDL: insight into particle metabolism and function. Prog Lipid Res 2015; 58: 14–25 [DOI] [PubMed] [Google Scholar]
- 41. Dutheil F, Walther G, Chapier R. et al. Atherogenic subfractions of lipoproteins in the treatment of metabolic syndrome by physical activity and diet—the RESOLVE trial. Lipids Health Dis 2014; 13: 112–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fahri A. Changes in serum lipid profile following moderate exercise. Afr J Pharm Pharmacol 2010; 4: 829–833 [Google Scholar]
- 43. Kannan U, Vasudevan K, Balasubramaniam K. et al. Effect of exercise intensity on lipid profile in sedentary obese adults. J Clin Diagn Res 2014; 8: BC08–BC10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vaziri N. Causes of dysregulation of lipid metabolism in chronic renal failure. Semin Dial 2009; 22: 644–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Attman PO, Samuelsson O, Alaupovic P.. Lipoprotein metabolism and renal failure. Am J Kidney Dis 1993; 21: 573–92 [DOI] [PubMed] [Google Scholar]
- 46. Vaziri ND, Deng G, Liang K.. Hepatic HDL receptor, SR-B1 and Apo A-I expression in chronic renal failure. Nephrol Dial Transplant 1999; 14: 1462–1466 [DOI] [PubMed] [Google Scholar]
- 47. Tofighi A, Rahmani F, Qarakhanlou BJ. et al. The effect of regular aerobic exercise on reverse cholesterol transport A1 and apo lipoprotein A-1 gene expression in inactive women. Iran Red Crescent Med J 2015; 17: e26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Collins M, Varady K, Jones P.. Modulation of apolipoprotein A1 and B, adiponectin, ghrelin, and growth hormone concentrations by plant sterols and exercise in previously sedentary humans. Can J Physiol Pharmacol 2007; 85: 903–910 [DOI] [PubMed] [Google Scholar]
- 49. Buyukyazi G. Differences in blood lipids and apolipoproteins between master athletes, recreational athletes and sedentary men. J Sports Med Phys Fitness 2005; 45: 112–120 [PubMed] [Google Scholar]
- 50. Vaziri N. Role of dyslipidemia in impairment of energy metabolism, oxidative stress, inflammation and cardiovascular disease in chronic kidney disease. J Clin Exp Nephrol 2014; 18: 265–268 [DOI] [PubMed] [Google Scholar]
- 51. Vaziri N, Sato T, Liang K.. Molecular mechanisms of altered cholesterol metabolism in rats with spontaneous focal glomerulosclerosis. Kidney Int 2003; 63: 1756–1763 [DOI] [PubMed] [Google Scholar]
- 52. Khabazian B, Ghanbari-Niaki A, Safarzadeh-Golpordesari AR. et al. Endurance training enhances ABCA1 expression in rat small intestine. Eur J Appl Physiol 2009; 107: 351–358 [DOI] [PubMed] [Google Scholar]
- 53. Lehmann R, Engler H, Honegger R. et al. Alterations of lipolytic enzymes and high-density lipoprotein subfractions induced by physical activity in type 2 diabetes mellitus. Eur J Clin Invest 2001; 31: 37–44 [DOI] [PubMed] [Google Scholar]
- 54. Williams P, Albers J, Krauss R. et al. Associations of lecithin: cholesterol acyltransferase (LCAT) mass concentrations with exercise, weight loss, and plasma lipoprotein subfraction concentrations in men. Atherosclerosis 1990; 82: 53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Brites F, Verona J, De Geitere C. et al. Enhanced cholesterol efflux promotion in well-trained soccer players. Metabolism 2004; 53: 1262–1267 [DOI] [PubMed] [Google Scholar]
- 56. Vaziri ND. Molecular mechanisms of lipid disorders in nephrotic syndrome. Kidney Int 2003; 63: 1964–1976 [DOI] [PubMed] [Google Scholar]
- 57. Trevisan R, Dodesini AR, Lepore G.. Lipids and renal disease. J Am Soc Nephrol 2006; 17(4 Suppl 2): S145–S147 [DOI] [PubMed] [Google Scholar]
- 58. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150. [DOI] [PubMed] [Google Scholar]
- 59. Moradi H, Yuan J, Ni Z. et al. Reverse cholesterol transport pathway in experimental chronic kidney disease. Am J Nephrol 2009; 30: 147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhao Y, Marcel YL.. Serum albumin is a significant intermediate in cholesterol transfer between cells and lipoproteins. Biochemistry 1996; 35: 7174–7180 [DOI] [PubMed] [Google Scholar]
- 61. Banz W, Maher M, Thompson W. et al. Effects of resistance versus aerobic training on coronary artery disease risk factors. Exp Biol Med 2003; 228: 434–440 [DOI] [PubMed] [Google Scholar]
- 62. LeMura L, von Duvillard S, Andreacci J. et al. Lipid and lipoprotein profiles, cardiovascular fitness, body composition, and diet during and after resistance, aerobic and combination training in young women. Eur J Appl Physiol 2000; 82: 451–458 [DOI] [PubMed] [Google Scholar]
- 63. Kelley G, Kelley K, Roberts S. et al. Comparison of aerobic exercise, diet or both on lipids and lipoproteins in adults: a meta-analysis of randomized controlled trials. Clin Nutr 2012; 31: 156–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fisher G, Brown A, Bohan Brown M. et al. High intensity interval- vs moderate intensity- training for improving cardiometabolic health in overweight or obese males: a randomized controlled trial. PLoS One 2015; 10: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Liou K, Ho S, Fildes J. et al. High intensity interval versus moderate intensity continuous training in patients with coronary artery disease: a meta-analysis of physiological and clinical parameters. Heart Lung Circ 2016; 25: 166–174 [DOI] [PubMed] [Google Scholar]
- 66. Paoli A, Pacelli Q, Moro T. et al. Effects of high-intensity circuit training, low-intensity circuit training and endurance training on blood pressure and lipoproteins in middle-aged overweight men. Lipids Health Dis 2013; 12: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sheikholeslami Vatani D, Ahmadi S, Ahmadi Dehrashid K. et al. Changes in cardiovascular risk factors and inflammatory markers of young, healthy, men after six weeks of moderate or high intensity resistance training. J Sports Med Phys Fitness 2011; 51: 695–700 [PubMed] [Google Scholar]
- 68. Durstine JL, Grandjean PW, Davis PG. et al. Blood lipid and lipoprotein adaptations to exercise: a quantitative analysis. Sports Med 2001; 31: 1033–1062 [DOI] [PubMed] [Google Scholar]
- 69. Dattilo A, Kris-Etherton P.. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am J Clin Nutr 1992; 56: 320–328 [DOI] [PubMed] [Google Scholar]
- 70. Shaw K. Exercise for overweight or obesity. Cochrane Database Syst Rev 2006; 4: CD003817. [DOI] [PMC free article] [PubMed] [Google Scholar]