Abstract
Peripheral somatosensory neurons are frequently exposed to mechanical forces. Strong stimuli result in neuronal activation of high-threshold mechanosensory afferent neurons, even in the absence of tissue damage. Among these neurons, fast-conducting nociceptors (A-fiber high-threshold mechanoreceptors (AHTMRs)) are normally resistant to sustained activation, transiently encoding the mechanical stimulus intensity but not its full duration. This rapidly adapting response seems to depend on changes in the electrical excitability of the membrane of these afferent neurons during sustained stimulation, a restraint mechanism that disappears following sensitization. Here, we examine the mechanism by which strong peripheral activation of mechanoreceptors elicits this control process in the absence of tissue injury and temporally silences afferent neurons despite ongoing stimulation. To study this, mechanoreceptors in Sprague–Dawley rats were accessed at the soma in the dorsal root ganglia from T11 and L4/L5. Neuronal classification was performed using receptive field characteristics and passive and active electrical properties. Sustained mechanical nociceptive stimulation in the absence of tissue damage of AHTMRs induces a rapid membrane hyperpolarization and a period of reduced responsiveness to the stimuli. Moreover, this phenomenon appears to be unique to this subset of afferent neurons and is absent in slow-conducting C-mechanonociceptors (C-fiber high-threshold mechanoreceptors) and rapidly adapting fast-conducting low-threshold mechanoreceptors. Furthermore, this mechanism for rapid adaptation and reducing ongoing input is ablated by repeated strong stimuli and in sensitized AHTMRs after chronic neuropathic injury. Further studies to understand the underling molecular mechanisms behind this phenomenon and their modulation during the development of pathological conditions may provide new targets to control nociceptive hyperexcitability and chronic pain.
Keywords: Primary sensory neurons, in vivo electrophysiology, membrane hyperpolarization
Introduction
The peripheral somatosensory system is exposed to a variety of stimuli of different modalities over a wide range of intensities. Although the force of some of these stimuli is great enough to prompt a discrete discharge of nociceptive afferent neurons, the perception of a given stimulus as painful will depend on intensity, duration, and consequent threat to damage the innervated tissues. Fast-conducting high-threshold mechanoreceptors (A-fiber high-threshold mechanoreceptor (AHTMRs)) are vitally important in detecting and transmitting mechanically induced nociceptive information. These AHTMRs conduct predominantly in the Aδ range, but may also be found in the Aβ range. A division of AHTMRs has been proposed based on their adaptation rate: slow-adapting (SA) AHTMRs (A-HT[SA]) and rapidly adapting (RA) ATHMR (A-HT[RA]). The A-HT[RA] are thought to only play a role in the initial encoding phase of nociceptive mechanical stimulation, while the slow-conducting nociceptive afferent neurons (C-fiber high-threshold mechanoreceptors (CHTMRs)) and A-HT[SA] are thought to be responsible for the steady nociceptive response and post-discharge after stimulation.1,2 While this distinction is frequently observed, whether it persists in pathological conditions where AHTMR activation plays a key role in sustained nociceptive input,3,4 is unclear. Herein, we test the hypothesis that these putative subtypes are in fact different physiological states of the same neuron and that these afferent neurons can transition from one state (A-HT[RA]) to the next (A-HT[SA]) depending on history of repeated activation, sensitization, and changes in their electrical excitability.
Sustained membrane hyperpolarization in response to cellular activation (evoked action potentials (APs)) has been observed in AHTMRs innervating both thoracic and lumbar dermatomes in different species.5–10 Although there is experimental evidence showing that AHTMRs can only be activated for a few seconds during constant stimulation,5 no explanation has been offered for this transient desensitization and resistance to sustained discharge. In addition, whether this desensitization is altered after neuropathic injury has not been examined. Moreover, some observations suggest the existence of electrical mechanisms which reduce the likelihood of post-discharges when activated.5,8,9 To better understand this paradox, the current study hypothesizes that this post-discharge hyperpolarization (PDH) effectively induces a transient inhibitory state in AHTMR nociceptors that is both normal and necessary for their timely and physiologic responses. We show that this mechanism is unique to the AHTMR nociceptive neuron subset, as it is absent from all other primary sensory neurons (nociceptive or non-nociceptive). Finally, we demonstrate an acute shift in AHTMR responses from RA to sustained following repeated application of strong stimuli, accompanied by loss of PDH and explore the potential consequences of the absence (or failure) of PDH and its correlation with AHTMR sensitization in hyper excitable states after nerve injury (L5-partial spinal nerve ligation (pSNL)).
Materials and methods
Animals
Fifty-eight Sprague–Dawley rats (4–6 weeks of age) were used (21 females and 37 males). Some of these animals were studied in protocols examining the effect of thermal stimulation,11 differences between mechanosensory afferent neurons innervating glabrous and hairy skin12 or the effects of pSNL.3 When a cell was obtained meeting criteria for study in the current protocol, it was examined according to the methods below. None of the neurons in the current study have been previously reported and all neurons were recorded prior to any other experimental manipulation of their receptor field (RF). Animals were housed together in pairs, in a climate-controlled room under a 12-h light/dark cycle. The use and handling of animals were in accordance with guidelines provided by the National Institutes of Health and the International Association for the Study of Pain and all procedures and experiments were approved by the Institutional Animal Care and Use Committee of Wake Forest University Health Sciences.
L5-partial spinal nerve ligation
Nine animals were deeply anesthetized with isoflurane, and, under aseptic conditions, the skin was incised at the midline over the lumbar spine. The right L5 spinal nerve was identified and approximately 1/3 to 1/2 thickness of the L5 spinal nerve was ligated with 9-0 nylon suture under a dissecting microscope, as previously described.13 Care was taken not to pull the nerve or contact the intact L4 spinal nerve. After hemostasis was achieved, the muscle layer was approximated with 4-0 synthetic absorbable suture (Look, Reading, PA) and the skin closed with absorbable suture. After the surgery, the rats were returned to their cages, kept warm under a heat lamp (∼32℃) and monitored during recovery
Electrophysiology
Animals were deeply anesthetized with isoflurane 3%. The trachea was intubated and the lungs ventilated using pressure controlled ventilation (Inspira PCV, Harvard Apparatus, Holliston, MA) with humidified oxygen. Heart rate and noninvasive blood pressure were monitored throughout as a guide to depth of anesthesia. Anesthetized animals were immobilized with pancuronium bromide (2 mg/kg) and inspired and end tidal isoflurane concentration maintained at 2% throughout the study (Teva Pharmaceuticals, North Wales, PA). A dorsal incision was made in the thoraco-lumbar midline and either L4, L5, or T11 dorsal root ganglion (DRG) and adjacent spinal cord were exposed by laminectomy as described previously.12 The tissue was continuously superfused with oxygenated artificial cerebrospinal fluid (aCSF (in mM): 127.0 NaCl, 1.9 KCl, 1.2 KH2PO4, 1.3 MgSO4, 2.4 CaCl2, 26.0 NaHCO3, and 10.0 D-glucose). The spinal column was secured using custom clamps and the preparation was transferred to a preheated (32–34℃) recording chamber where the superfusate was slowly raised to 37℃ (±0.2℃) (MPRE8, Cell MicroControls, Norfolk, VA). Pool temperature adjacent to the DRG was monitored with a thermocouple (IT-23, Physitemp, Clifton, NJ). Rectal temperature (RET-3, Physitemp) was maintained at 34 ± 1℃ with radiant heat.
The electrophysiological recordings from each animal were limited to a maximum duration of 75 min in order to diminish the likelihood that experimental manipulation would result in sensitization. DRG neuronal somata were impaled with quartz micropipettes (80–250 MΩ) containing 1 M potassium acetate. Direct current output from an Axoclamp 2B amplifier (Axon Instruments/Molecular Devices, Sunnyvale, CA) was digitized and analyzed off-line using Spike2 (CED, Cambridge, UK). Sampling rate for intracellular recordings was 21 kHz throughout (MicroPower1401, CED).
Inclusion criteria
All but one cell included in this study were the first nociceptive afferent recordings in that animal to satisfy the following requirements: RF not previously stimulated or manipulated in any way that may cause nociceptor sensitization, resting membrane potential more negative than −40 mV, AP amplitude ≥ 30 mV, the presence of afterhyperpolarization (AHP) and appropriate location of RF, as described below. Every cell was mechanosensitive and its response and properties evaluated after threshold and suprathreshold activation. Any change in cellular electrical excitability was documented after both manipulations (on average 5–10 min).
Cellular classification protocol
The procedure used in this study to classify primary sensory afferent neurons in vivo has been described in detail.4 Briefly, RFs were located with the aid of a stereomicroscope using increasing mechanical stimulation; the latter progressed from light touch with a fine sable hair paintbrush to searching with blunt probe (back of the paintbrush) and ultimately gentle to strong pinch with fine-tipped forceps. Although cellular RFs were found across the entire dermatome in these intact preparations, only those along the medial portion of the dermatome were used in this study (flank (T11); posterior-lateral aspect of the leg and paw (L4/L5)). The study was limited to these areas to avoid effects from the DRG surgical exposure toward the midline and/or the inability to access the full extent of the RF at this location. Based on the combination of their mechanical threshold, conduction velocity (CV) and dynamic response (phasic: on–off; tonic) neurons were classified into three groups: LTMR (low-threshold mechanoreceptors), AHTMR, and CHTMR. Specific cellular subtypes such as SA tactile afferent neurons (SAI and SAII), C-polymodal nociceptor (nociceptors which saturate their responses well below the mechanical nociceptive thresholds in human8,9,14–16) and mechanoinsensitive afferent neurons were excluded from the current study.
Mechanical sensitivity and cellular excitability
Peripheral and somatic cellular excitability were measured at three stages (Figure 1): (1) Cell and RF characterization, (2) initial response to suprathreshold stimulation, and (3) response to suprathreshold stimulus after repeated cellular activation.
Cell and RF characterization: Mechanical thresholds were determined with calibrated von Frey filaments (Stoelting, Wood Dale, IL) and the area of responsiveness to this threshold stimulus was marked using a red fine point marker. Adaptation rate was evaluated using suprathreshold stimulation from probes mounted in a micromanipulator; skin stretch and vibratory stimuli (tuning forks (TF) of 256 and 512 Hz; SKLAR instruments, West Chester, PA) were also tested.
Initial response to sustained suprathreshold stimulation: Immediately following the cell and RF characterization, all afferent neurons were exposed to suprathreshold activation by pinching with fine-tipped forceps in a gentle manner to avoid damaging the skin (as assessed visually by lack of development of erythema, edema, glossiness, etc.). The stimulus was maintained for 2 s after the initial cellular response. Only afferent neurons that showed clear membrane hyperpolarization during this manipulation were analyzed further.
Response to suprathreshold stimulus after repeated cellular activation: After suprathreshold cellular mechanical activation (three trials of 2 s stimulations each with a resting period of 1 min), the same suprathreshold activation was used as described previously. Only neurons with no evident skin damage were analyzed further.
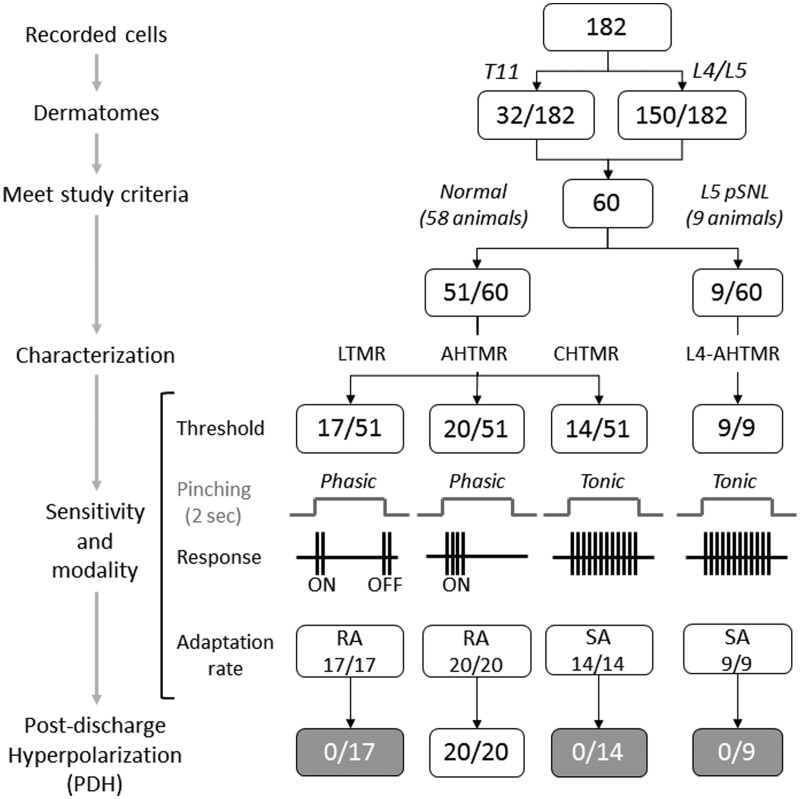
Figure 1.
Flow diagram of the distribution of neurons recorded and analyzed by group in the study. LTMR: low-threshold mechanoreceptor; AHTMR: A-fiber high-threshold mechanoreceptor; CHTMR: C-fiber high-threshold mechanoreceptor; L4-AHTMR: A-fiber high-threshold mechanoreceptor recorded from the L4 ganglia.
During these manipulations, three parameters were evaluated: (1) Number of APs during stimulation, (2) duration of the response (time between the first and the last AP during stimulation), and (3) maximal instantaneous frequency (IFmax) in Hz. The force applied during the pinch stimuli was not quantified but in all cases was adequate to generate APs
Somatic electrical properties
Active membrane properties of all excitable neurons were analyzed at the beginning and end of every experiment. These parameters included amplitude and duration of the AP and AHP of the AP, along with the maximum rates of spike depolarization and repolarization; AP and AHP durations were measured at half-amplitude (D50 and AHP50, respectively) to minimize hyperpolarization-related artifacts. Passive properties were analyzed including resting membrane potential (Em), input resistance (Ri), time constant (Tau), inward rectification, and, where possible, rheobase; all but the latter were determined by injecting incremental hyperpolarizing current pulses (≤0.1 nA, 500 ms) through balanced electrodes.
Conduction velocity
Because intact thoracic and lumbar DRGs serve multiple nerves, spike latency was obtained by stimulating the RF at the skin surface using a bipolar electrode (0.5 Hz, current range: 0.1–1.2 mA) and a stimulus isolator (A360LA, WPI, Sarasota, FL, USA); this was performed following all natural stimulation to prevent potential alterations in RF properties by electrical stimulation. All measurements were obtained using the absolute minimum intensity required to excite neurons consistently without jitter; this variability (jitter) in the AP generation latency (particularly at significantly shorter latencies), seen at traditional (i.e. two- to three-fold threshold) intensity has been presumed to reflect spread to more proximal sites along axons. Stimuli ranged in duration from 50 to 100 µs; utilization time was not taken into account. Conduction distances were measured for each afferent on termination of the experiment by inserting a pin through the RF (marked with ink at the time of recording) and carefully measuring the distance to the DRG along the closest nerve.
Statistical analysis
Prior to analysis, parametric assumptions were evaluated for all variables using histograms, identification of outliers with boxplots, descriptive statistics, and the Shapiro–Wilk test for normality. Data are reported as medians (range) if not normally distributed or means (standard error) if normally distributed. Student’s t-test and repeated measures analysis of variance (ANOVA) were used for normally distributed data and Friedman test and Mann Whitney U-test were used for not normally distributed data. Specifically, with regard to the analyses of changes in somatic excitability over time in AHTMR, the Em outcome was analyzed using repeated measures ANOVA with Greenhouse & Geisser sphericity correction as distributions at each time point proved to be parametric and there were no significant outliers. Friedman tests were run on number of APs per stimuli and duration data as the distributions were non-parametric at one or more time points in each dependent variable. For all analyses, p was set at 0.05 for statistical significance. All post hoc analyses were Bonferroni adjusted. Analyses were carried out using SPSS Statistics for Windows, version 22 (IBM Corp, Armonk, NY).
Results
Intracellular recordings were obtained in 182 well characterized mechanosensitive neurons innervating the skin of T11 (32/182) and L4/L5 (150/182) dermatomes from 58 animals. Of the 182 neurons, 60 met study inclusion criteria. These neurons were obtained from normal animals and classified as: LTMR (17/60) (all RA), AHTMR (20/60), and CHTMR (14/60). The remaining neurons, 9/60 L4-AHTMRs nociceptors, were obtained from 9 L5-pSNL animals one week after nerve injury (Figure 1).
Cell and RF characterization
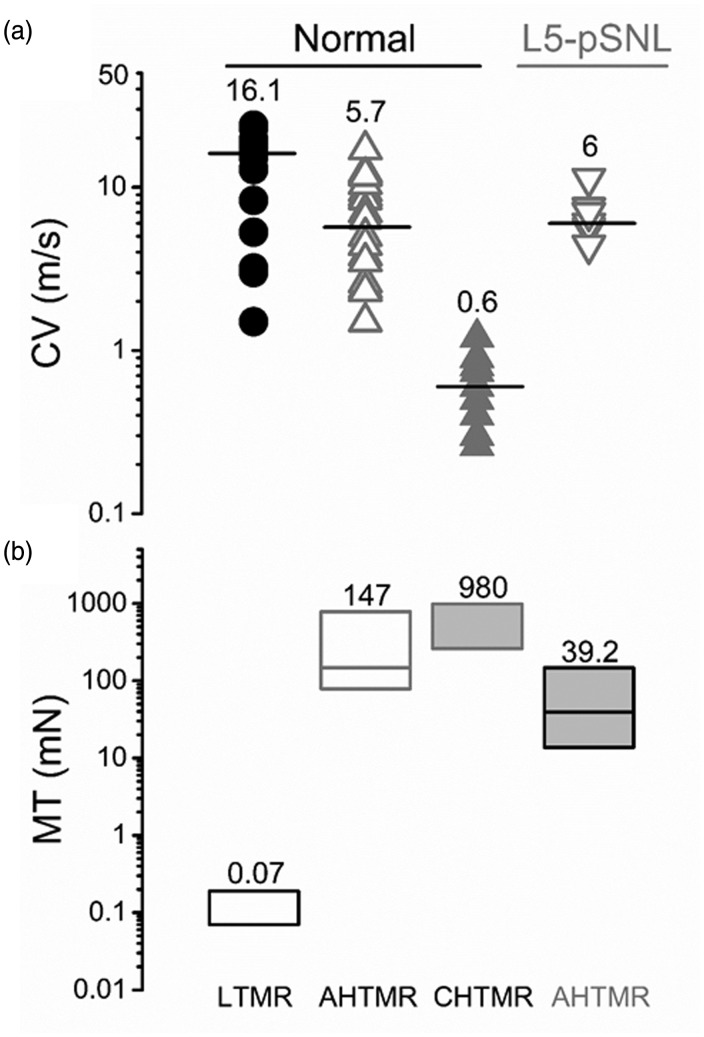
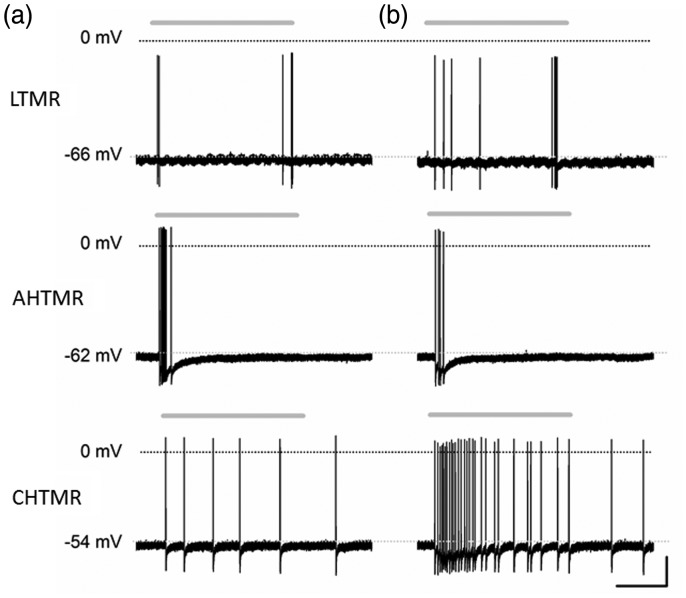
CHTMRs and LTMRs were unambiguously identified by their CV and mechanical thresholds (Figure 2). AHTMRs and LTMRs exhibit overlapping CVs, but were unambiguously separated by their mechanical thresholds and response to 256 and 512 Hz vibration (data not shown). At threshold mechanical stimulation, LTMRs showed phasic on/off (beginning and end of the stimulus), AHTMRs only responded to the initial application of the stimulus (on), and CHTMRs showed a sustained (tonic) response followed by post-discharges (Figure 3).
Figure 2.
(a) Conduction velocity by classification. Values and bars on the scatter points are medians. LTMR, AHTMR, CHTMR, L4 AHTMR after L5-pSNL. (b) Mechanical threshold of neurons by classification. Numbers indicate medians (on top), with boxes representing the 25 and 75 percentiles. LTMR: low-threshold mechanoreceptor; AHTMR: A-fiber high-threshold mechanoreceptor; CHTMR: C-fiber high-threshold mechanoreceptor; L4-AHTMR: A-fiber high-threshold mechanoreceptor recorded from the L4 ganglia.
Figure 3.
Typical examples of responses of different types of DRG neurons to (a) threshold (von Frey filaments) and (b) supra-threshold (pinch) stimuli. Both stimuli were applied consecutively (<1 min apart) for approximately 2 s (gray above each trace) in normal animals. LTMR: low-threshold mechanoreceptors; ATHMR: A-fiber high-threshold mechanoreceptor; CHTMR: C-fiber high-threshold mechanoreceptors. Scale bars: 0.67 s, 20 mV.
Somatic excitability
When stable cellular impalements were achieved and the peripheral mechanical threshold established, passive and active cellular membrane properties were evaluated.
LTMR
These neurons showed an Em with a mean of −66.6 ± 7.2 mV. Their narrow (D50 median: 0.6 ms (0.5–0.9)), small amplitude APs (mean: 39.6 ± 5.6 mV) showed fast rates of depolarization (median: 154 V/s (76–206)) and hyperpolarization (median: −67 V/s (−104 to −42)) with small amplitude, short duration AHPs (amplitude mean: 9.7 ± 3 mV; AHP50 median: 4 ms (2–8); AHP75 median: 6 ms (3–12)).
AHTMR
These neurons showed a less hyperpolarized Em (mean: −60.6 ± 7.2 mV) than the tactile afferent neurons and significantly bigger amplitude (61.8 ± 7.7 mV) and broader APs (D50 median: 1.35 ms (0.8–82)) (p < 0.01). On the other hand, they showed a slower depolarization rate (median: 104 V/s (64–165)) (p < 0.05). Their hyperpolarization rate (median: −66 V/s (−106 to −50)) was not different when compared to the LTMRs. Their AHPs were also both significantly greater in depth (amplitude mean: 11.8 ± 5.5 mV) (p < 0.05) and longer in duration (AHP50 median: 8 ms (3–65) (p < 0.01); AHP75 median: 19 ms (6–110) (p < 0.01)) when compared to the LTMRs.
CHTMR
These slow-conducting nociceptors presented an even less negative Em (mean: −48 ± 10 mV) than the AHTMR nociceptive group (p < 0.001). Although their AP amplitude (mean: 61.3 ± 8 mV) was similar to the AHTMR group, their D50 was significantly longer (median: 1.6 ms (1–5.6)) (p < 0.05) with similar depolarization rate (median: 108 V/s (57–183)) but significantly slower hyperpolarization rate (median: −57 V/s (−105 to −31)) (p < 0.01).
Initial response to suprathreshold stimulation
Peripheral excitability
Transient suprathreshold activation of these afferent neurons did not induce any perceptible change in on–off pattern of activation of LTMRs, whereas it increased the number of AP per stimulus in CHTMRs without change in the post-stimulus discharge or in the rate of spontaneous activity (Figure 3(b)). In contrast, the suprathreshold stimulus reduced the number of APs per stimulus in AHTMRs and the duration of their response (Figure 3(b) and 4(a)) (p < 0.001). There was no significant change in the cellular IFmax (median: 36 Hz (1–195)).
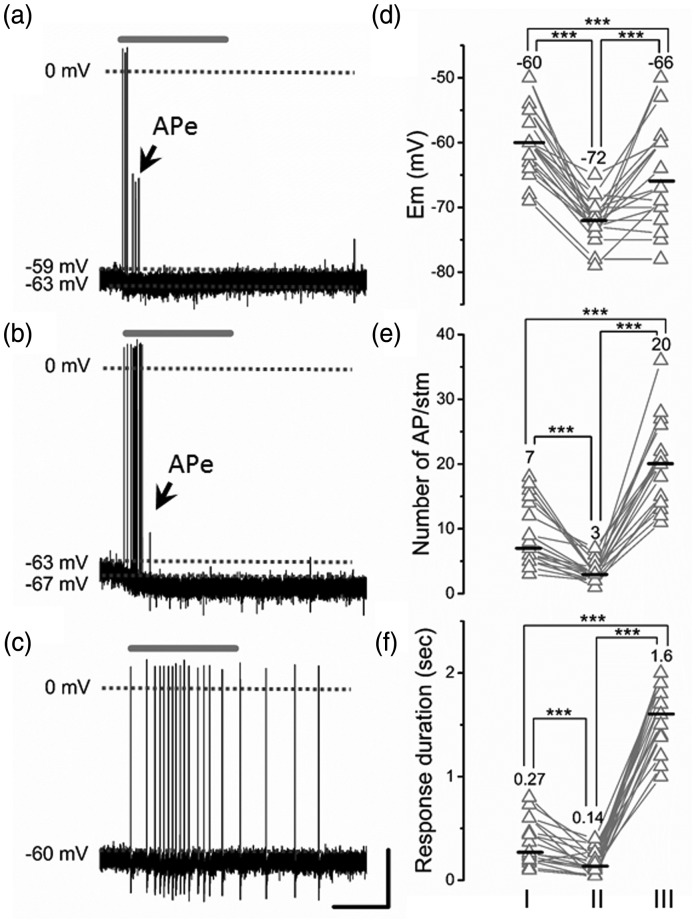
Figure 4.
Representative AHTMR response after three consecutive pinching stimuli. (a) Initial pinch (I), (b) second pinch hyperpolarization (II), and (c) third pinch (III). Note the cumulative nature of the hyperpolarization and initial incomplete development of AP (APe: electrotonic propagated APs) concluding with membrane potential recovery and the shift of the response for phasic (RA) to tonic (SA). Effect of the latest described patterns of activation on the AHTMR response and properties: membrane potential (Em) (d), number of AP/stimuli (stm) (e), and duration of response (f). ATHMR. Values and middle bars on the cell’s scatter points are means for the Em and medians for the others. Scale bars: 1 s, 20 mV. One-way repeated measures ANOVA with Bonferroni correction for multiple comparisons was used for the Em while the Friedman test was used for AP/stm and duration of the response with Bonferroni corrections for multiple comparisons, ***p < 0.001.
Somatic excitability
Transient suprathreshold activation of LTMR and CHTMR afferent neurons did not affect somatic electrical properties (data not shown). In contrast, mechanical suprathreshold activation of AHTMRs for 2 s resulted in early onset cellular membrane hyperpolarization (mean: −12.4 ± 1.1 mV) (Figure 4), with a recovery over 1–5 s (median: 2 s).
Response of AHTMRs to suprathreshold stimulus after repeated cellular activation
Peripheral excitability
Five minutes after three consecutive peripheral activations (2 s pinch) with an inter-stimulus interval of 1-min there was an increased responsiveness to subsequent activation, including increased number of APs, a switch from a phasic On responsiveness to an almost entirely tonic peripheral responsiveness (Figures 4(c), (e), and (f)) without change in IFmax (median: 47 Hz (8–118)).
Somatic excitability
Following three consecutive pinch stimuli, Em partially recovered but remained hyperpolarized compared to the initial state prior to repeated activation. However, PDH failed to develop: Em did not become more hyperpolarized at this time during a pinch stimulus (Figure 4(c) and (d)). At this time, the amplitude of APs was unaffected, while the duration was significantly shorter (p < 0.01) (Figure 5(a) and (b)). Their depolarization rate was significantly faster than before repeated activation (p < 0.01), while their hyperpolarization rate remained unchanged (Figure 5(c)). Finally, in these neurons neither the amplitude of the AHP nor the duration (at 50% and 75% of their amplitude) was changed (data not shown).
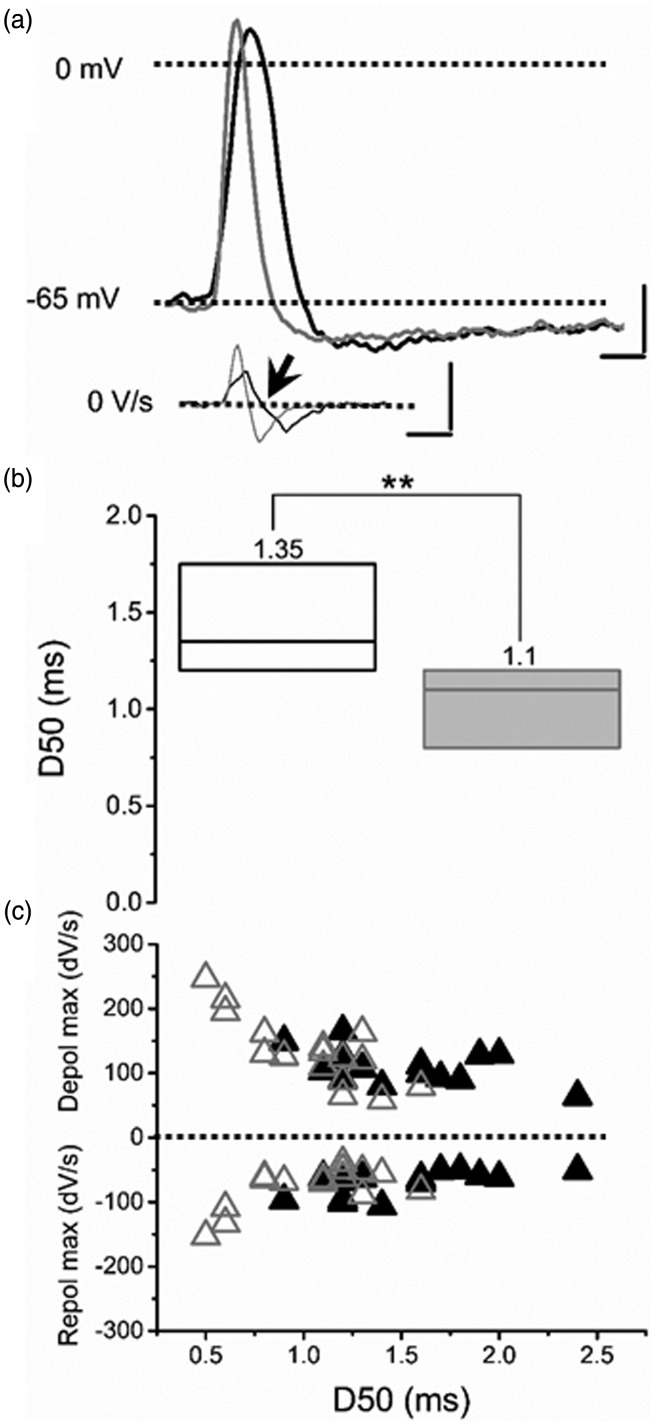
Figure 5.
Example of the effects of consecutive pinching (three stimuli) on the active electrical properties of AHTMR afferent neurons. (a) Typical AP shape before (black) and after stimulation (gray) presented with their dV/dt. Arrow shows the inflection point in the first derivative of the voltage Scale bars. 20 mV, 100 V/s, 1 ms. (b) Cumulative effect of repeated pinching stimulation on the duration of AP at 50% of their amplitude (D50). No change in amplitude was noted despite the shorter duration of the D50. Data are medians (on top), with boxes representing the 25 and 75 percentiles, Wilcoxon signed rank was used to test significance, **p < 0.001. (c) Changes in the maximal depolarizing and hyperpolarizing rates before (black triangles) and after pinching stimulation (open triangles) and it relations with the AP duration at 50% of their amplitude (D50).
Cellular excitability of L4-AHTMR after L5 pSNL during mechanical activation
Peripheral excitability
As previously described,3 AHTMRs in L4 after L5 injury showed a reduced mechanical threshold compared to normal animals (Figure 2(b)). The response to threshold mechanical stimulation (median: 12 APs per stm (8–34)) and activation time (median: 1.5 s (1.2–1.8)) were also significantly enhanced compared to the normal responses (median: 3APs per stm (3–18) and 0.27 s (0.1–0.8) (p < 0.01), while their IFmax (Median: 27 Hz; 18–73 Hz) did not differ from uninjured animals. In contrast to what was observed in normal animals, a 2 s suprathreshold mechanical activation did not induce a reduction in responsiveness, but instead induced a significant (p < 0.001) increase in the number of evoked APs/stm (median: 20 AP per stm (15–59)). Both the activation time and the IFmax of these neurons remained unchanged.
Somatic excitability
The active properties of these neurons were similar to those observed in normal animals after repeated pinching. In addition, their AP durations were significantly shorter (p < 0.01) than normal neurons with significantly greater amplitude (p < 0.05) at comparable membrane potentials (Figure 6). Similarly, depolarizing and hyperpolarizing rates were also significantly (p < 0.01) faster than AHTMRs from normal animals (depolarization rate median: 182 V/s (102–308); hyperpolarization rate median: −101 V/s (−173 to −58)). The peripheral activation (threshold and/or suprathreshold) of these neurons did not trigger any observable change in membrane potential (e.g. PDH was absent).
Figure 6.
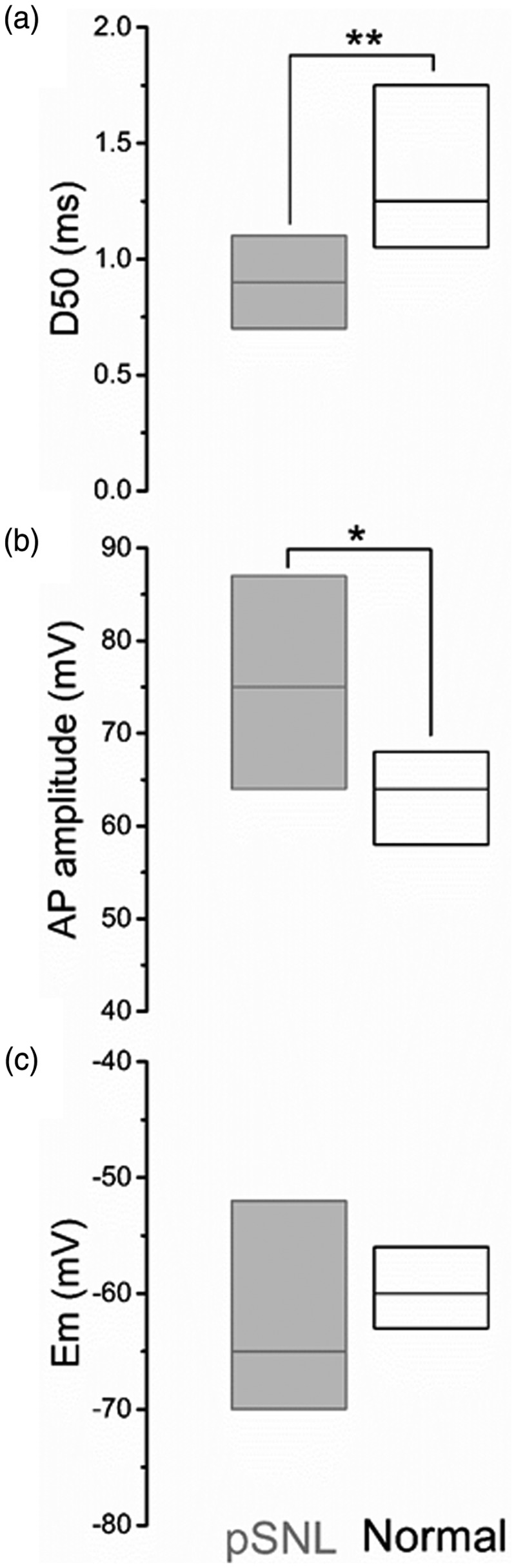
Comparison of somatic electrical properties of nociceptive AHTMR afferent neurons, obtained after L5 pSNL (gray) and normal naïve (black). Data show as medians (bars) and 25th and 75th percentiles (boxes). *p < 0.05; **p < 0.01.
Discussion
In this study, we report the acute and subacute effects of repeated mechanical suprathreshold activation on the membrane potential, responsiveness, and overall electrical excitability of different subtypes of mechanoreceptors. The principal observations and conclusions are (1) short-term (2 s) suprathreshold stimulation induces an early hyperpolarization of the membrane potential in fast-conducting nociceptors (AHTMRs) lasting several seconds; (2) this hyperpolarizing response is a unique feature of AHTMR afferent neurons; and (3) paradoxically, acute initial activation reduces cellular response to suprathreshold stimulation. Although brief, this cellular response was present in all 20 neurons studied. This “braking” mechanism (the rapid adaptation) in the face of initial sustained stimulation disappears after repeated suprathreshold stimuli in normal animals or is absent after damage (rendering the response slowly adapting and enhancing total activity) (e.g. L4-AHTMR after L5 pSNL) and may be one contributor to the development of peripheral sensitization in AHTMRs.
Technical considerations
Although this report addresses the early effect of suprathreshold mechanical stimulation of primary sensory afferent neurons, there are methodological aspects that limit its interpretation. Clearly the main problem arises from the nociceptive suprathreshold stimulation of normal neurons. While tactile afferent neurons respond to low intensity mechanical stimulation, nociceptors require greater forces. This fact effectively prevents detailed measurement of further increments in mechanical thresholds (already extremely high) and adequate (more precise) control of the stimulation time. While this study focuses on the cellular responsiveness of AHTMRs, improved stimulation methods may be required to better understand all of the nuances of the change in responsiveness from activation. In addition, our recordings were made in the cellular body of the afferent neurons to infer a process that likely takes place in the neuronal terminals within the RF. The temporal course and magnitude of these effects cannot be appropriately addressed without the direct recording of excitability within the terminals, something that remains beyond the current technology.
We also recognize that the pinch suprathreshold stimulus, although commonly used, is not calibrated. It is conceivable that greater responsiveness (more APs) of AHTMRs, after consecutive activation could reflect unconscious bias and more force applied, but this would not explain the lack of hyperpolarization, sustained discharge during the pinch, and the presence of after discharges observed at this time.
Nociceptive mechanical excitability and its modulation
As we know, pain may be caused by stimulation of multiple modalities. Although progress has been made in understanding nociceptive heat sensitivity and responsiveness,17–19 far less is known about afferent encoding of mechanical forces. Particularly important are observations that nociceptive discharges fail to match the pain felt during sustained mechanical stimulation20,21 and division of fast-conducting nociceptors (AHTMR) into two phenotypes based on response pattern to mechanical stimulation. In general, extracellular recordings indicate that ATHMRs can be subclassified into two different groups: A-HT[SA] and A-HT[RA] based on their patterns of adaptation (SA and RA nociceptive units, respectively) to sustained mechanical stimulation.1,2 Our data using intracellular recordings suggest that these putative subgroups may be merely different states of cellular excitability of the same neuron rather than two distinct classes of neurons. Our recordings of the first AHTMRs collected in each animal showed higher mechanical thresholds than a mixture of first and subsequent AHTMRs characterized in the same DRG, previously reported by our group,3,6 suggesting that despite all the precautions to reduce or prevent sensitization used previously, even modest activation of the periphery to characterize one AHTMR may alter mechanical sensitivity in subsequent neurons. This difference in thresholds likely results from initial stages of cellular sensitization and the disappearance of the PDH from repeated stimulation. This further supports the thesis that the PDH acts as an acute and initial braking mechanism allowing an initial response (avoiding or removing the stimulus by the organism or animal) without reducing responsivity during ongoing increasing activity as in chronic or subsequent activation. Our data also indicate that the transition from one state (A-HT[RA]) to the other (A-HT[SA]) depends not only on the magnitude of the stimulus but the recent history of cellular activation and its duration. This last statement is important not only because it can explain the reported inconsistency in the relationship between nociceptive activation and pain sensation20,21 but also because only one excitable state (A-HT[SA]) can be observed in afferent neurons after a presumed painful neuropathic injury has been performed (L5 pSNL).4 Together, our data indicate that the normal non-excitable state of fast-conducting nociceptors is almost entirely phasic (A-HT[RA]), encoding only the beginning (On response) of any mechanonociceptive stimulus and its magnitude (based on mechanical threshold). This also indicates that temporal encoding of the stimulus duration (at least initially or acutely) may rely heavily on the activity of slow-conducting mechanonociceptor (CHTMRs), afferent neurons with exclusively tonic responsiveness (SA). Peripheral suprathreshold stimulation (without apparent damage to tissues) triggers the activation of transient electrical compensatory mechanisms (see below) thereby limiting responsiveness and information transmission. This process should ultimately reduce the cell encoding capabilities and as such, partially block AP generation and limit nociceptive signaling from AHTMRs.
Post-discharge hyperpolarization
As stated above, our data show that AHTMR activation eventually triggers hyperpolarization of the membrane potential. This process seems to have a threshold (does not occur immediately after the first stimulus) is cumulative in nature (further cellular activation induces discrete increments in the membrane hyperpolarization), is transient (it disappears after cellular intense activation) and is only observed in fast-conducting nociceptors (AHTMRs). This effect does not occur in damaged neurons that are already hyperexcitable (e.g. L4-AHTMR after L5-pSNL). Together, these observations suggest PDH is a primordial endogenous cellular mechanism that reduces or prevents sustained activity of the AHTMR in the event of transient activation when no damage occurs. To our knowledge, this is the first time this electrical mechanism of PDH in AHTMR is documented in detail and correlated with afferent responsiveness, but not the first time the hyperpolarization has been observed. Moreover, it is the first time that a role in reducing excitability and possibly changing cellular adaptation has been observed. Woodbury and Koerber noticed similar electrical events in AHTMRs recorded intracellularly in their ex vivo neonatal mouse model.10 The fact that this process takes place in similar nociceptive afferent neurons in two different species along a developmental axis (mice and rats, newborn to adult) further suggests that the PDH is a basic intrinsic property for the appropriate modulation of AHTMR excitability.
The molecular mechanisms for this PDH are not known. Based on the observed changes in AHTMR electrical signature (reduction in the AP duration and increased voltage change rate), it is conceivable that calcium conductance and intracellular signaling are involved in the genesis of this process. As we know, AHTMR nociceptors have wider APs than tactile afferent neurons within the same CV ranges due the presence of an inflexion on the falling AP phase7–9,11,12,22–24 largely attributed to the presence of calcium conductance.25 On the other hand, it is also well known that calcium-activated potassium conductances play a role in the long AHP which is a distinctive characteristic of AHTMR afferent neurons.6,8,9 Taken together it seems plausible to hypothesize the involvement of Ca2+-activated potassium channels (KCa) as the PDH driving mechanism. Indeed, the activity of these channels may explain the changes that we have observed in the electrical signature of the recorded AHTMR APs. Moreover, KCa channels are widely expressed in nociceptive neurons.26 These channels are activated by increases in the intracellular calcium and in some cases also voltage sensitive (big conductance BKCA).27,28 The fact that these channels are activated by an increase in intracellular Ca2+ may be relevant to our observations on the AHTMR electrical changes. This increase in intracellular Ca2+ should induce the efflux of K+ which ultimately (via re/hyperpolarization of the membrane potential) should feed back onto intracellular Ca2+ by limiting the Ca2+ influx. Likely, this process should modify the membrane excitability either through deactivation of voltage-gated calcium (Cav) channels or through increased transport activity of Na+/Ca+ exchangers.29 Further studies will be needed to delineate the exact mechanism of the PDH and to determine if it can be induced or inhibited pharmacologically. This will help accelerate our understanding of the inhibitory nature of the PDH and its possible role in the sensitization process.
Conclusions
Here, we have described a RA response to non-tissue injuring noxious mechanical stimuli that is present during the initial stimulus in AHTMRs and yet disappears with continued activation or sensitization. This “braking” phenomenon seems to depend on the PDH and may serve to protect these neurons against paradoxical firing, likely tuning the peripheral primary nociceptive system toward inactivity unless justified by legitimate threat from repeated intense stimuli or from injury.
Acknowledgments
We wish to thank Dr Ken-ichiro Hayashida for performing the pSNL surgeries and Carol Aschenbrenner for her assistance with the statistical analysis.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Eisenach has received consulting fees from Adynxx and Teva Pharmaceuticals regarding clinical development of analgesics.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by grants R37-GM48085 (JCE) and R01-GM104249 (DGR, MDB) from the National Institutes of Health.
References
- 1.Andrew D, Greenspan JD. Peripheral coding of tonic mechanical cutaneous pain: comparison of nociceptor activity in rat and human psychophysics. J Neurophysiol 1999; 82: 2641–2648. [DOI] [PubMed] [Google Scholar]
- 2.Dubin AE, Patapoutian A. Nociceptors: the sensors of the pain pathway. J Clin Invest 2010; 120: 3760–3772. DOI: 10.1172/JCI42843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boada MD, Gutierrez S, Aschenbrenner CA, et al. Nerve injury induces a new profile of tactile and mechanical nociceptor input from undamaged peripheral afferents. J Neurophysiol 2015; 113: 100–109. DOI: 10.1152/jn.00506.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boada MD, Martin TJ, Peters CM, et al. Fast-conducting mechanoreceptors contribute to withdrawal behavior in normal and nerve injured rats. Pain 2014; 155: 2646–2655. DOI: 10.1016/j.pain.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boada MD. Relationship between electrophysiological signature and defined sensory modality of trigeminal ganglion neurons in vivo. J Neurophysiol 2013; 109: 749–757. DOI: 10.1152/jn.00693.2012. [DOI] [PubMed] [Google Scholar]
- 6.Boada MD, Gutierrez S, Giffear K, et al. Skin incision-induced receptive field responses of mechanosensitive peripheral neurons are developmentally regulated in the rat. J Neurophysiol 2012; 108: 1122–1129. DOI: 10.1152/jn.00399.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boada MD, Gutierrez S, Houle T, et al. Developmental differences in peripheral glabrous skin mechanosensory nerve receptive field and intracellular electrophysiologic properties: phenotypic characterization in infant and juvenile rats. Int J Dev Neurosci 2011; 29: 847–854. DOI: 10.1016/j.ijdevneu.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boada MD, Woodbury CJ. Physiological properties of mouse skin sensory neurons recorded intracellularly in vivo: temperature effects on somal membrane properties. J Neurophysiol 2007; 98: 668–680. DOI: 10.1152/jn.00264.2007. [DOI] [PubMed] [Google Scholar]
- 9.Boada MD, Woodbury CJ. Myelinated skin sensory neurons project extensively throughout adult mouse substantia gelatinosa. J Neurosci 2008; 28: 2006–2014. DOI: 10.1523/JNEUROSCI.5609-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodbury CJ, Koerber HR. Widespread projections from myelinated nociceptors throughout the substantia gelatinosa provide novel insights into neonatal hypersensitivity. J Neurosci 2003; 23: 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boada MD, Eisenach JC, Ririe DG. Mechanical sensibility of nociceptive and non-nociceptive fast-conducting afferents is modulated by skin temperature. J Neurophysiol 2016; 115: 546–553. DOI: 10.1152/jn.00796.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boada MD, Houle TT, Eisenach JC, et al. Differing neurophysiologic mechanosensory input from glabrous and hairy skin in juvenile rats. J Neurophysiol 2010; 104: 3568–3575. DOI: 10.1152/jn.00415.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan Y, Yuan F, Carteret AF, et al. A partial L5 spinal nerve ligation induces a limited prolongation of mechanical allodynia in rats: an efficient model for studying mechanisms of neuropathic pain. Neurosci Lett 2010; 471: 43–47. DOI: 10.1016/j.neulet.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garell PC, McGillis SL, Greenspan JD. Mechanical response properties of nociceptors innervating feline hairy skin. J Neurophysiol 1996; 75: 1177–1189. [DOI] [PubMed] [Google Scholar]
- 15.Greenspan JD, McGillis SL. Stimulus features relevant to the perception of sharpness and mechanically evoked cutaneous pain. Somatosens Mot Res 1991; 8: 137–147. [DOI] [PubMed] [Google Scholar]
- 16.Slugg RM, Meyer RA, Campbell JN. Response of cutaneous A- and C-fiber nociceptors in the monkey to controlled-force stimuli. J Neurophysiol 2000; 83: 2179–2191. [DOI] [PubMed] [Google Scholar]
- 17.Raja SN, Meyer RA, Campbell JN. Peripheral mechanisms of somatic pain. Anesthesiology 1988; 68: 571–590. [DOI] [PubMed] [Google Scholar]
- 18.Treede RD, Meyer RA, Campbell JN. Myelinated mechanically insensitive afferents from monkey hairy skin: heat-response properties. J Neurophysiol 1998; 80: 1082–1093. [DOI] [PubMed] [Google Scholar]
- 19.Treede RD, Meyer RA, Raja SN, et al. Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. J Physiol 1995; 483(Pt 3): 747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koltzenburg M, Handwerker HO. Differential ability of human cutaneous nociceptors to signal mechanical pain and to produce vasodilatation. J Neurosci 1994; 14: 1756–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Hees J, Gybels J. C nociceptor activity in human nerve during painful and non painful skin stimulation. J Neurol Neurosurg Psychiatry 1981; 44: 600–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ritter AM, Mendell LM. Somal membrane properties of physiologically identified sensory neurons in the rat: effects of nerve growth factor. J Neurophysiol 1992; 68: 2033–2041. [DOI] [PubMed] [Google Scholar]
- 23.Djouhri L, Bleazard L, Lawson SN. Association of somatic action potential shape with sensory receptive properties in guinea-pig dorsal root ganglion neurones. J Physiol 1998; 513: 857–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fang X, McMullan S, Lawson SN, Djouhri L. Electrophysiological differences between nociceptive and non-nociceptive dorsal root ganglion neurones in the rat in vivo. J Physiol 2005; 565: 927–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blair NT, Bean BP. Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. J Neurosci 2002; 22: 10277–10290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ocana M, Cendan CM, Cobos EJ, et al. Potassium channels and pain: present realities and future opportunities. Eur J Pharmacol 2004; 500: 203–219. DOI: 10.1016/j.ejphar.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 27.Mongan LC, Hill MJ, Chen MX, et al. The distribution of small and intermediate conductance calcium-activated potassium channels in the rat sensory nervous system. Neuroscience 2005; 131: 161–175. DOI: 10.1016/j.neuroscience.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 28.Zhang XF, Gopalakrishnan M, Shieh CC. Modulation of action potential firing by iberiotoxin and NS1619 in rat dorsal root ganglion neurons. Neuroscience 2003; 122: 1003–1011. [DOI] [PubMed] [Google Scholar]
- 29.Berkefeld H, Fakler B, Schulte U. Ca2+-activated K+ channels: from protein complexes to function. Physiol Rev 2010; 90: 1437–1459. DOI: 10.1152/physrev.00049.2009. [DOI] [PubMed] [Google Scholar]