Abstract
Collapsing glomerulopathy (CGP) is a pattern of kidney injury seen on renal biopsy with multiple associations and etiologies. It is most commonly described in African-Americans and others with recent African ancestry. The disease is rapidly progressive and often presents with abrupt onset of renal failure and nephrotic-range proteinuria. Since its description 30 years ago, this entity has transformed from a morphologic diagnosis typically seen in the setting of HIV infection to a complicated diagnosis with numerous etiologies, many of which are associated with underlying apolipoprotein L1 (APOL1)-risk variants or other genetic disorders. We review the evolution of CGP, and its history and proposed pathomechanisms. We also present the disease spectrum from our experience with emphasis on recognizing the lesion, distinguishing from mimics and linking the histopathological pattern to a specific cause. Our understanding continues to evolve as clinicians and scientists work toward a more complete understanding of the molecular pathways of injury in this disease and how these might be disrupted for therapeutic purposes. Much still remains to be discovered in CGP as the molecular underpinnings leading to disease are still not completely understood and no effective treatment exists despite the high morbidity. Based on this rapid evolution, CGP is a modern template of how we diagnose and think about kidney disease. The story of CGP represents the current shift in nephrology and nephropathology from morphology-alone-based diagnosis to a comprehensive approach including molecular diagnostics. We believe this new, holistic approach will lead to pathogenesis-centered diagnoses that will help to individualize risk stratification and treatment protocols.
Keywords: APOL1, collapsing glomerulopathy, HIVAN, mitotic catastrophe, pathology
Birth and evolution of a new morphologic entity: collapsing glomerulopathy
In the mid-1980s, the first descriptions of a very aggressive proteinuric disease with a glomerular pattern of collapse were published. Weiss et al. wrote the first clinicopathologic findings of what we now view as collapsing glomerulopathy (CGP) [1]. They described six African-American patients who developed rapidly progressive renal failure, nephrotic syndrome, glomerular tuft collapse, podocyte hyperplasia and significant tubulointerstitial damage (Figure 1A–C). Differently from the previously reported human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) phenotype [2, 3], these patients had negative serology for the HIV. Using a computerized medical record system, they convincingly argued that these patients represented a new disease entity [2, 3]. The association between HIV and CGP largely came about following a report by Cohen and Nast. describing nine HIV-positive patients with proteinuria and rapidly progressive renal disease [4]. Morphologically, these patients consistently showed unique segmental tuft collapse with overlying podocyte hypertrophy and hyperplasia. In addition, microcystic tubular dilatation with inspissated, proteinaceous material and extensive protein resorption droplets within proximal tubular epithelium were described. While this entity shared many similarities with the entity Weiss et al. reported, it was termed HIV-associated nephropathy (HIVAN) due to the strong correlation with HIV/AIDS infection. In 1994, Detwiler et al. reported 16 predominantly African-American patients, with rapidly progressive renal failure, proteinuria and segmental to global glomerular tuft collapse [5]. These findings shared extensive morphologic overlap with the patients described by Weiss et al., and Cohen et al. and the findings were corroborated by a comprehensive study of 30 cases reported by D’Agati et al. in 1989 [6]. In the mid-1990s, the original observations were confirmed by several authors. In 1994, Detwiler et al. reported 16 predominantly African-American, HIV-negative, patients with features similar to those described by Weiss etal. [1], including rapidly progressive renal failure, proteinuria and segmental to global glomerular tuft collapse [5]. Subsequently, others corroborated these results and with a larger study by Valeri etal., the term collapsing focal segmental glomerulosclerosis was introduced in the literature [7, 8].
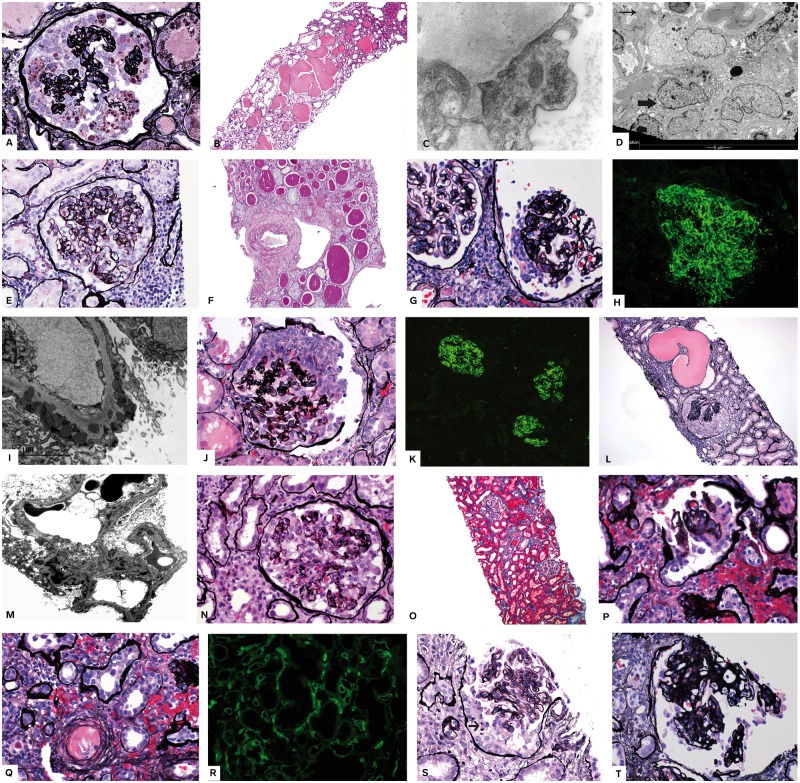
Fig. 1.
CGP, morphologic indicators of APOL1-related nephropathy, underlying etiologies and mimics. (A–D) HIV–CGP. Biopsy from a 31-year-old African-American (AA) woman, HIV-positive, who presented with nausea and vomiting, hematuria and proteinuria, acute and chronic renal failure, creatinine 3.4 mg/dL. Serum albumin 2.1 g/L. (A) Global capillary loop wrinkling (collapse) and massive podocyte proliferation. Podocytes are filled with lipid droplets (silver stain ×200). (B) Markedly dilated tubules filled with eosinophilic material (H + E ×100). (C) Tubular reticular inclusions in endothelial cell [electron microscopy (EM) ×20 000]. (D) Multinucleated podocytes (thick arrow) lining collapsed capillary loops (thin arrow). Also present are cytoplasmic osmiophilic inclusions (lysosomes). (E, F) APOL1 nephropathy. Renal biopsy is from a 51-year-old AA man who presented with hypertension, nephrotic-range proteinuria and chronic kidney disease. He was subsequently found to have two APOL1 risk alleles. (E) Biopsy shows segmental glomerular loop collapse and podocyte proliferation over the collapsed loops (silver ×200). Numerous solidified glomeruli were present (not shown). (F) Tubular atrophy thyroidization type and severe arteriosclerosis (PAS ×100). (G–I) Lupus membranous with CGP. Patient is a 27-year-old woman with nephrotic syndrome recently diagnosed with lupus. (G) CGP is shown. There are no spikes (silver stain ×200). (H) Immunofluorescence showed diffuse granular capillary loop deposits [(immunoglobulin G IgG) ×200]. (I) EM shows subepithelial and early intramembranous deposits. (J–M) Lupus with APOL1. The patient was an AA woman with well documented lupus serology seen for follow-up because of persistent proteinuria in spite of aggressive therapy. APOL1 genotyping revealed two risk alleles (Figure 2). (J) Segmental pilling up of visceral podocytes mimicking epithelial crescent (silver ×200). (K) Immunofluorescence showed diffuse full house immune deposits [immunofluorescence (IF) ×50]. (L) Crescent-like proliferation and marked tubular dilatation (silver ×100). (M) EM shows subendothelial and mesangial deposits. (N, O) Interferon-induced CGP. A 54-year-old man with multiple sclerosis treated with interferon beta1 alpha. Presented with proteinuria more than 5 g/24 h and preserved renal function. (N) Segmental capillary loop collapse and podocyte proliferation (silver ×200). (O) No tubulointerstitial damage (trichrome ×100). (P–R) Ischemic CGP in allograft kidney. The patient is a 43-year-old AA with deceased donor kidney 5 years prior to this biopsy. He presented with severe hypertension (HTN) and creatinine 4.5 mg/dL and increasing proteinuria. (P) Retracted glomerulus shows podocyte proliferation (silver ×200). (Q) Arteriolar thrombosis is identified adjacent to the glomerulus in (P) (silver stain ×200). (R) Diffuse C4d positivity in peritubular capillaries consistent with antibody mediated rejection (IF ×100). (S) Epithelial crescents mimic CGP. Shown is a glomerulus with podocyte proliferation (arrow points to mitotic podocyte) with no necrosis or fibrin deposits and vague capillary loop collapse. Patient was a 62-year-old man with rapidly progressing glomerulonephritis and pending serologies at the time of biopsy. No known predisposing factors for CGP. (T) Diabetes with CGP. The patient was a known diabetic for 10 years with acute onset nephrotic-range proteinuria. Biopsy shows podocyte proliferation over glomerular diabetic nodules (silver stain ×200).
Later, studies by Barisoni et al. suggested a common pathologic mechanism between CGP and HIVAN that leads to a dysregulated podocyte phenotype in both HIVAN and CGP [9]. This concept of common mechanisms of injury was based on three lines of evidence. First, both entities showed complete loss of normal podocyte phenotype utilizing known markers of podocytes (CALLA, GLEPP1, Podocalyxin, Synaptopodin, WT1, P27 and p57 were decreased while Cyclin D1, Cyclin E, Cyclin A, Ki-67, Desmin, Cytokeratin and CD68 were increased). Second, comparable numbers of podocytes were noted to enter the cell cycle in both diseases, in contrast to normal podocytes that are physiologically arrested, post-mitotic cells. Lastly, both diseases showed identical ultrastructural cytoarchitecture. Barisoni et al. subsequently proposed the term podocytopathy for diseases of the podocyte and developed a classification structure [normal glomerular histology—minimal change disease (MCD); segmental glomerulosclerosis (FSGS); mesangial sclerosis—diffuse mesangial sclerosis (DMS); and capillary loop collapse—CGP] [10].
More recently, a concept has been proposed that is termed podocyte mitotic catastrophe (MC) that attempts to explain the etiology of podocytopathies such as collapsing lesions [11]. To explain this concept, we can look to collapsing lesions in HIVAN. There is evidence that HIV can infect podocytes (and parietal epithelial cells), leading to podocyte mitosis and a switch to a proliferative podocyte phenotype where proliferating podocytes attempt to divide unsuccessfully. The morphologic result of this switch in phenotype is podocyte multi-nucleation (Figure 1D), previously thought of as a compensatory mechanism of podocyte repair. However, this phenotype switch and podocyte multi-nucleation has now been suggested to be a distinct podocyte death mechanism—MC [11, 12]. MC is believed to be a conflict in the podocyte cell cycle that prevents podocytes from dividing and leads to their detachment from the glomerular basement membrane (GBM). This is a separate mechanism from crescent formation where ruptured capillary loops allow blood leakage into Bowman’s space, which promotes epithelial cell proliferation (both visceral and parietal) [13]. The connection of podocyte proliferation and aberrant cell death through attempted mitosis (MC) is still a new concept that may potentially help explain the pathogenesis of CGP. However, to date, little has been studied experimentally or clinically on this topic despite its intriguing implications.
Throughout the discovery phase of CGP, several secondary etiologies/associations were reported such as renal vascular ischemia, infection other than HIV (hepatitis C, HTLV-1, parvovirus B19 and loa loa filariasis), systemic lupus erythematosus, drugs [such as pamidronate, interferon (Figure 1N and O), anabolic steroids and heroin], hematologic neoplasia and familial types [14–22]. Throughout the 2000s, CGP saw mostly growth in its associations without significant change in its morphologic descriptions.
CGP in the era of genetics
The discovery of the apolipoprotein L1 (APOL1) G1 and G2 risk variants (located on the long arm of chromosome 22 at position 13.1) would prove to be tremendously important in shaping our understanding of CGP [23]. These commonly occurring variants in the APOL1 gene are responsible for the increased burden of non-diabetic renal disease affecting African-Americans [24]. Patients with any combination of two of these risk alleles inherit a markedly increased risk of renal disease and progression to end-stage renal disease.
The G1 variant is a pair of two non-synonymous single nucleotide polymorphisms (SNPs) in almost complete linkage disequilibrium. The G2 variant is an in-frame deletion of the two amino acid residues, N388 and Y389 [25]. The gene product, APOL1 protein, is a minor component of high-density lipoprotein that is found in vascular endothelium, liver, heart, lung, placenta, podocytes, proximal tubules and arterial cells [26]. The protein also has a secreted form that circulates in the blood and is known for its roles in trypanosomal lysis, autophagic cell death, lipid metabolism and other biological activities [27]. The APOL1 risk variants are common in the African-American population due to selection pressure related to the protection they confer from Trypanosoma brucei rhodesiense infection [27].
The role of APOL1 in kidney health is still not entirely understood but there is at least one report of an APOL1 null Indian man who had no evidence of kidney disease while infected with trypanosomes [28]. This suggests that APOL1 may be dispensable for normal kidney function and that APOL1 risk variants may acquire toxic functions that damage the kidney. CGP has been shown to be associated with the presence of homozygosity for APOL1 risk alleles in a number of disease settings including HIV, lupus nephritis, membranous glomerulopathy and in association with interferon and pamidronate treatment [17, 19, 29–31]. It appears that both glomerular injury and inflammatory ‘second hits’ may potentiate kidney damage in patients with APOL1 risk variants [25, 32]. In addition, while identification of APOL1 risk variants requires genetic testing, Larsen et al. have recently described key morphologic features [microcystic tubular dilatation, thyroid-type tubular atrophy (Figure 1E and F) and a predominance of solidified/disappearing-type global glomerulosclerosis] that, when found in combination, have significant association with underlying APOL1 gene risk variant homozygosity [33].
CGP in children
While uncommon, CGP has been reported in children, especially in the setting of underlying APOL1 risk variants [34]. Kopp et al. described a pediatric subset of their cohort with two underlying APOL1 risk variants [34]. While the pediatric patients showed less FSGS than their older cohorts, CGP was identified within this population, usually after 12 years of age [34]. Subsequent studies of APOL1 in children have shown a more aggressive course of renal disease in those with homozygosity for APOL1 risk alleles [35].
In addition to the APOL1 association, other genetic diseases may be associated with CGP in children, especially ones with underlying mitochondrial dysfunction (action myoclonus—renal failure syndrome, ZMPSTE24 mandibuloacral dysplasia/action myoclonus, mitochondrial cytopathy coenzyme Q10 (CoQ10), CoQ2 deficiency and sickle cell anemia) [36]. Whether mitochondria in general may represent a common pathogenic pathway for CGP is intriguing because mitochondrial damage induces cell death and cytochrome C release, which have also been shown to play a role in CGP development [36, 37].
Another point to be considered in childhood CGP is the potential morphologic resemblance of CGP and DMS. While these entities are often straightforward to distinguish, both CGP and DMS may show podocyte proliferation and differentiation may be difficult in rare cases of DMS where mesangial sclerosis is not present. However, this is a very rare occurrence as DMS frequently shows additional glomerular findings (e.g. fetal glomeruli) and clinical manifestations (e.g. Denys–Drash syndrome) that may help in differentiation [38]. As DMS may be caused by treatable entities (CoQ2, CoQ10 and other mitochondrial enzyme deficiency), differentiation is imperative [36, 37].
CGP in the allograft kidney
CGP in the renal allograft has a similar set of potential etiologies to CGP in the native kidney [39]. However, two potential scenarios deserve special attention. First, APOL1 risk variants are still an important etiology of CGP in the renal allograft; however, it is the APOL1 status of the allograft donor that is associated with disease [40]. And, should CGP that is associated with donor APOL1 risk variants be identified, correlation with the other transplanted kidney (if both of the donor’s kidneys were transplanted) should be attempted as both will be affected [40]. The occurrence of APOL1-associated kidney disease in renal transplants has raised the important question of APOL1 gene testing of allografts from high-risk populations. However, this topic is controversial and would currently be difficult to implement due to utility and turnaround-time limitations associated with genetic testing [41].
Next, is the issue of how to interpret focal collapsing lesions in the renal allograft in the setting of ischemia and microangiopathy (Figure 1P–R). Although primarily reported in native kidneys, collapsing lesions can be seen in thrombotic microangiopathy (TMA) and other ischemic conditions in the allograft, and often occur in glomeruli with significant microangiopathic injury (Figure 1Q) [42–44]. In this setting, there should be a high threshold for the diagnosis of CGP. Features we find helpful to suggest a diagnosis of CGP include involvement of glomeruli that are seemingly uninvolved by microangiopathy and background tubulointerstitial changes typically seen in CGP (Figure 1G and H). Clinical symptoms supportive of CGP (nephrotic-range proteinuria and acute renal failure) are not uncommon in TMA and may not be helpful. Descriptive diagnoses or comments explaining the morphology in this setting are potentially useful and alerts the nephrologist to monitor the patient during and after treatment of the TMA to see if they have additional clinical features to corroborate the presence of a concurrent CGP (such as an abnormal clinical course, APOL1 risk alleles or a known secondary disease such as HIV).
CGP: causes and distribution from a large database
In our renal biopsy, database over a 15.5-year period, 1201 cases of CGP were identified representing 1.4% of renal biopsies performed over that time period (∼70 000). Over our study interval (the last 6 months of 2015) a total of 88 sequential CGP cases were retrieved as a focused subset. These cases represent 1.3% of the total renal biopsies over this period, a frequency similar to that of the entire database. Our biopsy database was queried for cases containing the term ‘CGP’ within the diagnosis or comment (July–December 2015). Inclusion criteria for this study consisted of: native and adequate renal biopsy tissue for diagnosis, unequivocal diagnosis of CGP and absence of proliferative lupus nephritis with crescents. A total of 88 cases met inclusion criteria and were included in the cohort.
Average patient age was 44 (11–83 range) years old with a nearly equal male:female ratio (48% male, 52% female). As in previous studies, a marked predilection for African ancestry was noted, with 84% of patients being African-American (see Table 1). The reasons for renal biopsy varied, with the most common presentation being nephrotic-range proteinuria and acute renal failure (51% of patients). Chronic kidney disease was noted in a fraction (17%) of patients. Markedly elevated serum creatinine values were common, with an average of 4.15 mg/dL (0.8–17.8 mg/dL range), and proteinuria was most often of the nephrotic range (72% of patients) with an average of 11.2 g/day (0.8–31 g/day range). Hematuria was noted in 28% of patients (however, this data point may not be reliable as <50% of patients had this data available for review). Hypertension was frequently present (92% of patients) at the time of biopsy.
Table 1.
Clinical findings and etiologies
| Average age | 44 years (11–83 range) |
| Male:female ratio | 1:1 |
| Ethnicity (%) | |
| African-American | 84 |
| Caucasian | 13 |
| Hispanic | 1 |
| Average serum creatinine (mg/dL) | 4.15 (0.8–17.8 range) |
| Average proteinuria (g/day) | 11.2 (0.8–31 range) |
| Nephrotic syndrome (%) | 86 |
| Hypertension (%) | 92 |
| Etiology of collapsing glomerulopathy (%) | |
| Idiopathic disease (APOL1 not tested) | 77 |
| HIV/AIDS (HIVAN) | 17 |
| Chronic ischemic vascular disease | 3 |
| APOL1-associated nephropathy | 2 |
| Heroin nephropathy | <1 |
| Hepatitis C | <1 |
While a potential etiology of CGP was identified in a small subset of patients, 77% of patients (68 patients) had idiopathic disease (see Table 2); however, none of these patients was tested for APOL1 risk variants. The most common etiology identified in these patients was HIV/AIDS (17%; 15 patients) and among these 15 patients with HIV/AIDS, 10 were African-American, one was Caucasian and four had unknown ancestry. Three non-African ancestry patients (3%) showed ischemic glomerular and tubulointerstitial changes in addition to arteriosclerosis, and a history of hypertension and an ischemic etiology was favored. A single patient (1%) had a history of recent heroin use and likely represented heroin nephropathy while another single patient (1%) had a history of active hepatitis C infection in the absence of other known etiologies of CGP. Additionally, eight patients had a history of systemic lupus erythematosus, with four showing membranous lupus nephritis (ISN/RPS Class V) (Figure 1G–I). APOL1 genetic testing was only requested and performed in two patients, both with systemic lupus, and both (2%) showed homozygosity for APOL1 risk alleles (Figure 1J–M and Figure 2). This overall distribution of etiologies is comparable to other, similar case series in CGP [5, 7, 8, 45, 46].
Table 2.
CGP associations from published studies and our data
| Infectious micro-organisms |
| HIV, parvovirus 19, cytomegalovirus, hepatitis C |
| Tuberculosis, Campylobacter enteritis |
| Autoimmune diseases |
| Lupus, lupus-like disease, connective tissue disease, Still’s disease |
| Hereditary |
| APOL1-related nephropathy |
| Mitochondrial cytopathies CoQ2 and CoQ10 deficiency, action myoclonous |
| Drugs |
| Interferon |
| Bisphosphonates |
| Anabolic steroids |
| Heroin |
| Valproic acid |
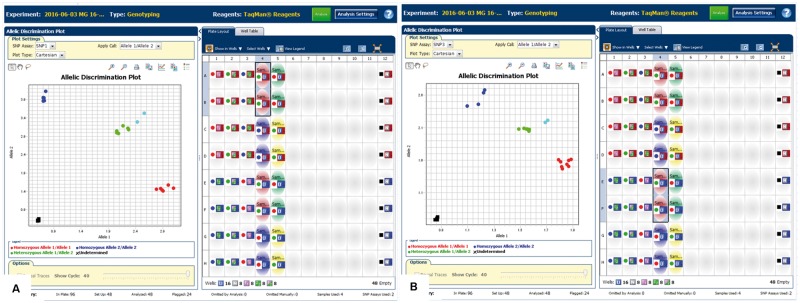
Fig. 2.
APOL1 genotyping. Taqman SNP genotyping data performed on a real-time PCR system with primers designed to detect the APOL1 risk alleles G1 (rs73885319) and G2 (rs71785313). The genotypes cluster according to whether they are homozygous for the risk allele being tested (dark blue), homozygous wild type (red) or heterozygous for the risk allele (green). The black boxes represent no-template controls. This patient (light blue) is heterozygous for each risk allele, indicating that she is compound heterozygous for G1 (A) and G2 (B) APOL1 risk alleles and, therefore, at risk for APOL1-associated nephropathy.
A second biopsy diagnosis was present in 52% (46/88) of patients. The most common secondary diagnosis by a wide margin was acute tubular injury followed by glomerular immune complex deposition, acute/chronic tubulointerstitial nephritis, diabetic glomerulopathy and non-proliferative lupus nephritis.
The difficult diagnosis; entities mimicking CGP
In most CGP cases, morphologic diagnosis is straightforward when histopathologic criteria are applied. However, cases do arise that are more nuanced and raise considerable disagreement even among experienced renal pathologists as to whether a diagnosis of CGP should be invoked. These cases often show some features of CGP (Figure 1S) but lack convincing glomerular morphologic changes or the characteristic accompanying tubulointerstitial changes are not present. Also, glomerular epithelial hyperplasia can become particularly florid, appearing as crescentic glomerulonephritis. This particular difficulty is often encountered on a background of membranous glomerulopathy, diabetic glomerulopathy, or membranous lupus nephritis or TMA, further complicating interpretation [30, 31, 47]. As no data exist detailing any specific test to differentiate crescents from CGP this formidable question is often relegated to experience and opinion. In the case of underlying diabetic glomerulopathy, Salvatore et al. have recently addressed the question of collapsing lesions superimposed on diabetic glomerulopathy (Figure 1T) and their study suggested that these lesions might be attributable to ischemia [47]. Similarly, CGP in TMA is thought to be due to ischemia. The recent study by Buob etal. addressed the association from the endothelial injury point of view, bringing this mechanism into the pathogenesis of CGP [42].
Overall, we find the presence of fibrinoid necrosis, karyorrhexis, glomerular basement membrane rupture and red blood cell casts to be helpful indicators of crescent formation while the absence of these findings with the presence of protein resorption droplets admixed with the hypertrophied and hyperplastic podocytes, significant tubular intracytoplasmic protein resorption drops, microcystic tubular dilatation, thyroid type tubular atrophy and a predominance of solidified or disappearing-type global glomerulosclerosis suggests CGP.
Conclusions
CGP is a morphologic lesion representing a common endpoint from multiple etiologies. It is a podocytopathy that is often secondary to APOL1 risk variants but has also been associated with infection, drugs, ischemia, hematologic neoplasia and autoimmune disease. Morphological features of CGP are time-tested and well-recognized today. However, there are several confounding morphologies that may provide diagnostic difficulty. The discovery of APOL1 risk variants changed the way we understand and classify CGP and provide, in part, a unifying etiology for some of the underlying associations, leading to a more pathogenesis-based approach to this multifaceted diagnosis.
Acknowledgements
We wish to thank Dr Fred Silva at Arkana Laboratories for reviewing our manuscript and commenting on the early history of CGP.
Conflict of interest statement
None declared.
References
- 1. Weiss MA, Daquioag E, Margolin EG. et al. Nephrotic syndrome, progressive irreversible renal failure, and glomerular “collapse”: a new clinicopathologic entity? Am J Kidney Dis 1986; 7: 20–28 [DOI] [PubMed] [Google Scholar]
- 2. Rao TK, Filippone EJ, Nicastri AD. et al. Associated focal and segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N Engl J Med 1984; 310: 669–673 [DOI] [PubMed] [Google Scholar]
- 3. Pardo V, Aldana M, Colton RM. et al. Glomerular lesions in the acquired immunodeficiency syndrome. Ann Intern Med 1984; 101: 429–434 [DOI] [PubMed] [Google Scholar]
- 4. Cohen AH, Nast CC.. HIV-associated nephropathy. A unique combined glomerular, tubular, and interstitial lesion. Mod Pathol 1988; 1: 87–97 [PubMed] [Google Scholar]
- 5. Detwiler RK, Falk RJ, Hogan SL et al.. Collapsing glomerulopathy: a clinically and pathologically distinct variant of focal segmental glomerulosclerosis. Kidney Int 1994; 45: 1416–1424 [DOI] [PubMed] [Google Scholar]
- 6. D’Agati V, Suh JI, Carbone L. et al. Pathology of HIV-associated nephropathy: a detailed morphologic and comparative study. Kidney Int 1989; 35: 1358–1370 [DOI] [PubMed] [Google Scholar]
- 7. Haas M, Spargo BH, Coventry S.. Increasing incidence of focal-segmental glomerulosclerosis among adult nephropathies: a 20-year renal biopsy study. Am J Kidney Dis 1995; 26: 740–750 [DOI] [PubMed] [Google Scholar]
- 8. Valeri A, Barisoni L, Appel GB. et al. Idiopathic collapsing focal segmental glomerulosclerosis: a clinicopathologic study. Kidney Int 1996; 50: 1734–1746 [DOI] [PubMed] [Google Scholar]
- 9. Barisoni L, Kriz W, Mundel P et al.. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 1999; 10: 51–61 [DOI] [PubMed] [Google Scholar]
- 10. Barisoni L, Schnaper HW, Kopp JB.. A proposed taxonomy for the podocytopathies: a reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol. 2007; 2: 529–542 [DOI] [PubMed] [Google Scholar]
- 11. Liapis H, Romagnani P, Anders HJ.. New insights into the pathology of podocyte loss: mitotic catastrophe. Am J Pathol 2013; 183: 1364–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tharaux PL, Huber TB.. How many ways can a podocyte die? Semin Nephrol 2012; 32: 394–404 [DOI] [PubMed] [Google Scholar]
- 13. Ryu M, Migliorini A, Miosge N. et al. Plasma leakage through glomerular basement membrane ruptures triggers the proliferation of parietal epithelial cells and crescent formation in non-inflammatory glomerular injury. J Pathol 2012; 228: 482–494 [DOI] [PubMed] [Google Scholar]
- 14. Pakasa NM, Nseka NM, Nyimi LM.. Secondary collapsing glomerulopathy associated with loa filariasis. Am J Kidney Dis 1997; 30: 836–839 [DOI] [PubMed] [Google Scholar]
- 15. Moudgil A, Shidban H, Nast CC. et al. Parvovirus B19 infection-related complications in renal transplant recipients: treatment with intravenous immunoglobulin. Transplantation 1997; 64: 1847–1850 [DOI] [PubMed] [Google Scholar]
- 16. Tanawattanacharoen S, Falk RJ, Jennette JC et al.. Parvovirus B19 DNA in kidney tissue of patients with focal segmental glomerulosclerosis. Am J Kidney Dis 2000; 35: 1166–1174 [DOI] [PubMed] [Google Scholar]
- 17. Markowitz GS, Appel GB, Fine PL. et al. Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol 2001; 12: 1164–1172 [DOI] [PubMed] [Google Scholar]
- 18. Palma Diaz MF, Pichler RH, Nicosia RF. et al. Collapsing glomerulopathy associated with natural killer cell leukemia: a case report and review of the literature. Am J Kidney Dis 2011; 58: 855–859 [DOI] [PubMed] [Google Scholar]
- 19. Markowitz GS, Nasr SH, Stokes MB et al.. Treatment with IFN-{alpha}, -{beta}, or -{gamma} is associated with collapsing focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 2010; 5: 607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nasr R, Johns C, Gertner E.. Collapsing glomerulopathy in collagen vascular-like disease. Lupus 2014; 23: 75–80 [DOI] [PubMed] [Google Scholar]
- 21. Herlitz LC, Markowitz GS, Farris AB. et al. Development of focal segmental glomerulosclerosis after anabolic steroid abuse. J Am Soc Nephrol 2010; 21: 163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Avila-Casado MC, Vargas-Alarcon G, Soto ME. et al. Familial collapsing glomerulopathy: clinical, pathological and immunogenetic features. Kidney Int 2003; 63: 233–239 [DOI] [PubMed] [Google Scholar]
- 23. Genovese G, Tonna SJ, Knob AU. et al. A risk allele for focal segmental glomerulosclerosis in African Americans is located within a region containing APOL1 and MYH9. Kidney Int 2010; 78: 698–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Freedman BI, Kopp JB, Langefeld CD. et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol 2010; 21: 1422–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McNicholas BA, Nelson PJ.. Immunity unmasks APOL1 in collapsing glomerulopathy. Kidney Int 2015; 87: 270–272 [DOI] [PubMed] [Google Scholar]
- 26. Nichols B, Jog P, Lee JH. et al. Innate immunity pathways regulate the nephropathy gene Apolipoprotein L1. Kidney Int 2015; 87: 332–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Genovese G1, Friedman DJ, Ross MD. et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010; 329: 841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnstone DB, Shegokar V, Nihalani D. et al. APOL1 null alleles from a rural village in India do not correlate with glomerulosclerosis. PLoS One 2012; 7: e51546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kopp JB, Nelson GW, Sampath K. et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 2011; 22: 2129–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Larsen CP, Beggs ML, Saeed M et al.. Apolipoprotein L1 risk variants associate with systemic lupus erythematosus-associated collapsing glomerulopathy. J Am Soc Nephrol 2013; 24: 722–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Larsen CP, Beggs ML, Walker PD. et al. Histopathologic effect of APOL1 risk alleles in PLA2R-associated membranous glomerulopathy. Am J Kidney Dis 2014; 64: 161–163 [DOI] [PubMed] [Google Scholar]
- 32. Couser WG, Johnson RJ.. The etiology of glomerulonephritis: roles of infection and autoimmunity. Kidney Int 2014; 86: 905–914 [DOI] [PubMed] [Google Scholar]
- 33. Larsen CP, Beggs ML, Saeed M. et al. Histopathologic findings associated with APOL1 risk variants in chronic kidney disease. Mod Pathol 2015; 28: 95–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kopp JB, Winkler CA, Zhao X. et al. Clinical features and histology of apolipoprotein L1-associated nephropathy in the FSGS clinical trial. J Am Soc Nephrol 2015; 26: 1443–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ng DK, Robertson CC, Woroniecki RP. et al. APOL1-associated glomerular disease among African-American children: a collaboration of the Chronic Kidney Disease in Children (CKiD) and Nephrotic Syndrome Study Network (NEPTUNE) cohorts. Nephrol Dial Transplant 2016 Apr 27. doi:10.1093/ndt/gfw061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barisoni L, Diomedi-Camassei F, Santorelli FM. et al. Collapsing glomerulopathy associated with inherited mitochondrial injury. Kidney Int 2008; 74: 237–243 [DOI] [PubMed] [Google Scholar]
- 37. Bouchier-Hayes L, Lartigue L, Newmeyer DD.. Mitochondria: pharmacological manipulation of cell death. J Clin Invest 2005; 115: 2640–2647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liapis H. Molecular pathology of nephrotic syndrome in childhood: a contemporary approach to diagnosis. Pediatr Dev Pathol 2008; 11: 154–163 [DOI] [PubMed] [Google Scholar]
- 39. Stokes MB, Davis CL, Alpers CE.. Collapsing glomerulopathy in renal allografts: a morphological pattern with diverse clinicopathologic associations. Am J Kidney Dis 1999; 33: 658–666 [DOI] [PubMed] [Google Scholar]
- 40. Shah PB, Cooper JE, Lucia MS. et al. APOL1 polymorphisms in a deceased donor and early presentation of collapsing glomerulopathy and focal segmental glomerulosclerosis in two recipients. Am J Transplant 2016; 16: 1923–1927 [DOI] [PubMed] [Google Scholar]
- 41. Freedman BI, Julian BA.. Should kidney donors be genotyped for APOL1 risk alleles? Kidney Int 2015; 87: 671–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buob D, Decambron M, Gnemmi V. et al. Collapsing glomerulopathy is common in the setting of thrombotic microangiopathy of the native kidney. Kidney Int 2016; 90: 1321–1331 [DOI] [PubMed] [Google Scholar]
- 43. Canaud G, Bruneval P, Noël LH. et al. Glomerular collapse associated with subtotal renal infarction in kidney transplant recipients with multiple renal arteries. Am J Kidney Dis 2010; 55: 558–565 [DOI] [PubMed] [Google Scholar]
- 44. Nadasdy T, Allen C, Zand MS.. Zonal distribution of glomerular collapse in renal allografts: possible role of vascular changes. Hum Pathol 2002; 33: 437–441 [DOI] [PubMed] [Google Scholar]
- 45. Kanodia KV, Vanikar AV, Patel RD. et al. Collapsing glomerulopathy: a single centre clinicopathologic study of seven years. J Clin Diagn Res 2016; 10: EC15–EC17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ferreira AC, Carvalho D, Carvalho F. et al. Collapsing glomerulopathy in Portugal: a review of the histological and clinical findings in HIV and non-HIV patients. Nephrol Dial Transplant 2011; 26: 2209–2215 [DOI] [PubMed] [Google Scholar]
- 47. Salvatore SP, Reddi AS, Chandran CB. et al. Collapsing glomerulopathy superimposed on diabetic nephropathy: insights into etiology of an under-recognized, severe pattern of glomerular injury. Nephrol Dial Transplant 2014; 29: 392–399 [DOI] [PubMed] [Google Scholar]