Abstract
Background
Quantification of cytomegalovirus (CMV) deoxyribonucleic acid (DNA) has important diagnostic, prognostic, and therapeutic implications in the management of transplant recipients. We aimed to assess a viral load in plasma and whole blood that distinguishes CMV disease from asymptomatic infection in a cohort of solid organ and hematopoietic stem cell transplantation.
Methods
We prospectively measured and compared CMV viral load in paired plasma and whole blood samples collected from transplant recipients with CMV infection and disease. Cytomegalovirus viral loads were determined by a commercially available US Food and Drug Administration-approved quantitative assay (COBAS AmpliPrep/COBAS TaqMan CMV Test [CAP/CTM CMV]) calibrated to the first World Health Organization International Standard for CMV DNA quantification.
Results
Moderate agreement of CMV viral load was observed between plasma and whole blood, with 31% of samples having discordant findings, particularly among samples with low DNA levels. Among the subset of samples where both paired samples had quantifiable levels, we observed a systematic bias that reflected higher viral load in whole blood compared with plasma. Based on receiver operating curve analysis, an initial plasma CMV viral load threshold of 1700 IU/mL in solid organ transplant recipients (sensitivity 80%, specificity 74%) and 1350 IU/mL in allogeneic hematopoietic stem cell transplant recipients (sensitivity 87%, specificity 87%) distinguished CMV disease and asymptomatic infection.
Conclusions
This study identifies standardized viral load thresholds that distinguish CMV disease from asymptomatic infection using CAP/CTM CMV assay. We propose these thresholds as potential triggers to be evaluated in prospective studies of preemptive therapy. Plasma was better than whole blood for measuring viral load using the CAP/CTM CMV assay.
Keywords: CMV DNA, cytomegalovirus, transplantation, viral load
Cytomegalovirus (CMV) causes significant morbidity after solid organ transplantation (SOT) and hematopoietic stem cell transplantation (HSCT). Quantitative nucleic acid testing (QNAT) is the most common diagnostic method used to guide initiation of antiviral therapy and to assess response to treatment [1, 2]. Current consensus guidelines recommend continuation of treatment until viral suppression is confirmed by at least 2 subsequent CMV viral load (VL) tests, because persistent DNAemia at end of treatment was associated with higher recurrence rates and delayed disease resolution [1–4].
The widespread applicability of treatment guidelines has been hampered by a lack of standardized assays for CMV QNAT [5]. Previous studies have suggested that the major contributor to interassay VL variability is the calibration standard. Hence, the first World Health Organization (WHO) International Standard for CMV QNAT was released in 2010. This has allowed diagnostic laboratories to transition the reporting of CMV VL results in international units per milliliter [6]. The COBAS AmpliPrep/COBAS TaqMan CMV Test (CAP/CTM CMV; Roche Molecular Systems, Inc., Branchburg, NJ) was the first commercially available CMV QNAT approved by the US Food and Drug Administration (FDA) for monitoring transplant patients during treatment of CMV disease. However, there is limited data available to distinguish VL of patients with asymptomatic CMV infection and disease. Likewise, the data on the utility of this standardized assay in HSCT recipients is limited.
The CAP/CTM CMV is marketed for quantifying VL in plasma (PL) samples. However, several studies, using nonstandardized, laboratory-developed CMV QNAT, have suggested that whole blood (WB) may be more sensitive, because it generally yields higher CMV deoxyribonucleic acid (DNA) levels when compared with PL and potentially earlier detection [7–9]. As a result, some diagnostic laboratories have routinely used WB for monitoring. There is currently limited data on the performance of CAP/CTM CMV using WB samples. To date, only a limited number of studies have correlated VL measurements in PL and WB using a standardized CMV QNAT [8, 9].
To determine the ideal blood compartment for VL measurement using standardized CMV QNAT, we prospectively quantified CMV DNA in paired PL and WB samples from transplant recipients with CMV infection and disease. In addition, we aimed to define VL thresholds to distinguish CMV disease from asymptomatic infection, and we propose values that could be evaluated in prospective clinical trials of preemptive antiviral therapy.
METHODS
Patients and Specimen Collection
Adult SOT and HSCT recipients (>18 years of age) diagnosed and treated for CMV syndrome (among SOT), asymptomatic DNAemia, or organ-specific disease were included. All patients were identified by a positive CMV QNAT in PL. Clinical laboratory personnel alerted the study team, who then followed the patients prospectively without any study-specific interventions. Plasma measurements were performed as part of routine clinical care, either for surveillance or clinical suspicion. Whole blood samples were obtained from leftover specimens collected for other indications. For inclusion into this study, paired PL and WB should be available at the time of CMV diagnosis (before initiation of antiviral therapy), on a weekly basis while on antiviral therapy, and at least once after discontinuation of therapy. Paired samples were tested using CAP/CTM CMV assay. Results of WB samples were not available to the primary clinical team. Demographic and clinical data were obtained by electronic chart review. All data were stored in a password-protected database. This study protocol was approved by the Mayo Clinic Institutional Review Board. Consent was waived because our study qualified as minimal risk, and only “waste WB samples” were retrieved for CMV VL testing (after all clinically indicated testing have been performed).
Cytomegalovirus Definition and Treatment
Definitions of CMV infection and disease were according to published guidelines [1, 2] and concurred with the most recent iteration [10]. This included asymptomatic infection, CMV syndrome (only in SOT), and organ-invasive disease. Diagnosis and treatment were implemented by the primary clinical team, using a protocol guided by CMV QNAT in PL, without study-associated interventions. Initial VL was defined as the first VL before initiation of therapy; peak VL was the highest documented VL.
Sample Storage and Processing
Plasma samples were subjected to CMV VL testing in real-time using CAP/CTM CMV according to manufacturer’s instructions. In PL, the lower limit of detection (LoD) is 91 IU/mL (95% detection rate), whereas the lower limit of quantification (LLoQ) is 137 IU/mL and the upper LoQ (ULoQ) is 9100000 IU/mL. Thus, CMV DNA levels >0 but <137 IU/mL are detectable but not quantifiable; quantification is possible between 137 and 9100000 IU/mL; levels >9100000 IU/mL are detectable but not quantifiable.
Whole blood samples were retrieved and stored at 2°C to 8°C for a maximum of 3 days (CMV DNA is stable for up to 14 days at this temperature [11]), then frozen at −70°C until batch testing was performed using the assay manufacturer’s recommended pre-extraction modifications. Because the CAP/CTM CMV assay was designed to test only PL samples, such modifications were necessary to allow WB samples to be tested with this assay and generate valid results. Briefly, samples were diluted 1:6 in Specimen Pre-Extraction Reagent (Roche Molecular Systems, Inc.) followed by incubation at 56°C for 10 minutes with continuous shaking at 1000 rpm. Because of the initial 1:6 dilution, all quantifiable results were multiplied by 6. Based on this, the calculated quantification range for WB was 822 IU/mL to 54600000 IU/mL. Finally, a previously determined assay calibration correction factor (−0.43 log10 IU/mL) was applied to each log-transformed result. This correction factor was determined by testing dilutions of a CMV quantification standard (AcroMetrix CMVtc Panel; Microgenics Corp., Fremont, CA) prepared in triplicate at each of 7 levels spanning the reportable range of the assay (6.00, 5.70, 5.00, 4.70, 4.00, 3.70, and 3.00 log10 IU/mL) and calculating the mean difference between observed and expected results (data not shown). The LoD for WB was experimentally determined to be 240 IU/mL by testing replicate dilutions of the same CMV quantification standard (AcroMetrix CMVtc Panel) spiked in WB followed by Probit analysis with a 95% detection rate (data not shown).
Statistical Analysis
Descriptive statistics on baseline characteristics were reported as percentages or quartiles (median, interquartile range [IQR]) as appropriate. To express the agreement between paired PL and WB samples, Cohen’s weighted kappa (κ) was presented for discrete levels of detection (VL categorized as follows: undetectable vs detectable, but not quantifiable vs quantifiable), whereas Lin’s concordance correlation coefficient (CCC) was reported for numerical values among the subset of paired results in which both yielded quantifiable results. In addition, within this subset, the relationship between PL and WB logarithmically transformed measurements was modeled with Deming regression. The derived regression line was superimposed on a scatterplot to represent the linear trend in relation to the line of symmetry (ie, perfect concordance). Additional analysis of agreement was performed by the Bland-Altman method to facilitate assessment of systematic patterns. To assess differences between groups with CMV disease versus asymptomatic infection, the Wilcoxon rank-sum test was used. A single optimal threshold was identified from receiver operating curve (ROC) analysis as the VL value that best distinguishes between symptomatic and asymptomatic groups according to sensitivity and specificity. Analyses were performed using SAS statistical software (version 9.4; SAS Institute, Inc., Cary, NC).
RESULTS
Patient Characteristics
A total of 88 SOT and HSCT recipients were enrolled. The majority were male (61%), median age was 56.7 years (IQR, 47.8–63.0), with a nearly equal distribution between SOT (52%) and HSCT (48%) (Table 1). The types of CMV infection were asymptomatic DNAemia (57/88 [65%]), viral syndrome (11/88 [13%]; SOT recipients only), and tissue-invasive disease (20/88 [23%]). During a median follow-up of 7.4 months (IQR, 4.3–11.0) from the time of the initial CMV episode, there were a total of 16 virologic relapses (1-year event rate, 18%), all occurring within 8 months. The majority of patients were alive at 1-year follow-up after CMV infection (1-year survival, 73%).
Table 1.
Patient Demographics and Baseline Clinical Characteristics
| Characteristic | No. of Patients With Data Available | Result |
|---|---|---|
| Age at time of transplant (years) | 88 | 56.7 (47.8, 63.0)a |
| Male gender | 88 | 54 (61%) |
| White | 87 | 75 (86%) |
| Year of transplant | 88 | 2013 [1989, 2015]b |
| Type of transplant | 88 | |
| Solid organ | 46 (52%) | |
| HSCT, autologous | 3 (3%) | |
| HSCT, allogenic | 39 (44%) | |
| Type of SOT | 46 | |
| Heart | 8 (17%) | |
| Lung | 2 (4%) | |
| Kidney | 11 (24%) | |
| Liver | 21 (46%) | |
| Other | 1 (2%) | |
| Combined | 3 (7%) | |
| CMV status, pretransplant, SOT subgroup | 43 | |
| D+R− | 17 (40%) | |
| D+R+ | 18 (42%) | |
| D−R+ | 8 (19%) | |
| CMV status, pretransplant, HSCT subgroup | 35 | |
| D+R− | 0 (0%) | |
| D+R+ | 18 (51%) | |
| D−R+ | 17 (49%) | |
| Year of CMV infection | 88 | 2014 [2013, 2015]b |
| Type of CMV infection | 88 | |
| Asymptomatic | 57 (65%) | |
| Syndrome (all SOT recipients) | 11 (13%) | |
| Tissue-invasive | 20 (23%) |
Abbreviations: CMV, cytomegalovirus; D, donor; HSCT, hematopoietic stem cell transplantation; R, recipient; SOT, solid organ transplantation.
aMedian (25th, 75th percentiles).
bMedian [minimum, maximum].
Comparison of Cytomegalovirus Viral Load in Plasma and Whole Blood
A total of 403 pairs of PL and WB were collected (median of 4 [IQR, 2–6] samples per person). For detection of any level of CMV DNA, the overall agreement between PL and WB was moderate (κ = 0.61; 95% confidence interval [CI], 0.56–0.67) (Table 2). Of the 403 sample pairs, 279 (69.2%) had concordant levels of detection; there were 124 sample pairs (30.8%) with discordant results where CMV DNA was detected in either PL or WB alone (in these discordant samples, CMV DNA was more likely to be detected and quantifiable in PL than WB [80% vs 67%, P < .001]) (Table 2). In particular, PL appeared to be more sensitive for detecting low-level DNAemia. Cytomegalovirus DNA was undetectable in WB but detectable at low level in PL in 44 sample pairs (10.9%), in contrast to CMV DNA being undetectable in PL but at low level in WB in 9 sample pairs (2.2%). A total of 17 sample pairs (4.2%) contained quantifiable CMV DNA in PL but undetectable levels in WB; in contrast, no PL sample with undetectable CMV DNA had a corresponding quantifiable WB pair. Similar findings related to agreement were observed from subgroup analysis that evaluated samples only from SOT (κ = 0.58; 95% CI, 0.49–0.68) and HCST patients (κ = 0.63; 95% CI, 0.56–0.70).
Table 2.
Categorical Agreement Between CMV VL in WB and PL Among All Patient Groups
| CMV VL in PL | CMV VL in WB | Overall Agreement (%) |
Kappa (95% CI) |
||
|---|---|---|---|---|---|
| Target Not Detected | Detected, but <822 IU/mL | Quantifiable | |||
| Target not detected | 72 (17.9%) | 9 (2.2%) | 0 (0.0%) | 279/403 (69.2%) |
0.49 (0.43–0.56) |
| Detected, but <137 IU/mL | 44 (10.9%) | 22 (5.5%) | 5 (1.2%) | ||
| Quantifiable | 17 (4.2%) | 49 (12.2%) | 185 (45.9%) | ||
Abbreviations: CI, confidence interval; CMV, cytomegalovirus; PL, plasma; VL, viral load; WB, whole blood.
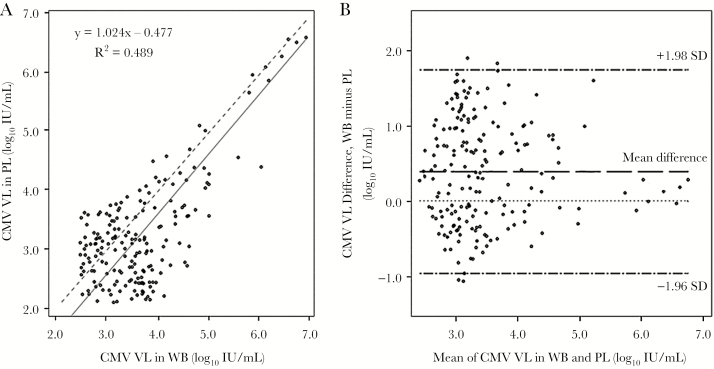
Among sample pairs with quantifiable results, higher VL was observed consistently in WB (Figure 1). Poor correlation of VL existed between PL and WB (R2 = 0.489), with an overall mean VL difference between WB and PL of 0.39 log10 IU/mL, and 95% of the differences falling within the range of −0.96 log10 to 1.74 log10 IU/mL. Notably, in subgroup analysis, CMV VL in paired PL and WB showed relatively high correlation among SOT (CCC = 0.81), but there was poor correlation among HSCT recipients (CCC = 0.14).
Figure 1.
Correlation and agreement of CMV VL results from PL and WB as determined by Deming regression analysis (A) and Bland-Altman plot (B). Dashed line in the regression plot represents the line of unity. Abbreviations: CMV, cytomegalovirus; PL, plasma; VL, viral load; WB, whole blood.
Viral Load Values in Cytomegalovirus Disease Versus Asymptomatic Infection
The overall median initial VL in PL and WB were 954 and 5480 IU/mL, respectively, whereas the median peak VL were 1380 and 6240 IU/mL (Table 3). In addition, the initial and peak CMV VL values were higher in patients with CMV disease versus asymptomatic infection, in both samples; these differences were statistically significant (Table 3).
Table 3.
Comparison of Initial and Peak CMV VL in PL and WB Based on Symptomatic and Asymptomatic Disease
| Patient Group | Overall Median VL (IU/mL)a | Symptomatic | Asymptomatic | P Valueb | ||
|---|---|---|---|---|---|---|
| N | Median VL (IU/mL)a | N | Median VL (IU/mL)a | |||
| All Patients | ||||||
| Initial VL, PL | 954 (363, 4880) | 29 | 4880 (1570, 20800) | 54 | 470 (290, 1140) | <.001 |
| Initial VL, WB | 5480 (1740, 18200) | 29 | 14800 (1990, 85400) | 54 | 5000 (1040, 8250) | .005 |
| Peak VL, PL | 1380 (442, 5690) | 31 | 5450 (1570, 20800) | 57 | 825 (363, 2030) | <.001 |
| Peak VL, WB | 6240 (2020, 22400) | 31 | 27600 (2900, 100000) | 57 | 5350 (1630, 11600) | .002 |
| SOT Patients | ||||||
| Initial VL, PL | 1700 (428, 12500) | 20 | 9100 (2830, 32200) | 23 | 606 (297, 2530) | <.001 |
| Initial VL, WB | 6280 (1974, 30300) | 20 | 35200 (2440, 237000) | 23 | 4520 (779, 8870) | .004 |
| Peak VL, PL | 2280 (434, 13400) | 22 | 9100 (1700, 26300) | 24 | 863 (355, 3960) | .003 |
| Peak VL, WB | 7144 (2050, 36600) | 22 | 35200 (5080, 101000) | 24 | 5271 (1210, 9130) | .002 |
| HSCT Patients | ||||||
| Initial VL, PL | 537 (302, 1550) | 9 | 2790 (1530, 3870) | 31 | 466 (281, 954) | <.001 |
| Initial VL, WB | 5190 (1280, 9930) | 9 | 5550 (1740, 14800) | 31 | 5130 (1040, 8250) | .734 |
| Peak VL, PL | 1120 (449, 3230) | 9 | 3220 (1570, 4880) | 33 | 825 (414, 1900) | .016 |
| Peak VL, WB | 5520 (1740, 17900) | 9 | 6200 (1740, 37900) | 33 | 5350 (3190, 15000) | .490 |
Abbreviations: CMV, cytomegalovirus; HSCT, hematopoietic stem cell transplant; PL, plasma; SOT, solid organ transplant; VL, viral load; WB, whole blood.
aNumbers in parenthesis represent the 25th and 75th percentile values (ie, interquartile range).
bWilcoxon rank-sum test.
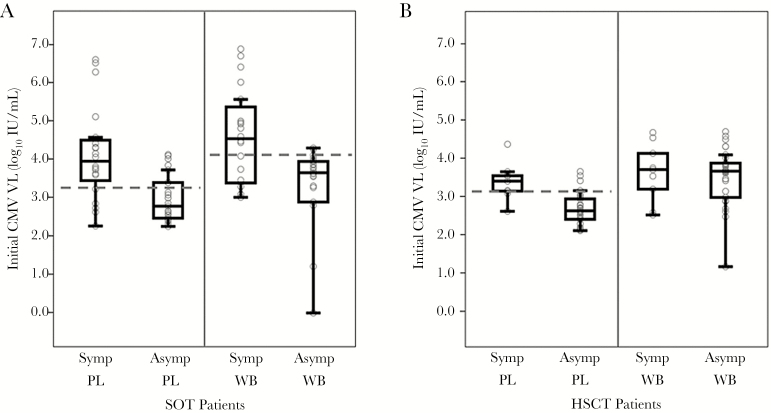
Among SOT recipients, median initial VL in PL was 9100 IU/mL (3.96 log10 IU/mL) in patients with CMV disease compared with 606 IU/mL (2.78 log10 IU/mL) with asymptomatic infection (P < .001). Likewise, the corresponding median initial VL in WB was 35200 IU/mL (4.55 log10 IU/mL) for patients with CMV disease and 4520 IU/mL (3.66 log10 IU/mL) for those with asymptomatic infection (P = .004) (Figure 2A).
Figure 2.
Boxplots of initial cytomegalovirus (CMV) viral load (VL) in plasma (PL) and whole blood (WB) among (A) solid organ transplant and (B) hematopoietic stem cell transplant recipients. Upper and lower bars on lines extending from each box represent maximum and minimum limits, respectively, of the result range. Top of each box indicates the third quartile; the horizontal line in the middle of each box indicates the median; the bottom of each box indicates the first quartile. Dashed lines represent the retrospectively determined VL thresholds for PL and WB that best distinguish between patients with symptomatic CMV disease and those with asymptomatic infection. Abbreviations: Asymp, asymptomatic; CMV, cytomegalovirus; HSCT, hematopoietic stem cell transplant; PL, plasma; SOT, solid organ transplant; Symp, symptomatic; VL, viral load; WB, whole blood..
Among HSCT recipients, the median initial VL in PL was 2790 IU/mL (3.45 log10 IU/mL) in those with CMV disease and 466 IU/mL (2.67 log10 IU/mL) in those with asymptomatic infection (P < .001). In contrast, there were no significant differences in initial VL in WB between those patients with CMV disease (5550 IU/mL or 3.74 log10 IU/mL) and asymptomatic infection (5130 IU/mL or 3.71 log10 IU/mL) (Figures 2B).
Viral Load Thresholds
Because initial CMV VL is the most clinically relevant result for guiding preemptive therapy, only these results were subjected to ROC analysis to statistically derive VL thresholds that distinguish CMV disease from asymptomatic infection. Using results obtained from PL in SOT, an initial VL threshold of 1700 IU/mL (3.23 log10 IU/mL), with sensitivity of 80% and specificity of 74%, was selected for the identification of CMV disease (Figure 2A). For WB, a threshold of 12400 IU/mL (4.09 log10 IU/mL) had sensitivity of 65% and specificity of 87% (Figure 2A).
Among HSCT recipients, a VL threshold of 1350 IU/mL (3.13 log10 IU/mL) in PL yielded the best differentiation between CMV disease and asymptomatic infection, based on sensitivity of 89% and specificity of 87% (Figure 2B). No VL threshold for WB was identified due to the lack of a statistically significant difference in VL between CMV disease and asymptomatic infection.
DISCUSSION
Using the FDA-approved WHO-calibrated CAP/CTM CMV assay, our study confirms previous observations that VL is significantly higher in WB than PL, likely due to detection of both cell-associated and free virus in WB [7, 8]. However, we also observed important differences in the categorical agreement of VL results between PL and WB samples, particularly at low VL; this resulted in higher assay sensitivity when using PL samples (eg, 15% of samples had undetectable VL in WB but either detectable or quantifiable values in PL). This finding contrasts previous studies, which showed higher sensitivity with WB [7–9]. This discordance at low VL levels is likely an assay-specific finding resulting from the modified sample processing methods necessary for testing WB with CAP/CTM CMV. In the clinical setting, this reduced sensitivity of WB when used as sample for the CAP/CTM CMV assay could adversely impact CMV surveillance, optimal initiation of preemptive antiviral therapy, and treatment duration [1, 12]. Based on these findings, we recommend PL as the preferred sample for CAP/CTM. Whether PL is also the preferred specimen for other standardized CMV VL assays is not known.
We demonstrated significant differences between initial VL in PL of SOT and HSCT recipients and among patients with CMV disease versus asymptomatic DNAemia. These differences were consistent among the SOT, HSCT, and combined patient groups. In addition, the difference between initial VL in patients with CMV disease and those with asymptomatic CMV infection permitted the selection of VL thresholds to distinguish between these 2 groups. These VL values identified in this study are proposed as potential thresholds that can be used for prospective clinical studies examining the utility of preemptive therapy for CMV disease prevention. In this regard, based on ROC analysis, we propose an initial VL threshold of 1700 IU/mL for SOT recipients when testing PL samples with CAP/CTM CMV (Figure 2A). This threshold is similar to the VL threshold of 1500 IU/mL proposed in a previous report of CMV disease in a cohort of at-risk kidney transplant recipients [13]. Another study identified a VL threshold of 3983 IU/mL among moderate-risk CMV-seropositive kidney recipients [14]. Of note, our proposed VL threshold (and those of the previous studies) falls between the observed median initial VL of 9100 and 606 IU/mL for a heterogeneous cohort of SOT recipients with CMV disease and asymptomatic CMV infection, respectively. Because this VL threshold of 1700 IU/mL was defined by ROC analysis to distinguish between CMV disease and asymptomatic infection, we favor initiating antiviral therapy in SOT recipients who have reached this VL threshold (because our study implied a high sensitivity and specificity for developing CMV disease). However, we emphasize that this VL threshold was derived from analysis of all SOT patients without risk stratification. The limited number of patients per risk stratum (eg, CMV D+/R−, lung recipients) did not allow us to perform ROC analysis to define a threshold for each subgroup. Accordingly, some high-risk groups, such as CMV D+/R− and lung transplant recipients, and those with augmented immunosuppression, may require treatment at lower VL. In our contemporary clinical practice, however, these high-risk SOT recipients are given antiviral prophylaxis and are not generally subjected to preemptive monitoring.
Hematopoietic stem cell transplantation recipients typically undergo surveillance with serial CMV QNAT to guide possible initiation of preemptive therapy. In this study, we found that a VL threshold of 1350 IU/mL for PL is optimal in distinguishing CMV disease from asymptomatic infection (Figure 2A). We therefore suggest that prospective studies should consider initiating antiviral treatment in HSCT patients whose serial CMV VL in PL reaches the proposed threshold of 1350 IU/mL. Again, preemptive antiviral therapy may be considered or necessary at lower VL for high-risk recipients, such as those with severe graft-versus-host disease or recipients of umbilical cord, T-cell depleted, or CMV-seronegative donor cells. Likewise, all symptomatic patients should be treated regardless of VL values. Of note, a recent study showed that treatment at lower VL (<1000 IU/mL) was associated with shorter duration of DNAemia and antiviral therapy, although this study did not assess risk of progression to clinical disease [15].
For WB measurements, ROC analysis yielded an initial VL threshold for WB of 12400 IU/mL that would best differentiate between CMV disease and asymptomatic infection among SOT patients (Figure 1A). However, a threshold could not be determined in HSCT recipients due to significant overlap in VL results of patients with CMV disease and asymptomatic infection. This lack of significant difference in initial VL in WB among HSCT recipients may be due to underlying hematologic pathology and effects of cytotoxic chemotherapy with consequent leukopenia, neutropenia, and lymphopenia. Because low white blood cell counts characterize the immediate and early period after HSCT, the degree of CMV DNAemia in these patients would be expected to be lower than that in transplant recipients with normal number of CMV-infected leukocytes. This reasoning could also explain the generally lower level of CMV DNAemia observed in HSCT compared with SOT patients (Figure 2A and 2B). Given the lack of a defined VL threshold for WB in HSCT recipients and the additional preanalytical modifications that reduced assay sensitivity (LLoQ of 822 IU/mL), we suggest to use PL sample for CMV surveillance in transplant recipients when using CAP/CTM CMV assay [16].
Our study was limited by its observational nature and lack of prospective evaluation of our proposed VL thresholds. Despite the relatively large number of study subjects, subgroup analyses according to risk strata were not possible. Another limitation is the lower sensitivity of the assay in testing WB due to the necessary preanalytical modifications of CAP/CTM CMV procedures required to test this sample type. Moreover, our results may not be extrapolated to other transplant centers utilizing other CMV QNAT. Recent studies have shown that despite improvement in harmonizing VL results from all assays calibrated to the WHO International Standard, there remain assay-specific performance characteristics that necessitate the use of the same assay for serial monitoring of CMV VL in a given patient [17]. Even among standardized assays, VL values may be affected by sample choice, extraction methods, primers and amplicon sizes, among others. The strength of this study is the inclusion of a relatively large heterogeneous cohort of SOT and HSCT recipients thus enabling a robust overall analysis of the resulting data. The nearly equal numbers of SOT and HSCT recipients allowed subgroup analysis of the 2 transplant cohorts.
CONCLUSIONS
In conclusion, CAP/CTM CMV usually generated higher CMV VL values with WB than PL, but the assay was less sensitive with WB in conditions characterized by low-level CMV DNAemia (likely due to the preanalytical modifications of WB). Therefore, our study findings support the use of PL with CAP/CTM CMV per manufacturer’s instructions and without modification for CMV surveillance. In this regard, VL thresholds in PL were established to distinguish CMV disease from asymptomatic CMV infection in SOT and allogeneic HSCT recipients. We propose VL thresholds of 1700 and 1350 IU/mL in PL for SOT and HSCT recipients, respectively, as potential triggers that could be evaluated and validated in prospective clinical trials of CMV disease prevention.
Acknowledgments
Disclaimer. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official view of National Institutes of Health (NIH). Roche Molecular Systems, Inc. did not provide input regarding study design, analysis, or interpretation of results.
Financial support. This work was funded by Clinical and Translational Science Award Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences, a component of the NIH. Roche Molecular Systems, Inc. (Pleasanton, CA) provided additional funding for this study.
Potential conflicts of interest. R. R. R. and J. D. Y. serve as advisors to Roche Diagnostics Corporation (Indianapolis, IN) and have received clinical research funding support from this Corporation (research funds directed to the Mayo Clinic).
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Razonable RR, Humar A. Cytomegalovirus in solid organ transplantation. Am J Transplant 2013; 13 (Suppl 4):93–106. [DOI] [PubMed] [Google Scholar]
- 2. Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Infect Dis Clin North Am 2010; 24:319–37. [DOI] [PubMed] [Google Scholar]
- 3. Sia IG, Wilson JA, Groettum CM et al. Cytomegalovirus (CMV) DNA load predicts relapsing CMV infection after solid organ transplantation. J Infect Dis 2000; 181:717–20. [DOI] [PubMed] [Google Scholar]
- 4. Razonable RR, Åsberg A, Rollag H et al. Virologic suppression measured by a cytomegalovirus (CMV) DNA test calibrated to the World Health Organization international standard is predictive of CMV disease resolution in transplant recipients. Clin Infect Dis 2013; 56:1546–53. [DOI] [PubMed] [Google Scholar]
- 5. Caliendo AM. The long road toward standardization of viral load testing for cytomegalovirus. Clin Infect Dis 2013; 56:374–5. [DOI] [PubMed] [Google Scholar]
- 6. Fryer JF, Heath AB, Minor PD. A collaborative study to establish the 1st WHO International Standard for human cytomegalovirus for nucleic acid amplification technology. Biologicals 2016; 44:242–51. [DOI] [PubMed] [Google Scholar]
- 7. Razonable RR, Brown RA, Wilson J et al. The clinical use of various blood compartments for cytomegalovirus (CMV) DNA quantitation in transplant recipients with CMV disease. Transplantation 2002; 73:968–73. [DOI] [PubMed] [Google Scholar]
- 8. Babady NE, Cheng C, Cumberbatch E et al. Monitoring of cytomegalovirus viral loads by two molecular assays in whole-blood and plasma samples from hematopoietic stem cell transplant recipients. J Clin Microbiol 2015; 53:1252–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Costa C, Sidoti F, Mantovani S et al. Comparison of two molecular assays for detection of cytomegalovirus DNA in whole blood and plasma samples from transplant recipients. New Microbiol 2016; 39:186–91. [PubMed] [Google Scholar]
- 10. Ljungman P, Boeckh M, Hirsch HH et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis 2017; 64:87–91. [DOI] [PubMed] [Google Scholar]
- 11. Abdul-Ali D, Kraft CS, Ingersoll J et al. Cytomegalovirus DNA stability in EDTA anti-coagulated whole blood and plasma samples. J Clin Virol 2011; 52:222–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kotton CN, Kumar D, Caliendo AM et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2013; 96:333–60. [DOI] [PubMed] [Google Scholar]
- 13. Martín-Gandul C, Pérez-Romero P, Blanco-Lobo P et al. Viral load, CMV-specific T-cell immune response and cytomegalovirus disease in solid organ transplant recipients at higher risk for cytomegalovirus infection during preemptive therapy. Transpl Int 2014; 27:1060–8. [DOI] [PubMed] [Google Scholar]
- 14. Martín-Gandul C, Pérez-Romero P, Sánchez M et al. Determination, validation and standardization of a CMV DNA cut-off value in plasma for preemptive treatment of CMV infection in solid organ transplant recipients at lower risk for CMV infection. J Clin Virol 2013; 56:13–8. [DOI] [PubMed] [Google Scholar]
- 15. Tan SK, Waggoner JJ, Pinsky BA. Cytomegalovirus load at treatment initiation is predictive of time to resolution of viremia and duration of therapy in hematopoietic cell transplant recipients. J Clin Virol 2015; 69:179–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hirsch HH, Lautenschlager I, Pinsky BA et al. An international multicenter performance analysis of cytomegalovirus load tests. Clin Infect Dis 2013; 56:367–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Preiksaitis JK, Hayden RT, Tong Y et al. Are we there yet? Impact of the first international standard for cytomegalovirus DNA on the harmonization of results reported on plasma samples. Clin Infect Dis 2016; 63:583–9. [DOI] [PubMed] [Google Scholar]