Abstract
The field of infectious disease is undergoing a paradigm shift as the intestinal microbiome is becoming understood. The aim of this review is to inform infectious disease physicians of the potential relevance of the intestinal microbiome to their practice. We searched Medline using both index and text words relating to infectious diseases, microbiome, and probiotics. Relevant articles published up through 2017 were reviewed within Rayyan. The review illustrates pathophysiologic concepts linking the microbiome and infectious diseases; specifically, the intestinal microbiome’s relevance to early immune development, the microbiome and enteric infections, the microbiome’s relevance in compromised hosts, and antimicrobial resistance. Within each subject, there are specific examples of diseases and at-risk patient populations where a role for the microbiome has been strongly established. This provides an overview of the significance of the intestinal microbiome to microbiology, pediatric and adult infectious diseases with an underpinning of concepts useful for the practicing clinician.
Keywords: immunology, infectious diseases, intestinal microbiome, microbiology
The fields of clinical microbiology and infectious diseases are undergoing a paradigm shift as the intricate interactions between the intestinal microbiome, the immune system, and human pathogens are slowly being untwined. The human microbiome is the collective genome of trillions of bacteria, archaea, fungi, viruses, and eukaryotes, which can be conceptualized as a complex ecosystem existing within and on the human host (see list of definitions in Table 1) [1]. The largest and most heterogeneous of these microbial communities is found in the gastrointestinal tract. Infectious disease physicians and microbiologists, long trained in recognizing and treating individual human pathogens, are increasingly recognizing a need to incorporate the findings arising from the nascent microbiome field into their daily clinical practice.
Table 1.
List of Definitions
| Term | List of Definitions |
|---|---|
| Microbiome | The collection of all genomes of microorganisms from a defined environment, such as the human intestine. |
| Microbiota | The collection of all microorganisms in a defined environment, such as the human intestine. Virome: The collection of all viruses in a defined environment, such as the human intestine. |
| Mycobiome | The collection of all fungi in a defined environment, such as the human intestine. |
| Resistome: | The collection of all antimicrobial resistance genomes derived from microorganisms from a defined environment, such as the human intestine. |
| Ecosystem | The complex of a community of organisms and its environment functioning as an ecological unit. |
| Ecology | The totality or pattern of relations between organisms and their environment. |
| Commensal microbiome | Often referred to as an ensemble of microorganisms that reside in close proximity and in mutualistic relation with the host. However, the more correct term describing the resident microbiota in the intestines may be “Amphibiont” organisms that may have a pathogenic (detrimental), commensal (neutral), or symbiotic relationship (beneficial) with the host. We therefore use the term, “resident microbiota” in this review to describe the aggregate (pathogenic, commensal, symbiotic) endogenous microbiota in the intestine. |
| Pathobionts | Potentially pathogenic microorganisms residing in the microbiota. |
| Dysbiosis | A perturbation that departs from an otherwise balanced ecology to prolong, exacerbate, or induce a detrimental health effect. |
| Prebiotics | Nutritional substrates that promote the growth of microbes that confer a health benefit on the host. |
| Probiotics | A live microorganism that, when administered in adequate amounts, confer a health benefit on the host. |
| Synbiotics | Formulations consisting of a combination of pre- and probiotics. |
| Fecal Microbiota Transplantation (FMT) | The introduction of a liquid filtrate of stools from a healthy donor into the gastrointestinal tract of an ill patient. |
| Selective Decontamination of the Digestive Tract (SDD) | Use of daily antibiotics with the aim of preventing hospital-acquired infections while preserving the anaerobic microbiota. |
Much of the current understanding of the intestinal microbiome is made possible by the application of culture-independent, high-throughput deoxyribonucleic acids equencing techniques to describe the community structures and functions of the microorganisms (microbiota) residing in the human intestinal tract. These techniques are described in detail in several excellent reviews [2, 3] and briefly in Table 2.
Table 2.
Techniques Used to Study the Structure and Function of the Intestinal Microbiome
| Name | Purpose | Method |
|---|---|---|
| Biomarker Sequencing | Studies of the sequence variation of 1 ubiquitous gene (eg, 16S ribosomal ribonucleic acid [RNA] for bacteria) to describe microbial composition within an environmental study | Next-generation sequencing |
| Metagenomics | Studies of the function of all genetic material within an environmental study | Next-generation sequencing |
| Metatranscriptomics | Studies of gene expression at the RNA level | Next-generation sequencing |
| Metaproteomics | Studies of gene expression at the protein level | Liquid or gas chromatography, mass spectometry |
| Metabolomics | Studies of metabolite formation by the microbiota | Liquid or gas chromatography, mass spectometry |
As the genetic composition and functionality of the bacterial intestinal microbiome is charted in greater detail, essential roles have emerged for the intestinal microbiome in human physiology. Conceptualized as “the last undiscovered human organ” [4], the intestinal microbiome influences the development and differentiation of the immune system (described in more detail below), it is critical in energy metabolism and catabolism, and it modulates bile and lipid metabolism, endocrine regulation, neurologic signaling, and drug metabolism, among other roles. Imbalance in the microbiota composition or function, or dysbiosis, also associate with numerous diseases ranging from inflammatory bowel disease and atopy to diabetes, obesity, and arthritis [5, 6]. Due to the infancy of intestinal microbiome research, animal studies and associative data predominate the field. Although numerous studies correlate microbiome composition and function with disease states, studies are widely heterogeneous, and few clearly demonstrate a clear pathophysiologic mechanism and causality.
This review attempts to offer the clinical microbiologist and infectious disease physician 2 things: (1) an overview of concepts that can provide a pathophysiologic frame of reference for the interaction between the microbiome and infectious diseases and (2) specific examples of infectious diseases and at-risk patient populations where a role for the microbiome has been strongly established. This is supplemented by a tabular (Table 4) and pictorial overview (Figure 1) of all those infectious diseases in which a clinical correlation between a disease and the microbiome exist.
Table 4.
Overview of the Infectious Diseases or Patient Groups With Risk of Infectious Disease in Which the Microbiome Has Been Targeted for Prevention or Treatment and Tested in Human Clinical Trials
| Patient Category | Disease | Target and Known Effects | Key Refs. |
|---|---|---|---|
| Critically ill, adult | VAP | SDD for the prevention of respiratory tract infections. Probiotics for the prevention of VAP. Moderate evidence supports the indication. | [1, 2] |
| Mortality | SDD and probiotics for the prevention of mortality. Strong evidence for SDD. No conclusions possible based on limited evidence for probiotics. | [2–5] | |
| Critically ill, neonatal | Necrotizing enterocolitis | Probiotics for the prevention of NEC. Strong evidence supporting derived from meta-analyses supporting probiotics for prevention of NEC severity and mortality. However, recent RCT, not included in the meta-analysis, showed no benefit of probiotic in NEC. | [6, 7, 39] |
| Candidemia | Probiotics tested for prevention of candidemia, Candida colonization, and candiduria. No conclusions possible based on limited evidence | [8] | |
| Late-onset sepsis | Probiotics for the prevention of late-onset sepsis in preterm infants. Moderate evidence supporting probiotics for the prevention of late-onset mortality. | [7, 9] | |
| Surgical | Trauma | Probiotics for the prevention of “infectious complications” and mortality. SDD for prevention of “infectious complications and mortality”. Heterogeneous studies, with some support for use, no conclusions possible based on limited evidence. | [10, 11] |
| Post-GI surgery | Probiotics for the prevention of infectious complications and mortality. Heterogenous studies, with some support for use. No conclusions possible based on limited evidence. | [12, 13] | |
| Oncology and hematology | HSCT and chemotherapy complications | Microbiome composition and diversity can act as a predictor for the risk of blood stream and other infections. FMT (study ongoing) and probiotics to decrease infectious complications and GvHD—no conclusions possible based on limited evidence to date. | [14–16] |
| Mucositis | Probiotics for the prevention and treatment of chemotherapy and radiation-induced mucositis and diarrhea. Few studies, significant heterogeneity, no conclusions possible based on limited evidence. | [17, 18] | |
| HIV | Disease progression | Probiotics and synbiotics for improvement of immune function. Small, heterogeneous studies, no conclusions possible based on limited evidence. | [19] |
| Diarrhea | Probiotics to decrease diarrhea in HIV patients. Small, heterogeneous studies, no conclusions possible based on limited evidence. | [20] | |
| Upper respiratory | Upper respiratory tract infection | Probiotics for prevention of URTI in children. Small, heterogeneous studies, some support of use, no conclusions possible based on limited evidence. | [21, 22] |
| Gastrointestinal | Clostridium difficile and antibiotic-associated diarrhea | FMT effective as treatment for refractory C difficile. Bacteriotherapy for prevention. Probiotics for prevention. Moderate quality evidence suggests probiotics are safe and effective. | [23–25] |
| Acute infectious gastroenteritis | Probiotics for the prevention and treatment of infectious gastroenteritis. Evidence supports probiotics use in the treatment of persistent diarrhea in pediatric patients and shortening and reducing stool frequency in adults and infants. | [26, 27] | |
| Traveler’s diarrhea | Probiotics for the prevention of traveler’s diarrhea. Limited and inconclusive evidence that probiotics prevent traveler’s diarrhea. | [28, 29] | |
| Amebiasis | Probiotics for the treatment of amebiasis in children. No conclusions possible based on limited evidence. | [30] | |
| Helicobacter pylori | Probiotics for the adjunctive treatment of H pylori. No evidence probiotics improve eradication. | [31] | |
| Spontaneous bacterial peritonitis | Probiotics for the prevention of SBP in patients with ascites. No evidence that probiotics prevent SBP. | [32] | |
| Urogenital | Urinary tract infection | Probiotics for the prevention of (recurrent) UTI. No significant benefit, no conclusions possible based on limited evidence. | [33] |
| Antimicrobial resistance | Multiresistant infections or colonization | FMT for treatment of multidrug-resistant colonization and AMR genes. FMT can reduce antibiotic-resistant organisms and genes, but evidence to date of clinical consequences only in case series and reports. | [34–36] |
| Vaccines | Polio vaccine, rotoavirus vaccine | Antibiotics (azithromycine) to improve oral polio vaccine efficacy showed no effect. There is a significant association between microbiome composition and rotavirus vaccine. | [37, 38] |
Abbreviations: AMR, antimicrobial resistance; FMT, fecal microbiota transplant; GI, gastrointestinal; GvHD, graft-versus-host disease; HIV, human immunodeficiency virus; HSCT, hematopoietic stem cell transplantation; NEC, necrotizing enterocolitis; SBP, spontaneous bacterial peritonitis; RCT, randomized control trial; SDD, selective digestive tract decontamination; URTI, upper respiratory tract infection; UTI, urinary tract infection; VAP, ventilator-associated pneumonia.
NOTE: Please see Supplementary Material for list of references.
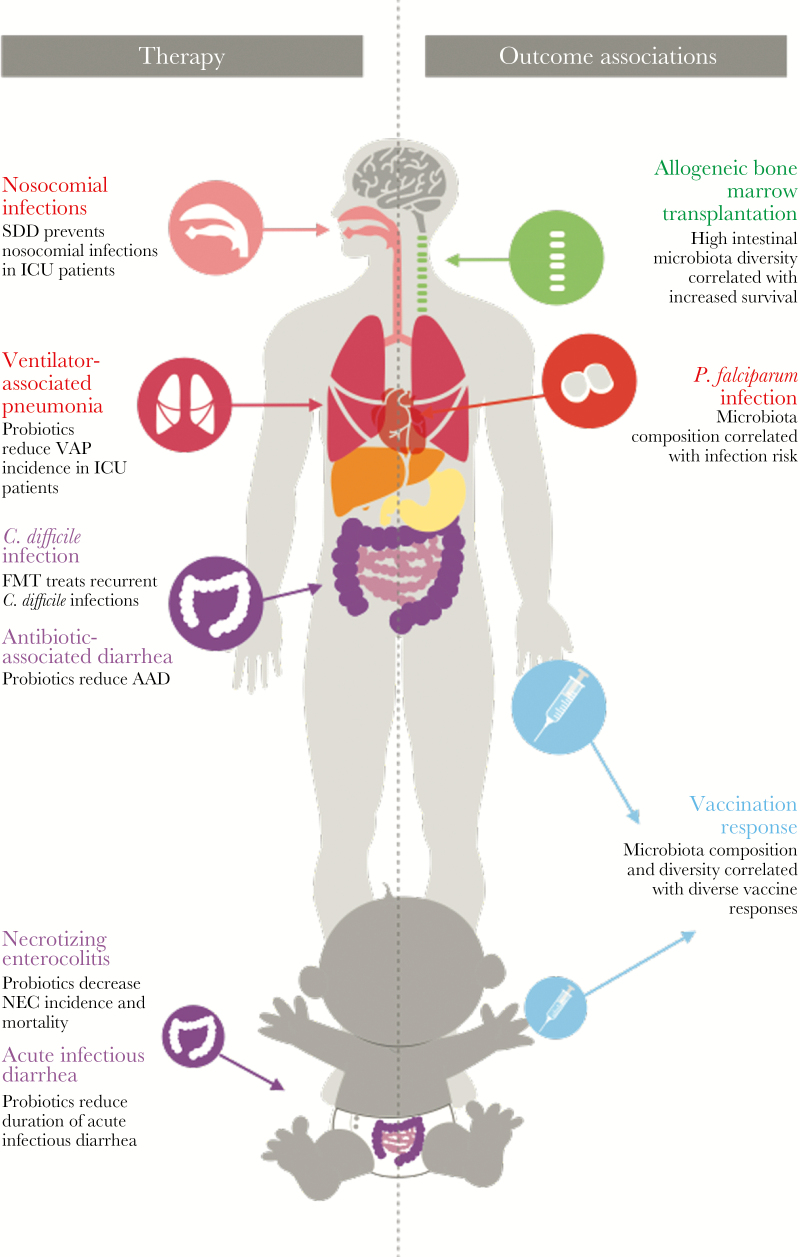
Figure 1.
A schematic overview of the infectious diseases in which there is a proven therapeutic role for microbiome manipulation through either probiotics or fecal microbiota transplantation (left panel) and an overview of the subjects for which there is a strong correlation between microbiome composition and risk of infectious disease (right panel). AAD, antibiotic-associated diarrhea; ICU, intensive care unit; NEC, necrotizing enterocolitis; P. falciparum, Plasmodium falciparum; SDD, selective digestive tract decontamination; VAP, ventilator-associated pneumonia.
SEARCH STRATEGY AND SELECTION CRITERIA
We searched Medline using both index and text words for infectious diseases, microbiome, and probiotics. The full search query and database details can be found in Table 3. Relevant articles published up through 2017 were reviewed within Rayyan. Articles published in English, French, German, and Dutch were included. Articles were screened by abstract and only included if there was a correlation between the risk, prevention, or treatment of an infectious disease and either the composition of the microbiome or manipulation of the microbiome. Manipulation included pre-, pro-, and synbiotics, fecal microbiota transplantation (FMT), or antimicrobial therapies. Only human studies were included. Table 4 provides a summary of the search findings, with an overview of all infectious diseases in which treatment targeting the microbiome has been tested.
Table 3.
Review Search Criteria
| No. | Review Search Criteria | Results |
|---|---|---|
| Searches | ||
| 1 | exp Enterocolitis, Necrotizing/ or exp Sepsis/ or exp Pneumonia, Ventilator-Associated/ or exp Pseudomonas aeruginosa/ or exp Critical Illness/ or exp Diarrhea/ or exp Clostridium difficile/ or exp Vaccination/ or exp Gastroenteritis/ or exp Stem Cell Transplantation/ or (“necrotizing enterocolitis” or NEC or sepsis or septic or “systemic infection” or “ventilator-associated pneumonia” or VAP or aeruginosa or (critical* adj ill*) or diarrhea or difficile or clostridium or vaccin* or Gastroenteritis or “stem cell”).ti,ab,kf. | 927 799 |
| 2 | “Gastrointestinal Microbiome”/ or dysbiosis/ or (“gut flora” or “fecal bacteria” or “commensal bacteria” or “fecal bacterial flora” or “enteric bacterial flora” or microbiome or “commensal microbes” or “gut microflora” or “intestinal microbial communities” or “intestinal bacteria” or dysbiosis or resistomes or metagenom*).ab. /freq=2 | 7082 |
| 3 | exp probiotics/ or exp prebiotics/ or exp synbiotics/ or exp Bifidobacterium/ or exp “Fecal Microbiota Transplantation”/ or (probiotic* or microbial supplement* or prebiotic* or synbiotic* or bifidobacterium or BBG-01 or clostridium scindens or bifidobacteria or bifidobacterium or “fecal microbiota transplantation” or FMT or fecal suspension or “fecal transplantation” or “fecal transfer” or “fecal infusion” or bacteriotherapy or “fecal donation” or selective decontamination).ab. /freq=2 | 18 450 |
| 4 | (2 or 3) and 1 | 5447 |
| 5 | exp animals/ not humans/ | 4 240 420 |
| 6 | 4 not 5 | 4463 |
| Search 01-02-2017 in Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R) 1946 to Present. |
THE MICROBIOME AND EARLY IMMUNE DEVELOPMENT
The microbiome plays a vital role in preventing infectious diseases as early as birth. Millions of years of evolution have shaped the interactions between bacterial communities and the human body, and there are elegant mutualistic relationships between the human host and microbiota. An infant may first be exposed to bacteria as early as in utero and upon delivery undergoes rapid intestinal colonization. The patterns of colonization are in part nonrandom and can be shaped by mode of delivery, breastfeeding, geography, genetics, antibiotics, and age [7]. Specific bacterial colonization is required for normal neonatal immune development [8], as is most clearly evidenced in germ-free mice who have highly aberrant gut-associated lymphoid tissue development in germ-free mice [9] and immunoglobulin A-producing B-cell maturation [10]. The commensal microbiome is implicated in shaping T-cell subsets, specifically effector T cell [11] and colonic T-regulatory cell generation [12, 13]. Finally, the microbiota and intestinal epithelium continually interact, leading to innate and adaptive immune signaling that likely maintain intestinal immune homeostasis throughout life.
NECROTIZING ENTEROCOLITIS
Necrotizing enterocolitis (NEC) illustrates how aberrant microbiome colonization in neonates can predispose to clinically relevant infectious disease. Although the pathophysiology of NEC is incompletely understood, a prevailing hypothesis is that NEC follows aberrant gut microbiome colonization, and factors such as premature birth and peri- or neonatal antibiotic administration may predispose for acquisition of NEC via perturbed microbial colonization patterns [14, 15]. Necrotizing enterocolitis disease risk primarily correlates with Proteobacteria (the phylum containing many aerobic Gram-negative pathogens) and anaerobic depletion [14, 16]. A body of literature also supports the use of probiotics (see Table 1 and Table 4) to prevent and or treat NEC, although the literature remains conflicting. Meta-analyses show significantly decreased relative risks for severe NEC and mortality only in preterm infants >1000 grams receiving enteral probiotic supplementation [17]. When used as medication, probiotics are not regulated by the US Food and Drug Administrations, and there are broad concerns about their content, infectious potential, and contamination, and inadequate data about the best form and duration of supplementation. These issues have limited the implementation of probiotic use in neonatal units in routine clinical practice and underscores the difficulty of modulating the intestinal microbiome safely in fragile populations [18].
THE MICROBIOME AND ENTERIC INFECTIONS
Enteric Viral Infections
The intestinal microbiome must increasingly be considered in host-pathogen interactions. Viral enteric infections illustrate the trilateral relationship binding infectious disease pathogens, intestinal microbiota, and host immunity. Numerous pediatric enteric viruses including polio, norovirus, and rotavirus have likely evolved to exploit the “bacterial” microbiota for immune evasion, entry, and replication in the gut. Polio virus (1) has diminished replication and cell entry in mice intestines whose bacteria have been depleted by antibiotics and (2) also uses bacterial surface polysaccharides (such as lipopolysaccharides) to enhance infectivity [19]. In antibiotic-depleted mice, rotavirus similarly has diminished replication and infectivity [20].
The bacterial microbiota can also calibrate innate immune responses to viruses. When flagellin derived from Escherichia coli is given to mice with rotavirus infections, the infection is cleared through Toll-like receptor-5 activation of innate immune defenses [21]. In addition, when the microbiota is disrupted with antibiotics during a murine norovirus infection, the innate immune system is capable of clearing the norovirus infection via interferon-λ signaling [22]. Although these findings are in mice, there is considerable potential relevance for the practicing clinician. The long-held belief that antibiotics have no effect on viral infections is challenged by these animal models demonstrating how antibiotic-induced alteration of the microbiota impacts both viral replication and host viral immunity.
Enteric Bacterial Infections
A key concept underpinning the importance of the intestinal microbiota to infectious diseases is termed colonization resistance. The microorganisms residing in the intestine can have pathogenic, commensal (lack of benefit or harm), or symbiotic relationships with the host. Enteric bacteria causing infections can be exogenous pathogens or pathobionts—potentially pathogenic microorganisms already part of the resident microbiota (Table 1). Along with providing protection against exogenous pathogens, an important function of the resident microbiota is to protect the host from enteric pathobiont overgrowth and eventual invasion [23]. This symbiotic conceptualization of the microbiota protecting the host against enteric infections and the host protecting the colonizing microbiota is termed colonization resistance [24]. The resistance provided by the microbiota against enteric pathogens can be divided into direct and indirect mechanisms. On the one hand, resident microbiota can inhibit or even kill pathogens directly via metabolic byproducts (bacteriocins, acids, peptides) [25], or it can outcompete pathogens for space, metabolites, and nutrients [26]. On the other hand, the microbiota can indirectly inhibit intestinal pathogens by calibrating host immune responses to them [13, 23]. Microbiota also can indirectly stimulate production of mucin, the protective mucin layer over the epithelium. Perturbation of this resident microbiota is therefore a common starting point for subsequent risk of true infection by pathobionts normally kept in check by these mechanisms. Antibiotic therapy is the most common cause of microbiome perturbation—rapidly and markedly decreasing bacterial microbiota diversity and abundance [27].
Clostridium difficile
The most well known example of this phenomenon is Clostridium difficile infection after antibiotic use. Clostridium difficile thrives after antibiotic use. Antibiotics deplete sensitive microbiota resulting in decreased microbiota signaling and diminished local and systemic immune responses to C difficile. Antibiotic use also increases the availability of primary bile acids, which the bacteria thrive upon, improving their colonization and triggering germination [28]. Diminished microbiota reduces C difficile’s need to compete for nutrients such as host carbohydrates, giving it a competitive advantage over non-pathogenic bacteria [28].
The clear relation between C difficile infection and antibiotic use makes it a prime target for microbiota-based therapy. It is, at the moment, the only infection (and disease) with a proven treatment indication with FMT. Fecal microbiota transplantation describes the introduction of a liquid filtrate of stools from a healthy donor into the gastrointestinal tract of an ill patient. A randomized, open-label trial demonstrated that FMT is significantly more effective for the treatment of recurrent C difficile infection than oral antibiotic therapy with vancomycin [29]. Fecal microbiota transplantation not only reconstitutes the bacterial diversity and richness of the microbiota but also transfers biologic products such as bile acids, proteins, and bacteriophages, which may contribute to its high success rate in C difficile infections [30]. Clostridium difficile infection is also one of the first infectious diseases where rationally designed microbial supplementation may become a feasible therapeutic alternative to FMT. Several studies demonstrate that specific bacteria or consortia can prevent C difficile infection [31, 32].
THE MICROBIOME AND COMPROMISED HOSTS
Specific sets of patients may have higher risks of microbiome perturbation leading to infectious disease. Those groups with the highest evidence for an etiologic role for the microbiome are critically ill and oncologic patients.
CRITICALLY ILL PATIENTS
Critically ill patients have a dramatically altered microbiome, and specific microbiome patterns have been associated with detrimental clinical outcomes [33]. Although antibiotics are key in treating infections and sepsis in critically ill patients, they may deplete protective microbiota [34]. In addition, critically ill patients’ microbiota sustain injury via hypoxic injury, disrupted epithelial permeability, altered gut motility, intraluminal pH values, and treatment with vasopressors, opioids, and parenteral or enteral nutrition [35]. These factors can facilitate rapid expansion of hospital-acquired pathogens or pathobionts, including vancomycin-resistant enterococcal infections [36] as well as Gram-negative Enterobacteriaceae invasion and infection [37].
Interventions aimed at altering the intestinal microbiome towards a protective phenotype in intensive care patients are understandably attractive. Probiotics exert unclear and heterogeneous influences in preventing adverse outcomes in critically ill patients (see Table 4). Selective decontamination of the digestive tract (SDD) (Table 4) is an alternative approach. The SDD is the use of daily antibiotics with the aim of preventing hospital-acquired infections while preserving the anaerobic microbiota [38]. Several studies demonstrate that SDD prevents nosocomial infections in critically ill patients and decreases overall mortality (see Table 4). However, broad implementation of SDD has been limited due to (perhaps unfounded) fear of selecting antibiotic-resistant bacteria and inducing long-lasting antibiotic resistance reservoirs [39, 40].
ONCOLOGY PATIENTS
Oncology patients undergoing chemotherapy or bone marrow transplantation also have specific microbiome-associated risks for infectious diseases [41]. Allogeneic hematopoietic stem cell transplantation is used as therapy in a range of hematologic malignancies and disorders. Ablation of the bone marrow through chemotherapy and radiation results in collateral gastrointestinal mucosal damage and alteration of the microbiome composition. Transplant patients also often receive antibiotic therapy. Those patients who can maintain a high microbiota diversity before stem cell engraftment have significantly lower (9% vs 53%) posttransplant mortality due to infection or graft-versus-host disease [42].
The microbiome also plays a role in the risk that neutropenic chemotherapy patients have for developing systemic blood stream infections. Antibiotics and chemotherapy-induced mucosal injury in combination with neutropenia can result in a progressive loss of colonization resistance [41, 43]. Sequential measurement of the microbiome before a blood stream infection in this population first shows loss of diversity and anaerobic microbiota, then microbiome domination by one bacterial strain, followed by a positive blood culture of the strain [44]. The most common pathobionts are vancomycin-resistant Enterococcus, Enterobacteriacae, and Streptococcus viridans. Preservation of, particularly, anaerobic microbiota, through limitation of the use of antianaerobic antibiotics in this population may protect against such expansion and subsequent infection [44]. Novel prevention and treatment options for oncologic patients includes identification of those exact microbiota that can inhibit specific pathobionts, which can then be developed and tested as rationally developed probiotics.
ANTIMICROBIAL RESISTANCE AND THE INTESTINAL MICROBIOME
The intestinal microbiome is both a barrier against and a potential repository for antimicrobial resistance. As described above, loss of colonization resistance can also lead to acquisition and/or expansion of antibiotic resistant-pathogens. In contrast, correction of a depleted microbiome via FMT can reverse resistant pathobiont dominance and even decrease the total numbers of antibiotic resistance genes present in the microbiome [45, 46].
The genomic study of the total antibiotic resistance harbored in the microbiome is just emerging, and, although not all resistance genes can disseminate, there is great potential for transfer of endogenous and acquired resistance genes within and to the microbiota. The aggregate of antibiotic resistance genes in the microbiome has been termed the resistome, and new genomic techniques are uncovering exactly how resistance genes are acquired and spread [47, 48]. Nearly identical resistance genes can be found, for example, in Gram-positive and Gram-negative bacterial species from the same host [49]. Clinically relevant resistance genes are present in children even without recent selective antibiotic pressure [50]. This implies that the resistome can expand due to indiscriminate antibiotic use but is also an intrinsic component of the resident microbiota, compounding the ever-expanding threat of antibiotic resistance in the field of infectious disease.
FUTURE DIRECTIONS
Research findings into the role of the gut microbiome in the field of infectious diseases remain rudimentary. The majority of work has focused upon the bacteria in the microbiome, yet there is an enormous knowledge gap pertaining to its other organisms and their transkingdom interactions. Very little is known about the role of archaea, viruses, fungi, helminths, and protozoa in the intestinal microbiome and their relationship to common infectious diseases. As knowledge gaps in the microbiome are filled, balances will shift towards new treatment opportunities. Simple addition of a probiotic or heavy-handed therapy with fecal transplantation will be supplanted by tailored microbiome therapy, with selected commensal repletion differing per host and targeted infectious disease. Human studies and randomized clinical trials, with an emphasis on therapeutic reproducibility and patient safety, are absolutely essential to translating heterogeneous basic research into new therapeutic paradigms. The intestinal microbiome will undoubtedly feed the fields of clinical microbiology and infection for years to come.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
We thank René Spijker for invaluable help and support in the search strategy for this article.
Financial support. This work was funded by the Netherlands Organization for Health Research Development (ZonMw, Vidi grant 91716475; to W. J. W.), the European Union (EU) through the Horizon 2020 Marie Sklodowska-Curie-European Sepsis Academy-Innovative Training Networks (MSCA-ESA-ITN) project (676129), and a Stichting Emma Foundation grant (to V. C. H.).
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Gordon JI. Honor thy gut symbionts redux. Science 2012; 336:1251–3. [DOI] [PubMed] [Google Scholar]
- 2. Fraher MH, O’Toole PW, Quigley EM. Techniques used to characterize the gut microbiota: a guide for the clinician. Nat Rev Gastroenterol Hepatol 2012; 9:312–22. [DOI] [PubMed] [Google Scholar]
- 3. Kuczynski J, Lauber CL, Walters WA et al. Experimental and analytical tools for studying the human microbiome. Nat Rev Genet 2011; 13:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baquero F, Nombela C. The microbiome as a human organ. Clin Microbiol Infect 2014; 18:2–4. [DOI] [PubMed] [Google Scholar]
- 5. Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med 2016; 375:2369–79. [DOI] [PubMed] [Google Scholar]
- 6. Young VB. The role of the microbiome in human health and disease: an introduction for clinicians. BMJ 2017; 356:j831. [DOI] [PubMed] [Google Scholar]
- 7. Yatsunenko T, Rey FE, Manary MJ et al. Human gut microbiome viewed across age and geography. Nature 2012; 486:222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chung H, Pamp SJ, Hill JA et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell 2012; 149:1578–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bouskra D, Brézillon C, Bérard M et al. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature 2008; 456:507–10. [DOI] [PubMed] [Google Scholar]
- 10. Kunisawa J, Gohda M, Hashimoto E et al. Microbe-dependent CD11b+ IgA+ plasma cells mediate robust early-phase intestinal IgA responses in mice. Nat Commun 2013; 4:1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ivanov II, Frutos Rde L, Manel N et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe 2008; 4:337–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Atarashi K, Tanoue T, Oshima K et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013; 500:232–6. [DOI] [PubMed] [Google Scholar]
- 13. Kamada N, Núñez G. Regulation of the immune system by the resident intestinal bacteria. Gastroenterology 2014; 146:1477–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Warner BB, Deych E, Zhou Y et al. Gut bacteria dysbiosis and necrotising enterocolitis in very low birthweight infants: a prospective case-control study. Lancet 2016; 387:1928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Morrow AL, Lagomarcino AJ, Schibler KR et al. Early microbial and metabolomic signatures predict later onset of necrotizing enterocolitis in preterm infants. Microbiome 2013; 1:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pammi M, Cope J, Tarr PI et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome 2017; 5:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. AlFaleh K, Anabrees J. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev 2014; 4:CD005496. [DOI] [PubMed] [Google Scholar]
- 18. Urben LM, Wiedmar J, Boettcher E et al. Bugs or drugs: are probiotics safe for use in the critically ill? Curr Gastroenterol Rep 2014; 16:388. [DOI] [PubMed] [Google Scholar]
- 19. Kuss SK, Best GT, Etheredge CA et al. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science 2011; 334:249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uchiyama R, Chassaing B, Zhang B, Gewirtz AT. Antibiotic treatment suppresses rotavirus infection and enhances specific humoral immunity. J Infect Dis 2014; 210:171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang B, Chassaing B, Shi Z et al. Viral infection. Prevention and cure of rotavirus infection via TLR5/NLRC4-mediated production of IL-22 and IL-18. Science 2014; 346:861–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baldridge MT, Nice TJ, McCune BT et al. Commensal microbes and interferon-λ determine persistence of enteric murine norovirus infection. Science 2015; 347:266–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buffie CG, Pamer EG. Microbiota-mediated colonization resistance against intestinal pathogens. Nat Rev Immunol 2013; 13:790–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. van der Waaij D, Berghuis-de Vries JM, Lekkerkerk-van der Wees JC. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J Hyg (Lond) 1971; 69:405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rea MC, Sit CS, Clayton E et al. Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc Natl Acad Sci U S A 2010; 107:9352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Abt MC, Pamer EG. Commensal bacteria mediated defenses against pathogens. Curr Opin Immunol 2014; 29:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci USA 2011; 108Suppl 1:4554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Britton RA, Young VB. Role of the intestinal microbiota in resistance to colonization by Clostridium difficile. Gastroenterology 2014; 146:1547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Nood E, Vrieze A, Nieuwdorp M et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013; 368:407–15. [DOI] [PubMed] [Google Scholar]
- 30. Cammarota G, Ianiro G, Gasbarrini A. Fecal microbiota transplantation for the treatment of Clostridium difficile infection: a systematic review. J Clin Gastroenterol 2014; 48:693–702. [DOI] [PubMed] [Google Scholar]
- 31. Buffie CG, Bucci V, Stein RR et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature. 2015; 517:205–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tvede M, Rask-Madsen J. Bacteriotherapy for chronic relapsing Clostridium difficile diarrhoea in six patients. Lancet 1989; 1:1156–60. [DOI] [PubMed] [Google Scholar]
- 33. Schuijt TJ, van der Poll T, de Vos WM, Wiersinga WJ. The intestinal microbiota and host immune interactions in the critically ill. Trends Microbiol 2013; 21:221–9. [DOI] [PubMed] [Google Scholar]
- 34. Lankelma JM, van Vught LA, Belzer C et al. Critically ill patients demonstrate large interpersonal variation in intestinal microbiota dysregulation: a pilot study. Intensive Care Med 2017; 43:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dickson RP. The microbiome and critical illness. Lancet Respir Med 2016; 4:59–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Donskey CJ, Chowdhry TK, Hecker MT et al. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 2000; 343:1925–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Donskey CJ. Antibiotic regimens and intestinal colonization with antibiotic-resistant Gram-negative bacilli. Clin Infect Dis 2006; 43Suppl 2:S62–9. [DOI] [PubMed] [Google Scholar]
- 38. D’Amico R, Pifferi S, Leonetti C et al. Effectiveness of antibiotic prophylaxis in critically ill adult patients: systematic review of randomised controlled trials. BMJ 1998; 316:1275–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vincent JL, Jacobs F. Effect of selective decontamination on antibiotic resistance. Lancet Infect Dis 2011; 11:337–8. [DOI] [PubMed] [Google Scholar]
- 40. Daneman N, Sarwar S, Fowler RA, Cuthbertson BH. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis 2013; 13:328–41. [DOI] [PubMed] [Google Scholar]
- 41. Taur Y, Pamer EG. Microbiome mediation of infections in the cancer setting. Genome Med 2016; 8(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Taur Y, Jenq RR, Perales MA et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 2014; 124:1174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ubeda C, Taur Y, Jenq RR et al. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest 2010; 120:4332–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Taur Y, Xavier JB, Lipuma L et al. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 2012; 55:905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stripling J, Kumar R, Baddley JW et al. Loss of vancomycin-resistant enterococcus fecal dominance in an organ transplant patient with Clostridium difficile colitis after fecal microbiota transplant. Open Forum Infect Dis 2015; 2:ofv078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Millan B, Park H, Hotte N et al. Fecal microbial transplants reduce antibiotic-resistant genes in patients with recurrent Clostridium difficile infection. Clin Infect Dis 2016; 62:1479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van Schaik W. The human gut resistome. Philos Trans R Soc Lond B Biol Sci 2015; 370:20140087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Crofts TS, Gasparrini AJ, Dantas G. Next-generation approaches to understand and combat the antibiotic resistome. Nature Rev Microbiol 2017; 67:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chung WO, Young K, Leng Z, Roberts MC. Mobile elements carrying ermF and tetQ genes in Gram-positive and Gram-negative bacteria. J Antimicrob Chemother 1999; 44: 329–35. [DOI] [PubMed] [Google Scholar]
- 50. Moore AM, Ashcraft MH. Children’s mathematical performance: five cognitive tasks across five grades. J Exp Child Psychol 2015; 135:1–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.