Abstract
This study aimed to assess the long-term outcomes of radiotherapy in patients with localized gastric mucosa–associated lymphoid tissue (MALT) lymphoma. Twenty-seven patients with Stage I gastric MALT lymphoma were treated with radiotherapy from 1999 to 2010. The median age was 65 years (range: 31–84). Fifteen patients were Helicobacter pylori–negative. Thirteen patients were treated with definitive radiotherapy alone. The other 14 patients who had refractory or residual disease following a prior treatment received salvage radiotherapy. The median dose of the radiotherapy was 30 Gy in 20 fractions (range: 30–39.5 Gy). The median follow-up period was 121 months (range: 8–176 months). The 5- and 10-year overall survival rates for all patients were 92% and 87%, respectively. No patients died from MALT lymphoma. Three patients died of other diseases at 8, 33 and 74 months after radiotherapy (myocardial infarction, pneumonia and hepatocellular carcinoma, respectively). No cases of local recurrence were observed during the follow-up period. There were no serious late gastric, liver or kidney complications during a median follow-up period of over 10 years. Two patients remain alive with distant metastases: a lung metastasis and an abdominal lymph node metastasis at 104 months and 21 months after radiotherapy, respectively. Excellent long-term local control was observed in patients with localized gastric MALT lymphoma after radiotherapy. However, lifelong follow-up should be conducted to detect cases of late recurrence, especially distant metastases.
Keywords: gastric lymphoma, MALT, radiotherapy, complication
INTRODUCTION
Extra-nodal marginal zone lymphomas of the mucosa-associated lymphoid tissue (MALT) are indolent, low-grade B-cell non-Hodgkin lymphomas, and most frequently affect the stomach. Gastric MALT lymphomas often result from Helicobacter pylori infection. The H. pylori positivity rate in the early stage of gastric MALT lymphoma is approximately 90% [1]. Eradication therapy has had excellent clinical results in patients with H. pylori–positive gastric MALT lymphoma and is accepted as the first-line treatment [2, 3].
For H. pylori–negative gastric MALT lymphomas or patients who do not respond to eradication therapy, radiotherapy has been accepted as a preferred organ-preserving local treatment modality. Several retrospective studies have reported that radiotherapy has excellent clinical outcomes and minimal toxicities in patients with gastric MALT lymphoma [4–6].
On the other hand, in the lifelong observation of patients with extra-nodal MALT lymphoma, relapse has been reported to occur in 37% of patients after a median period of 47 months (range: 14–307 months) [7]. Gastric MALT lymphomas are considered to have better clinical results than MALT lymphomas of other sites; however, late relapses have been observed more than 5 years after radiotherapy [8]. These results imply the necessity of long-term follow-up in MALT lymphoma patients.
In the present study, we performed a retrospective analysis to assess the long-term results, over a median follow-up period of 10 years, in patients with localized gastric MALT lymphoma who were treated with radiotherapy.
MATERIALS AND METHODS
Patient characteristics
From November 1999 to December 2010, 27 patients with gastric MALT lymphoma underwent radiotherapy treatment at our center. All the data collections were performed with approval from the institutional review board. All patients were histologically diagnosed with MALT lymphoma by endoscopic biopsy. Table 1 summarizes the patient characteristics. The median age was 65 years (range: 31–84). All patients were classified as clinical Stage I, according to the Lugano staging system [9]. Fifteen patients (56%) were H. pylori–negative. Eight patients (30%) were positive for a t(11:18)(q21;q21) chromosomal translocation. The proximal site of the stomach was most commonly involved (67%). Two patients (7%) were diagnosed with MALT lymphoma mixed with a high-grade transformation component.
Table 1.
Patient characteristics (n = 27)
| No. of patients (%) | |
|---|---|
| Gender | |
| Male | 11 (41) |
| Female | 16 (59) |
| Age, median (range) | 65 (31–84) |
| Clinical Stage I | 27 (100) |
| Helicobacter pylori infection | |
| Positive | 12 (44) |
| Negative | 15 (56) |
| t(11;18)(q21;q21) chromosomal translocation | |
| Positive | 8 (30) |
| Negative | 13 (48) |
| Unknown | 6 (22) |
| Dominant site of lesion | |
| Proximal | 18 (67) |
| Distal two-thirds | 7 (26) |
| Diffuse | 2 (7) |
| Dominant endoscopic type | |
| Superficial spreading | 17 (63) |
| Exophytic/Mass-forming | 4 (15) |
| Ulcerative/Diffuse infiltrating | 6 (22) |
| Histological grade | |
| Low | 25 (93) |
| Mixed with high-grade component | 2 (7) |
Treatments prior to radiotherapy
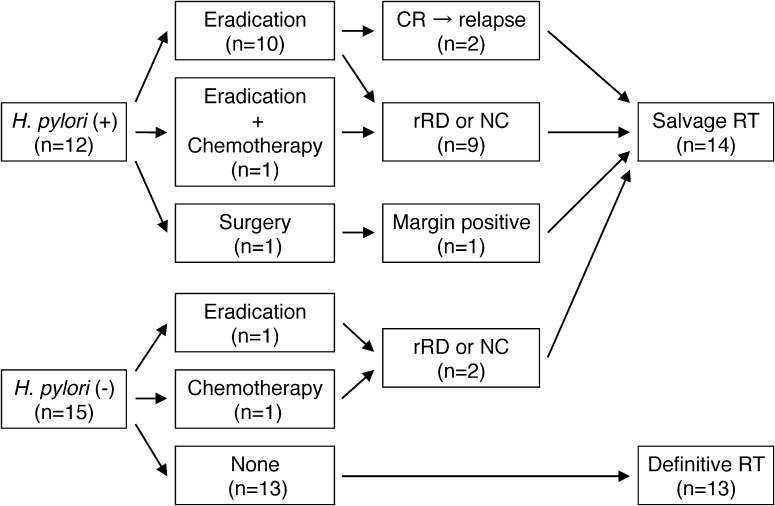
Figure 1 shows the initial treatments before radiotherapy. In 11 of the 12 patients with H. pylori infection, eradication therapy was administered using a proton-pump inhibitor plus two antibiotics. Among these patients, one patient received additional chemotherapy (cyclophosphamide) due to eradication resistance. Two patients who achieved a complete response (CR) with eradication therapy subsequently relapsed; another 9 patients showed persistent disease after eradication. One H. pylori–positive patient underwent surgery simultaneously due to the complication of a gastric adenocarcinoma. The postoperative pathological examination revealed an early-stage gastric adenocarcinoma; however, the surgical margin was positive for MALT lymphoma. Thus, adjuvant radiotherapy was administered.
Fig. 1.
The initial treatments before radiotherapy. H. pylori = Helicobacter pylori, CR = complete histological remission, rRD = responding residual disease, NC = No change, RT = radiotherapy.
Among the 15 H. pylori–negative patients, 13 received definitive radiotherapy alone. Another two patients underwent either eradication or chemotherapy (R-CHOP: rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone) prior to radiotherapy, and received salvage radiotherapy for their residual disease. R-CHOP was administered to one patient due to pathological findings, which included a high-grade lymphoma transformation component.
Among the 12 of 27 patients who underwent eradication therapy, the median interval from eradication therapy to the start of radiotherapy was 3.5 months (range: 2 to 39 months).
Methods of radiotherapy
All patients were treated in the supine position with an empty stomach. Before October 2003, 11 patients underwent an X-ray simulation using fluoroscopy for 2D planning. After November 2003, 16 patients underwent a CT scan for 3D conformal radiotherapy (3D-CRT). The clinical target volume (CTV) was defined as the entire stomach, including the perigastric lymph nodes. The planning target volume (PTV) included the CTV and additional margins to account for internal organ motion (which was estimated from the stomach movement on fluoroscopy after the ingestion of a barium suspension) and set-up error. Twenty of 27 patients (74%) were treated with a two-field treatment technique consisting of opposed anterior and posterior fields; in the other 7 patients, this was combined with the lateral opposed fields or four-fields technique to reduce the dose to the kidneys. In 2D planning, the shape of the kidney was evaluated on an intravenous pyelogram (IVP). The median dose of radiotherapy was 30 Gy in 20 fractions (range: 30–39.5 Gy). Although we used 30 Gy in principle, two patients with bulky tumors were treated with 6 Gy boost irradiation after 30 Gy of irradiation. Only one patient underwent 39.5 Gy irradiation (because of a bulky tumor and because radiotherapy was performed during the New Year holidays). Basically, the dose constraints were as follows: the mean dose to either kidney was kept to <18 Gy, and the mean liver dose was kept to <20 Gy.
Follow-up and statistical analysis
An endoscopic biopsy was used to histologically assess the treatment response in all patients. Overall survival (OS) was measured from the initiation of radiotherapy to death regardless of cause. Relapse-free survival (RFS) was defined as the time from the start of radiotherapy to relapse (including a local recurrence or a distant metastasis) or death from any cause. The OS and RFS rates were calculated using the Kaplan–Meier method.
Cases of acute toxicity were graded according to the Common Terminology Criteria for Adverse Events (CTCAE version 4.0). The Radiation Therapy Oncology / European Organization for Research and Treatment of Cancer (RTOG/EORTC) late radiation morbidity scoring scheme was used to evaluate late complications.
RESULTS
Treatment outcome
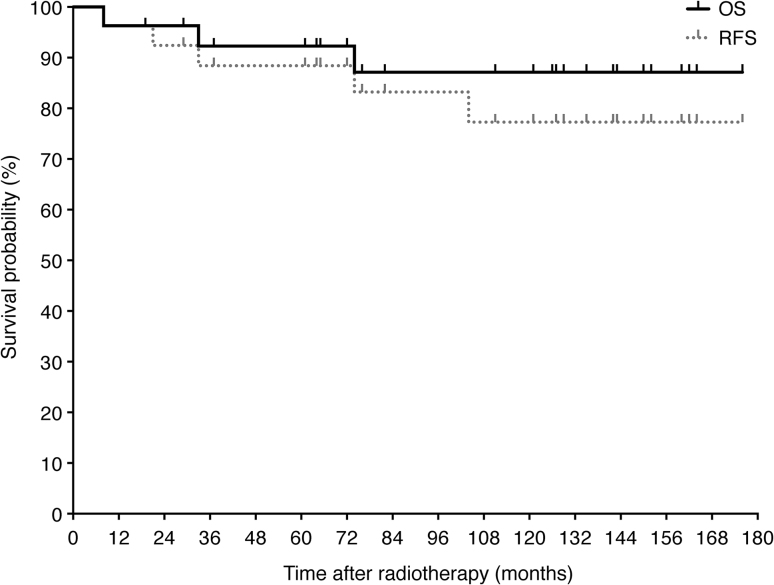
The median follow-up period was 121 months (range: 8–176 months). All patients achieved a CR at the post-treatment endoscopic biopsy. The 5- and 10-year OS rates were 92% and 87%, respectively. The 5- and 10-year RFS rates were 88% and 77%, respectively (Fig. 2). During the follow-up period, no cases of local recurrence were observed. Three patients died of intercurrent diseases without any evidence of relapse of the MALT lymphoma: one of myocardial infarction at 8 months after the start of radiotherapy, one of pneumonia at 33 months, and one of hepatocellular carcinoma from hepatitis C at 74 months.
Fig. 2.
Overall survival (OS) and relapse free survival (RFS) for all patients after radiotherapy as determined by the Kaplan–Meier method.
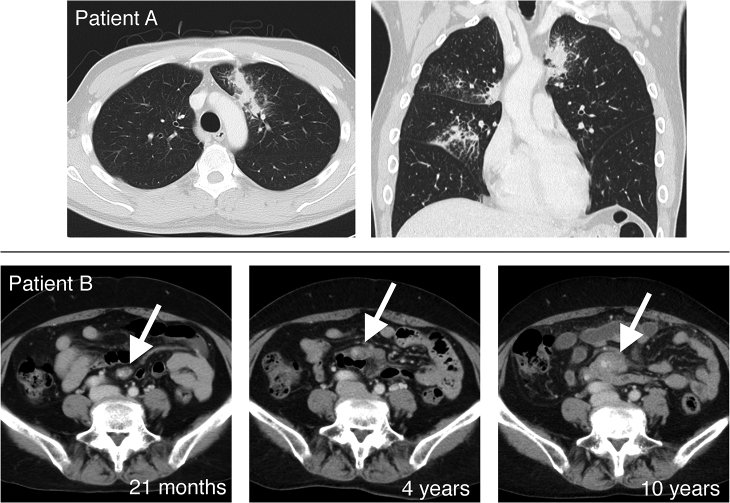
Two of the 27 patients suffered from a distant metastasis without a local recurrence (Fig. 3). Table 2 shows the details of the relapsed cases. One had a pulmonary metastasis with a mild cough at 104 months after radiotherapy. A pathological examination revealed that the metastasis was of the same type as the initial MALT lymphoma without any transformation to high-grade lymphoma. Another patient had a mesenteric lymph node metastasis 21 months after radiotherapy, which was diagnosed from changes in CT images without a pathological examination. This relapse tumor slowly progressed without symptoms over a period of 10 years. The patients with distant metastasis have been followed up without any additional treatment.
Fig. 3.
The computed tomography images of two patients who suffered from distant metastases. Upper column (Patient A): a late lung metastasis, which occurred 104 months after radiotherapy. Lower column (Patient B): a slowly progressive mesenteric lymph node metastasis.
Table 2.
The details of the relapsed cases
| #1 | #2 | |
|---|---|---|
| Gender | Male | Female |
| Age | 31 | 56 |
| Helicobacter pylori infection | Positive | Negative |
| t(11;18)(q21;q21) chromosomal translocation | Positive | Unknown |
| Dominant site of lesion | Proximal | Proximal |
| Dominant endoscopic type | Superficial | Superficial |
| Histological grade | Low | Low |
| Recurrence site | Lung | Mesenteric lymph node |
| Time to recurrence (after radiotherapy) | 104 months | 21 months |
Radiation-related complications and dose–volume histogram analysis
The acute non-hematological toxicities of the patients were categorized as Grade 1 (n = 15) and Grade 2 (n = 2), with complaints of anorexia, nausea or vomiting. White blood cell count decreases corresponding to Grade 2 and 3 were observed in 8 and 3 patients, respectively. In all patients, the full course of radiotherapy was completed without interruptions due to treatment-related acute toxicities.
A dose–volume histogram analysis was available for 15 of the 27 patients (56%). The percentages of liver volumes that received ≥10 Gy, ≥15 Gy and ≥20 Gy (V10, V15 and V20, respectively) were 45.9% (range: 13.7–75.1%), 33.4% (range: 10.3–67.3%) and 22.7% (range: 7.5–57.4%), respectively. The mean dose to the liver was 11.3 Gy (range: 3.8–20.0 Gy). The mean dose to the right kidney, left kidney and both kidneys were as follows: 7.4 Gy (range: 1.1–16.6 Gy), 13.1 Gy (range: 0.9–23.7 Gy) and 10.0 Gy (range: 1.2–16.5 Gy), respectively.
After the completion of radiotherapy, transient liver dysfunction was observed in 10 patients (37%); the median duration between the completion of radiotherapy and the onset of transient liver dysfunction was 1 month (range: 1–4 months). No cases of transaminase re-elevation or any other types of liver-dysfunction were observed within the follow-up period. Although two patients were diagnosed with hepatitis C prior to gastric MALT lymphoma treatment, no exacerbation of liver dysfunction was observed in these patients.
There were no serious (Grade ≥3) late gastric, liver or kidney complications during a median follow-up period of over 10 years. No patients suffered from gastrointestinal bleeding or perforation. Late renal complications of Grade 1 (n = 2, 7%) and 2 (n = 1, 4%) were observed; 2D treatment planning had been performed for both of these patients.
One patient had an early gastric adenocarcinoma 4 years after radiotherapy for gastric MALT lymphoma, and underwent endoscopic submucosal dissection. Another patient had cancer of the left breast 12 years after radiotherapy, which was thought to have arisen from outside the field of radiotherapy.
DISCUSSION
In current guidelines and reports, the first-line approach in gastric MALT lymphoma, regardless of clinical stage, is H. pylori eradication therapy [2, 3]. The complete remission rate with the eradication of H. pylori is reported to be approximately 73–94.4% [1, 10–12]. Furthermore, in the H. pylori–negative patients the eradiation therapy achieved a 29–46% response rate (complete and partial remission). The current guidelines recommend that H. pylori–negative patients with gastric MALT lymphoma also undergo anti-H. pylori treatment as a first-line treatment. [2, 3, 13–15]. However, some factors have been reported to be associated with eradication-resistance, including: an endoscopic finding of non-superficial type (mass-forming or diffuse infiltrating type), the presence of t(11;18)(q21;q21) chromosomal translocation, location in proximal or multiple areas (non-antral involvement), the absence of H. pylori infection, and a diffuse large B-cell lymphoma (DLBCL) component [1, 16–19]. Radiotherapy is a promising therapy for gastric MALT lymphomas that are residual or recurrent after eradication therapy. Several reports have mentioned that the 5-year cause-specific survival rates in patients with gastric MALT lymphoma who receive radiotherapy are 80–100% [4–6, 8, 20–22]. In our study, good long-term local control was achieved over a median follow-up period of 10 years. These results are in line with the results of other reports and indicate that radiotherapy may be a promising second-line treatment for eradication-resistant localized gastric MALT lymphoma.
Adverse events seem to be rare in radiotherapy for gastric MALT lymphoma [4–6]. In our study, severe renal toxicities were also not observed during a median follow-up period of over 10 years. The current radiation technique for the stomach includes 3D-CRT plans, consisting of three to four fields, and intensity-modulated radiation therapy (IMRT) plans, in order to reduce the dose to the left kidney. However, these techniques involve an increase in the liver dose in comparison with the traditional two-field treatment technique using parallel-opposed anterior and posterior fields [23]. Tanaka et al. reported that only transient or mild hepatic dysfunction occurred in gastric lymphoma patients who underwent low to moderate dose irradiation to the liver. They named this phenomenon ‘radiation-induced hepatic dysfunction (RIHD)’. RIHD is diagnosed in ~70% of gastric lymphoma patients after radiotherapy [24]. In our study, RIHD was observed in 37% of patients; all cases were transient and showed spontaneous improvement. Further study is necessary in order to determine the clinical importance of RIHD and dose restrictions for the liver. However, it seems to be important to pay careful attention to reduce the dose to the healthy liver and kidneys.
There is not yet an established consensus regarding the optimal second-line treatment for patients with gastric MALT lymphoma who do not respond to eradication treatment or who suffer from local recurrence [2, 3, 25]. Surgical resection has gradually declined because of the high incidence of post-operative complications and due to the absence of evidence to show an advantage of surgery in comparison with organ-preserving treatments [26, 27]. Radiotherapy alone and chemotherapy with/without a combination of anti-CD20 monoclonal antibodies have both achieved promising results as second-line treatments. Although immunotherapy using an anti-CD20 monoclonal antibody (rituximab) only achieved a 46–59.3% CR rate, the combination of rituximab plus chlorambucil therapy achieved a 78% CR rate [21, 28, 29]. The CR rate of chemotherapy was approximately 85–88%, which was lower than the remission rate of radiotherapy [1, 21]. On the other hand, low-grade MALT lymphoma with the DLBCL component has been reported to have a tendency to progress to distant metastasis after radiotherapy [30]. Furthermore, the presence of either large B-cell components or an exophytic growth pattern have been reported to be negative predictive factors of treatment failure after radiotherapy [4]. It is, therefore, suggested that patients with the DLBCL component should be treated by CHOP-based systemic chemotherapy rather than radiotherapy alone [31]. Two of the patients in our study had a high-grade lymphoma transformation component; one patient underwent R-CHOP chemotherapy as a first-line treatment before the radiotherapy. There was no local recurrence or evidence of metastasis at 74 or 135 months after the radiotherapy. On the other hand, one patient with a t(11;18)(q21;q21) chromosomal translocation has developed lung metastasis at 104 months after radiotherapy. These results suggest the necessity of long-term follow-up in MALT lymphoma patients after radiotherapy, and that further investigation is required to elucidate the risk factors that are related to the distant relapses (high-grade component and chromosomal translocation, etc.).
Some recent studies have proposed a ‘watch-and-wait’ strategy for patients with minimal residual gastric MALT after eradication [2, 14, 25, 32]. The time to the best response after eradication therapy was 3–9 months in the majority of responders, However, some patients took 2–3 years [14, 32]. The European Society of Medical Oncology (ESMO) has proposed that physicians wait for at least 12 months before starting other treatments in patients who achieve a clinical and endoscopic remission together with the eradication of H. pylori, but who still have persistent (residual) lymphoma at the histological level [3]. The European Gastro-Intestinal Lymphoma Study (EGILS) group has also recommended that patients with persistent histological lymphoma can be managed for up to 24 months by a watch-and-wait strategy unless progression or recurrent endoscopic lesions can be demonstrated [2]. They also mentioned that in relapse cases, even though a watch-and-wait strategy can be adopted if no signs of endoscopic or clinical progression are evident, in a few cases, local histological recurrence was reported to disappear spontaneously on the subsequent histological examination without any treatment [1, 10].
The present study was associated with a limitation in that the interval between eradication and radiotherapy was relatively short (median: 3.5 months). Additional investigations, which take into account the abovementioned factors that predict resistance to eradication therapy, might be required to determine the optimal timing for the initiation of radiotherapy for patients with eradication-resistant gastric MALT lymphoma.
In conclusion, radiotherapy for localized gastric MALT lymphoma achieved excellent local control without serious toxicities over a 10-year follow-up period. However, careful long-term follow-up should be performed after radiotherapy to identify late relapses, especially distant metastases.
FUNDING
Funding to pay the Open Access publication charges for this article was provided by Yu Ohkubo.
CONFLICT OF INTEREST
The authors state that there are no actual or potential conflicts of interest.
ACKNOWLEDGEMENTS
Parts of this study were presented at the International Lymphoma Radiation Oncology Group's (ILROG's) Meeting, ‘Modern Radiation For Lymphoma: Updated Role and New Rules,’ at Memorial Sloan Kettering Cancer Center (MSKCC), New York City, NY, 8–9 May 2015.
REFERENCES
- 1. Nakamura S, Sugiyama T, Matsumoto T, et al. Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: a multicentre cohort follow-up study of 420 patients in Japan. Gut 2012;61:507–13. [DOI] [PubMed] [Google Scholar]
- 2. Ruskoné-Fourmestraux A, Fischbach W, Aleman BM, et al. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut 2011;60:747–58. [DOI] [PubMed] [Google Scholar]
- 3. Zucca E, Copie-Bergman C, Ricardi U, et al. Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24Suppl 6:vi144–8. [DOI] [PubMed] [Google Scholar]
- 4. Wirth A, Gospodarowicz M, Aleman BM, et al. Long-term outcome for gastric marginal zone lymphoma treated with radiotherapy: a retrospective, multi-centre, International Extranodal Lymphoma Study Group study. Ann Oncol 2013;24:1344–51. [DOI] [PubMed] [Google Scholar]
- 5. Kim SW, Lim do H, Ahn YC, et al. Clinical outcomes of radiation therapy for early-stage gastric mucosa-associated lymphoid tissue lymphoma. World J Gastroenterol 2013;19:6062–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nam TK, Ahn JS, Choi YD, et al. The role of radiotherapy in the treatment of gastric mucosa-associated lymphoid tissue lymphoma. Cancer Res Treat 2014;46:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raderer M, Streubel B, Woehrer S, et al. High relapse rate in patients with MALT lymphoma warrants lifelong follow-up. Clin Cancer Res 2005;11:3349–52. [DOI] [PubMed] [Google Scholar]
- 8. Abe S, Oda I, Inaba K, et al. A retrospective study of 5-year outcomes of radiotherapy for gastric mucosa-associated lymphoid tissue lymphoma refractory to Helicobacter pylori eradication therapy. Jpn J Clin Oncol 2013;43:917–22. [DOI] [PubMed] [Google Scholar]
- 9. Rohatiner A, d'Amore F, Coiffier B, et al. Report on a workshop convened to discuss the pathological and staging classifications of gastrointestinal tract lymphoma. Ann Oncol 1994;5:397–400. [DOI] [PubMed] [Google Scholar]
- 10. Hong SS, Jung HY, Choi KD, et al. A prospective analysis of low-grade gastric malt lymphoma after Helicobacter pylori eradication. Helicobacter 2006;11:569–73. [DOI] [PubMed] [Google Scholar]
- 11. Zullo A, Hassan C, Cristofari F, et al. Effects of Helicobacter pylori eradication on early stage gastric mucosa-associated lymphoid tissue lymphoma. Clin Gastroenterol Hepatol 2010;8:105–10. [DOI] [PubMed] [Google Scholar]
- 12. Ono S, Kato M, Takagi K, et al. Long-term treatment of localized gastric marginal zone B-cell mucosa associated lymphoid tissue lymphoma including incidence of metachronous gastric cancer. J Gastroenterol Hepatol 2010;25:804–9. [DOI] [PubMed] [Google Scholar]
- 13. Nakamura S, Matsumoto T, Ye H, et al. Helicobacter pylori–negative gastric mucosa-associated lymphoid tissue lymphoma: a clinicopathologic and molecular study with reference to antibiotic treatment. Cancer 2006;107:2770–8. [DOI] [PubMed] [Google Scholar]
- 14. Raderer M, Wöhrer S, Kiesewetter B, et al. Antibiotic treatment as sole management of Helicobacter pylori–negative gastric MALT lymphoma: a single center experience with prolonged follow-up. Ann Hematol 2015;94:969–73. [DOI] [PubMed] [Google Scholar]
- 15. Asano N, Iijima K, Koike T, et al. Helicobacter pylori–negative gastric mucosa-associated lymphoid tissue lymphomas: a review. World J Gastroenterol 2015;21:8014–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steinbach G, Ford R, Glober G, et al. Antibiotic treatment of gastric lymphoma of mucosa-associated lymphoid tissue. An uncontrolled trial. Ann Intern Med 1999;131:88–95. [DOI] [PubMed] [Google Scholar]
- 17. Ruskoné-Fourmestraux A, Lavergne A, Aegerter PH, et al. Predictive factors for regression of gastric MALT lymphoma after anti-Helicobacter pylori treatment. Gut 2001;48:297–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Akamatsu T, Mochizuki T, Okiyama Y, et al. Comparison of localized gastric mucosa-associated lymphoid tissue (MALT) lymphoma with and without Helicobacter pylori infection. Helicobacter 2006;11:86–95. [DOI] [PubMed] [Google Scholar]
- 19. Kim JS, Chung SJ, Choi YS, et al. Helicobacter pylori eradication for low-grade gastric mucosa-associated lymphoid tissue lymphoma is more successful in inducing remission in distal compared to proximal disease. Br J Cancer 2007;96:1324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vrieling C, de Jong D, Boot H, et al. Long-term results of stomach-conserving therapy in gastric MALT lymphoma. Radiother Oncol 2008;87:405–11. [DOI] [PubMed] [Google Scholar]
- 21. Zullo A, Hassan C, Andriani A, et al. Treatment of low-grade gastric MALT-lymphoma unresponsive to Helicobacter pylori therapy: a pooled-data analysis. Med Oncol 2010;27:291–5. [DOI] [PubMed] [Google Scholar]
- 22. Ruskoné-Fourmestraux A, Matysiak-Budnik T, Fabiani B, et al. Exclusive moderate-dose radiotherapy in gastric marginal zone B-cell MALT lymphoma: results of a prospective study with a long term follow-up. Radiother Oncol 2015;117:178–82. [DOI] [PubMed] [Google Scholar]
- 23. Della Biancia C, Hunt M, Furhang E, et al. Radiation treatment planning techniques for lymphoma of the stomach. Int J Radiat Oncol Biol Phys 2005;62:745–51. [DOI] [PubMed] [Google Scholar]
- 24. Tanaka H, Hayashi S, Ohtakara K, et al. Hepatic dysfunction after radiotherapy for primary gastric lymphoma. J Radiat Res 2013;54:92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakamura S, Matsumoto T.. Helicobacter pylori and gastric mucosa-associated lymphoid tissue lymphoma: recent progress in pathogenesis and management. World J Gastroenterol 2013;19:8181–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yoon SS, Coit DG, Portlock CS, et al. The diminishing role of surgery in the treatment of gastric lymphoma. Ann Surg 2004;240:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Koch P, Probst A, Berdel WE, et al. Treatment results in localized primary gastric lymphoma: data of patients registered within the German multicenter study (GIT NHL 02/96). J Clin Oncol 2005;23:7050–9. [DOI] [PubMed] [Google Scholar]
- 28. Martinelli G, Laszlo D, Ferreri AJ, et al. Clinical activity of rituximab in gastric marginal zone non-Hodgkin's lymphoma resistant to or not eligible for anti-Helicobacter pylori therapy. J Clin Oncol 2005;23:1979–83. [DOI] [PubMed] [Google Scholar]
- 29. Zucca E, Conconi A, Laszlo D, et al. Addition of rituximab to chlorambucil produces superior event-free survival in the treatment of patients with extranodal marginal-zone B-cell lymphoma: 5-year analysis of the IELSG-19 Randomized Study. J Clin Oncol 2013;31:565–72. [DOI] [PubMed] [Google Scholar]
- 30. Taal BG, Boot H, van Heerde P, et al. Primary non-Hodgkin lymphoma of the stomach: endoscopic pattern and prognosis in low versus high grade malignancy in relation to the MALT concept. Gut 1996;39:556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nakamura S, Matsumoto T, Suekane H, et al. Long-term clinical outcome of Helicobacter pylori eradication for gastric mucosa-associated lymphoid tissue lymphoma with a reference to second-line treatment. Cancer 2005;104:532–40. [DOI] [PubMed] [Google Scholar]
- 32. Terai S, Iijima K, Kato K, et al. Long-term outcomes of gastric mucosa-associated lymphoid tissue lymphomas after Helicobacter pylori eradication therapy. Tohoku J Exp Med 2008;214:79–87. [DOI] [PubMed] [Google Scholar]