Abstract
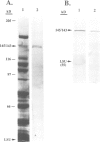
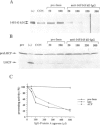
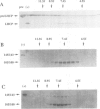
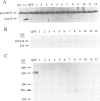
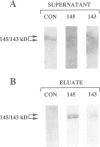
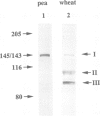
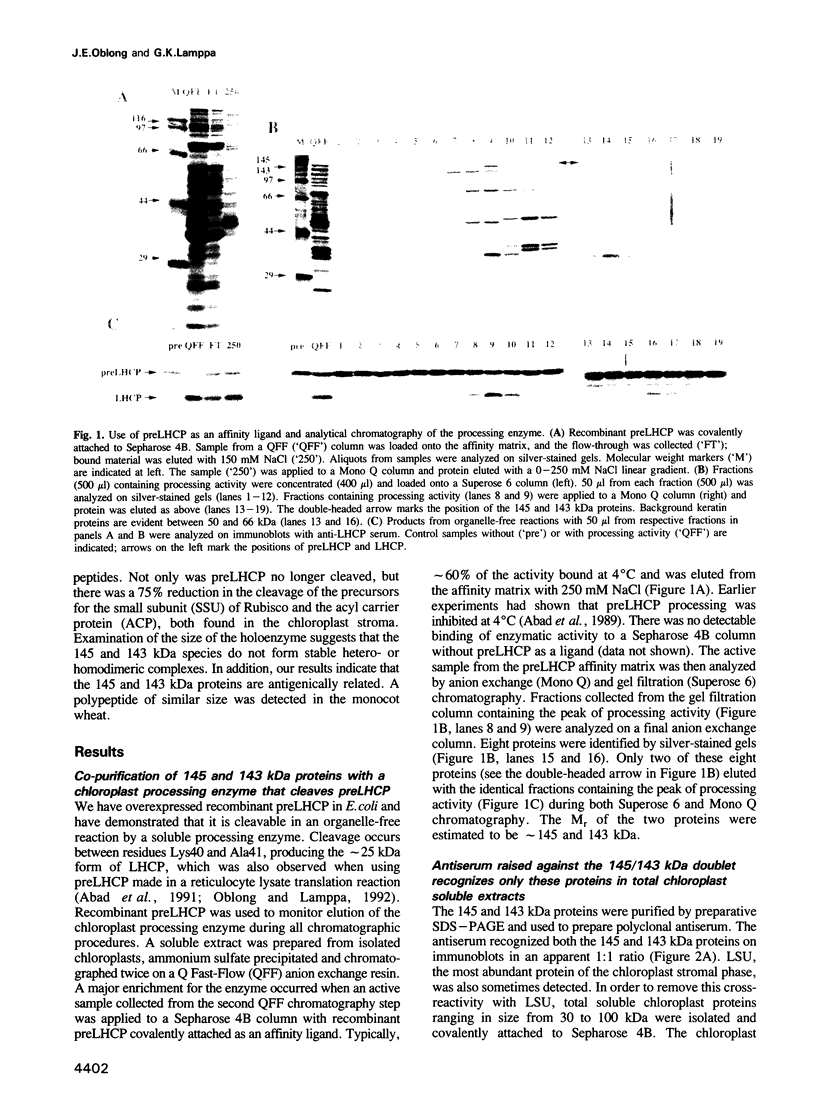
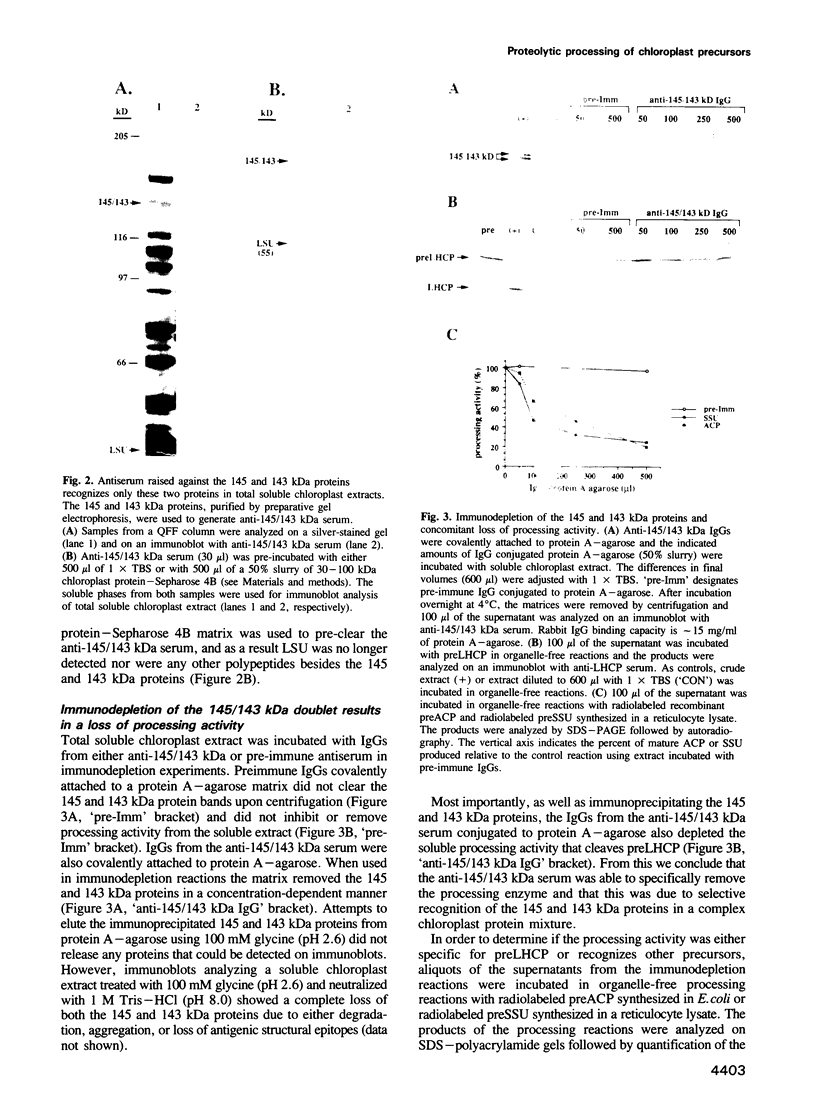
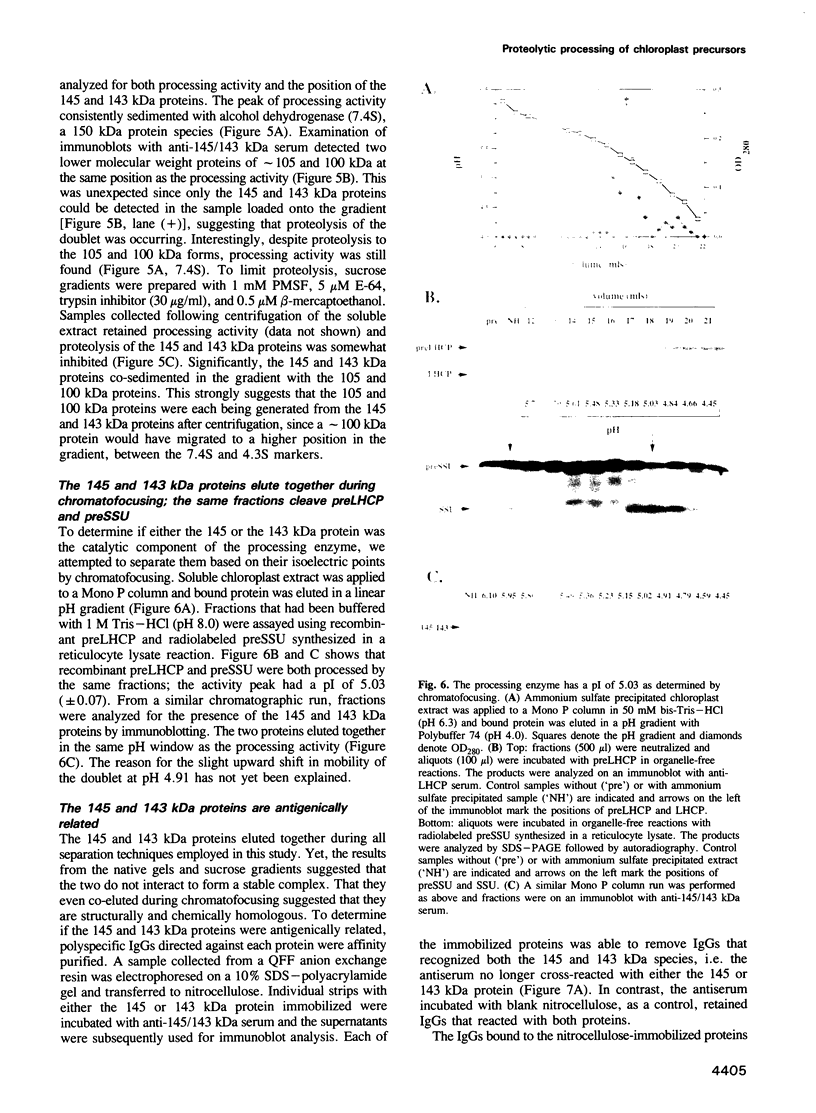
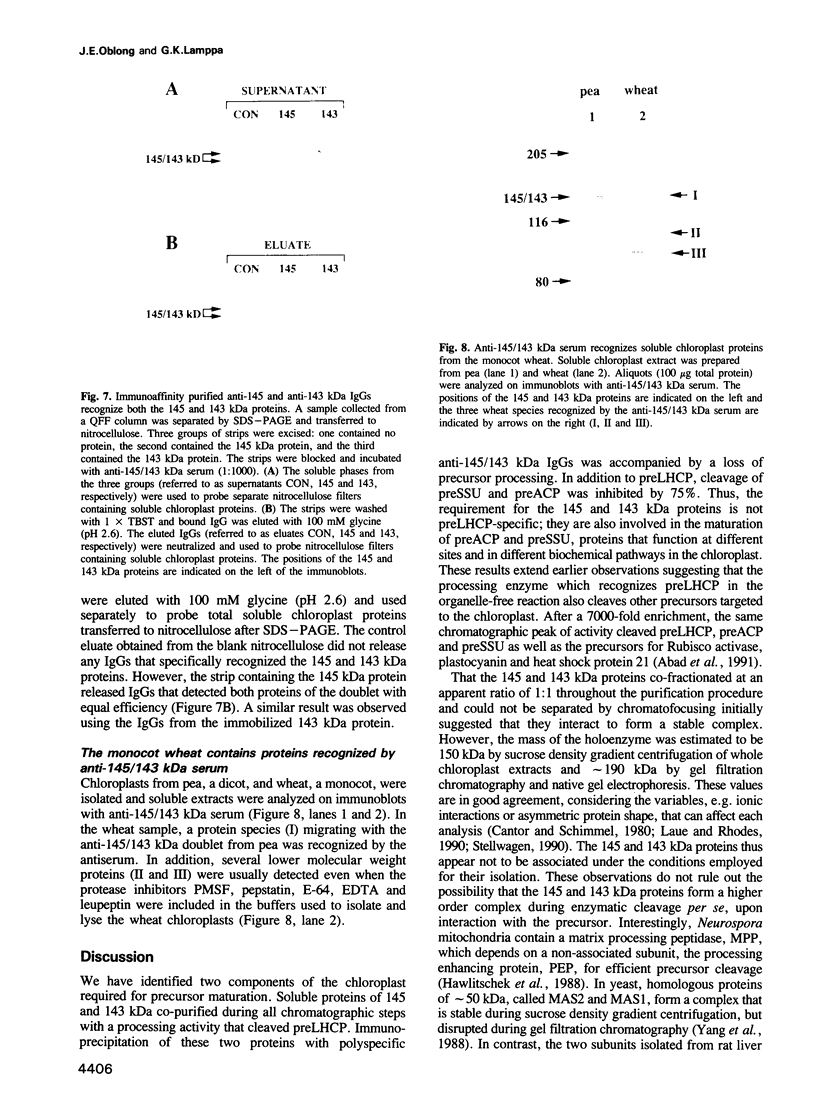
Two proteins of 145 and 143 kDa were identified in pea which co-purify with a chloroplast processing activity that cleaves the precursor for the major light-harvesting chlorophyll binding protein (preLHCP). Antiserum generated against the 145/143 kDa doublet recognizes only these two polypeptides in a chloroplast soluble extract. In immunodepletion experiments the antiserum removed the doublet, and there was a concomitant loss of cleavage of preLHCP as well as of precursors for the small subunit of Rubisco and the acyl carrier protein. The 145 and 143 kDa proteins co-eluted in parallel with the peak of processing activity during all fractionation procedures, but they were not detectable as a homo- or heterodimeric complex. The 145 and 143 kDa proteins were used separately to affinity purify immunoglobulins; each preparation recognized both polypeptides, indicating that they are antigenically related. Wheat chloroplasts contain a soluble species similar in size to the 145/143 kDa doublet.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abad M. S., Clark S. E., Lamppa G. K. Properties of a Chloroplast Enzyme that Cleaves the Chlorophyll a/b Binding Protein Precursor : Optimization of an Organelle-Free Reaction. Plant Physiol. 1989 May;90(1):117–124. doi: 10.1104/pp.90.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abad M. S., Oblong J. E., Lamppa G. K. Soluble Chloroplast Enzyme Cleaves preLHCP Made in Escherichia coli to a Mature Form Lacking a Basic N-Terminal Domain. Plant Physiol. 1991 Aug;96(4):1220–1227. doi: 10.1104/pp.96.4.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitnis P. R., Harel E., Kohorn B. D., Tobin E. M., Thornber J. P. Assembly of the precursor and processed light-harvesting chlorophyll a/b protein of Lemna into the light-harvesting complex II of barley etiochloroplasts. J Cell Biol. 1986 Mar;102(3):982–988. doi: 10.1083/jcb.102.3.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. E., Abad M. S., Lamppa G. K. Mutations at the transit peptide-mature protein junction separate two cleavage events during chloroplast import of the chlorophyll a/b-binding protein. J Biol Chem. 1989 Oct 15;264(29):17544–17550. [PubMed] [Google Scholar]
- Clark S. E., Lamppa G. K. Determinants for cleavage of the chlorophyll a/b binding protein precursor: a requirement for a basic residue that is not universal for chloroplast imported proteins. J Cell Biol. 1991 Aug;114(4):681–688. doi: 10.1083/jcb.114.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavel Y., von Heijne G. A conserved cleavage-site motif in chloroplast transit peptides. FEBS Lett. 1990 Feb 26;261(2):455–458. doi: 10.1016/0014-5793(90)80614-o. [DOI] [PubMed] [Google Scholar]
- Geli V., Yang M. J., Suda K., Lustig A., Schatz G. The MAS-encoded processing protease of yeast mitochondria. Overproduction and characterization of its two nonidentical subunits. J Biol Chem. 1990 Nov 5;265(31):19216–19222. [PubMed] [Google Scholar]
- Hawlitschek G., Schneider H., Schmidt B., Tropschug M., Hartl F. U., Neupert W. Mitochondrial protein import: identification of processing peptidase and of PEP, a processing enhancing protein. Cell. 1988 Jun 3;53(5):795–806. doi: 10.1016/0092-8674(88)90096-7. [DOI] [PubMed] [Google Scholar]
- Lamppa G. K., Abad M. S. Processing of a wheat light-harvesting chlorophyll a/b protein precursor by a soluble enzyme from higher plant chloroplasts. J Cell Biol. 1987 Dec;105(6 Pt 1):2641–2648. doi: 10.1083/jcb.105.6.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamppa G. K., Morelli G., Chua N. H. Structure and developmental regulation of a wheat gene encoding the major chlorophyll a/b-binding polypeptide. Mol Cell Biol. 1985 Jun;5(6):1370–1378. doi: 10.1128/mcb.5.6.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laue T. M., Rhodes D. G. Determination of size, molecular weight, and presence of subunits. Methods Enzymol. 1990;182:566–587. doi: 10.1016/0076-6879(90)82045-4. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Oblong J. E., Lamppa G. K. Precursor for the light-harvesting chlorophyll a/b-binding protein synthesized in Escherichia coli blocks import of the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase. J Biol Chem. 1992 Jul 15;267(20):14328–14334. [PubMed] [Google Scholar]
- Olmsted J. B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981 Dec 10;256(23):11955–11957. [PubMed] [Google Scholar]
- Ou W. J., Ito A., Okazaki H., Omura T. Purification and characterization of a processing protease from rat liver mitochondria. EMBO J. 1989 Sep;8(9):2605–2612. doi: 10.1002/j.1460-2075.1989.tb08400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain D., Kanwar Y. S., Blobel G. Identification of a receptor for protein import into chloroplasts and its localization to envelope contact zones. Nature. 1988 Jan 21;331(6153):232–237. doi: 10.1038/331232a0. [DOI] [PubMed] [Google Scholar]
- Perry S. E., Buvinger W. E., Bennett J., Keegstra K. Synthetic analogues of a transit peptide inhibit binding or translocation of chloroplastic precursor proteins. J Biol Chem. 1991 Jun 25;266(18):11882–11889. [PubMed] [Google Scholar]
- Pollock R. A., Hartl F. U., Cheng M. Y., Ostermann J., Horwich A., Neupert W. The processing peptidase of yeast mitochondria: the two co-operating components MPP and PEP are structurally related. EMBO J. 1988 Nov;7(11):3493–3500. doi: 10.1002/j.1460-2075.1988.tb03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C., Ellis R. J. Transport of proteins into chloroplasts. Partial purification of a chloroplast protease involved in the processing of important precursor polypeptides. Eur J Biochem. 1984 Jul 16;142(2):337–342. doi: 10.1111/j.1432-1033.1984.tb08291.x. [DOI] [PubMed] [Google Scholar]
- Schnell D. J., Blobel G., Pain D. Signal peptide analogs derived from two chloroplast precursors interact with the signal recognition system of the chloroplast envelope. J Biol Chem. 1991 Feb 15;266(5):3335–3342. [PubMed] [Google Scholar]
- Stellwagen E. Gel filtration. Methods Enzymol. 1990;182:317–328. doi: 10.1016/0076-6879(90)82027-y. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasmann C. C., Reiss B., Bohnert H. J. Complete processing of a small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase from pea requires the amino acid sequence Ile-Thr-Ser. J Biol Chem. 1988 Jan 15;263(2):617–619. [PubMed] [Google Scholar]
- Westwood J. T., Clos J., Wu C. Stress-induced oligomerization and chromosomal relocalization of heat-shock factor. Nature. 1991 Oct 31;353(6347):822–827. doi: 10.1038/353822a0. [DOI] [PubMed] [Google Scholar]
- Witte C., Jensen R. E., Yaffe M. P., Schatz G. MAS1, a gene essential for yeast mitochondrial assembly, encodes a subunit of the mitochondrial processing protease. EMBO J. 1988 May;7(5):1439–1447. doi: 10.1002/j.1460-2075.1988.tb02961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe M. P., Schatz G. Two nuclear mutations that block mitochondrial protein import in yeast. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4819–4823. doi: 10.1073/pnas.81.15.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Jensen R. E., Yaffe M. P., Oppliger W., Schatz G. Import of proteins into yeast mitochondria: the purified matrix processing protease contains two subunits which are encoded by the nuclear MAS1 and MAS2 genes. EMBO J. 1988 Dec 1;7(12):3857–3862. doi: 10.1002/j.1460-2075.1988.tb03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G., Steppuhn J., Herrmann R. G. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989 Apr 1;180(3):535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]