Abstract
Background.
Toca 511 (vocimagene amiretrorepvec) is a retroviral replicating vector encoding an optimized yeast cytosine deaminase (CD). Tumor-selective expression of CD converts the prodrug, 5-fluorocytosine (5-FC), into the active chemotherapeutic, 5-fluorouracil (5-FU). This therapeutic approach is being tested in a randomized phase II/III trial in recurrent glioblastoma and anaplastic astrocytoma (NCT0241416). The aim of this study was to identify the immune cell subsets contributing to antitumor immune responses following treatment with 5-FC in Toca 511–expressing gliomas in a syngeneic mouse model.
Methods.
Flow cytometry was utilized to monitor and characterize the immune cell infiltrate in subcutaneous Tu-2449 gliomas in B6C3F1 mice treated with Toca 511 and 5-FC.
Results.
Tumor-bearing animals treated with Toca 511 and 5-FC display alterations in immune cell populations within the tumor that result in antitumor immune protection. Attenuated immune subsets were exclusive to immunosuppressive cells of myeloid origin. Depletion of immunosuppressive cells temporally preceded a second event which included expansion of T cells which were polarized away from Th2 and Th17 in the CD4+ T cell compartment with concomitant expansion of interferon gamma–expressing CD8+ T cells. Immune alterations correlated with clearance of Tu-2449 subcutaneous tumors and T cell–dependent protection from future tumor challenge.
Conclusions.
Treatment with Toca 511 and 5-FC has a concentrated effect at the site of the tumor which causes direct tumor cell death and alterations in immune cell infiltrate, resulting in a tumor microenvironment that is more permissive to establishment of a T cell mediated antitumor immune response.
Keywords: 5-fluorouracil, cytosine deaminase, immunotherapy, myeloid-derived suppressor cell, retroviral gene transfer
Importance of the study
Reported here for the first time, we utilize the Tu-2449 syngeneic mouse model to show that treatment of Toca 511–expressing tumors with 5-FC alters the immune profile in the tumor microenvironment, which results in long-term immune protection. While it was historically believed that this methodology only resulted in direct tumor cytotoxicity, it is now appreciated that this approach also utilizes an immunotherapeutic mechanism. As such, we are positioned to determine whether alterations in immune profiles such as those reported here are correlative with outcomes in the clinical setting, which is why the findings from this work are being directly applied to the immune monitoring efforts for our randomized phase II/III trial in recurrent glioblastoma and anaplastic astrocytoma (NCT0241416).
Toca 511 selectively replicates and spreads in malignant cells.1 Once tumor cells are infected, cytosine deaminase (CD) enzyme is expressed, which converts the prodrug, 5-fluorocytosine (5-FC) into the pyrimidine analog, 5-fluorouracil (5-FU), leading to direct cytotoxicity of the cancer cells.2–4 We have shown that this treatment modality has broad applicability in multiple tumor types.5,6 Additionally, we and our colleagues (Hiraoka et al, accompanying article) show that this therapeutic approach also exerts an extended immunotherapeutic effect. These data are further supported by an abundance of evidence that cytotoxic anticancer drugs, including 5-FU, can promote antitumor immune responses.7–12 This can take place through a multitude of mechanisms, including immunogenic cell death (ICD) and depletion of immunosuppressive cell populations.13,14 Of note, 5-FU has been shown to selectively deplete myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment and circulation.11 MDSCs, and other immunosuppressive myeloid cells such as tumor associated macrophages (TAMs), have been extensively studied in the field of tumor immunology and play a clear role in suppressing the immune system to promote tumor evasion from immune detection.15 MDSCs have also been shown to support recruitment and expansion of other immunosuppressive cells as well as directly support tumor growth and metastasis.15 Selective MDSC killing by 5-FU is hypothesized to be due to low target expression of thymidylate synthase (TS) in MDSCs, a phenomenon that has also been shown to correlate with cancer cell sensitivity to 5-FU.11,16–19 Thymidylate synthase is the target of fluorodeoxyuridine monophosphate (FdUMP), an active metabolite of 5-FU. Inhibition of TS by FdUMP results in DNA damage and cell death, therefore lower expression levels of TS make MDSCs highly sensitive to this process.11
Chemotherapeutic agents such as 5-FU have dose limitations due to their effects on normal tissue. Off-tumor effects include hematologic and gastrointestinal toxicities. These dose limiting toxicities (i) hinder the amount of 5-FU that can be delivered to the patient, and thus the amount of cytotoxic agent that reaches the tumor, and (ii) reduce the number of healthy circulating immune cells that are available to participate in antitumor immunity. Therefore, a therapy that minimizes systemic effects by targeting 5-FU to within the tumor and its associated immunosuppressive microenvironment is of great value.20
The combination of Toca 511 and Toca FC, an extended release formulation of 5-FC, is under clinical investigation (NCT01156584, NCT01470794, NCT02414165, NCT01985256) in patients with recurrent high-grade glioma (HGG). Further, a randomized phase II/III trial in recurrent glioblastoma (GBM) and anaplastic astrocytoma is in progress (NCT0241416).
Materials and Methods
Toca 511 Generation and Cell Line Transfection
Materials and methods for generation and modification of the Toca 511 vector have been described in detail previously.1 A mouse glioma cell line, Tu-2449SC, was selected in vivo for its ability to grow subcutaneously and was transduced with Toca 511 in the presence of polybrene (4 µg/mL) (Sigma-Aldrich). Two percent of the Tu-2449SC Toca 511 transduced cells were admixed with 98% wild-type (WT) Tu-2449SC cells (termed Tu-2449SC 2% Toca 511) and immediately implanted subcutaneously on the flanks of mice. By admixing pretransduced tumor cells with uninfected tumor cells, we aimed to capture the biological relevance of vector spread without introducing variability among animals through exogenous administration of vector. Rechallenge was conducted with WT Tu-2449SC cells.
Animals and In vivo Studies
All animal studies were conducted under approval and oversight by the facility’s Animal Care and Use Committee. Female B6C3F1 mice (Harlan Laboratories) received subcutaneous implants of 2 × 106 Tu-2449SC 2% Toca 511 cells on the right flank. Once tumors reached an average of 100 mm3, treatment was initiated. 5-FC was administered s.i.d. (500 mg/kg) i.p. Control animals received phosphate buffered saline (PBS). Additional details are provided in the Supplementary material.
For adoptive transfer studies, recipient mice were given 2 mg/mouse cyclophosphamide i.p. one day before adoptive cell transfer (ACT). Adoptive transfer was 13 × 106 splenocytes, 5 × 106 purified T cells, or 8 × 106 T-deplete splenocytes administered as a single i.v. injection.
Pharmacokinetic Analysis of 5-FU and 5-FC
Quantitative determination of 5-FU and 5-FC in plasma and tumor was conducted by Southern Research Institute and accomplished by use of supported liquid extraction and hydrophilic interaction chromatography with tandem mass spectrometry detection. The lower limit of quantitation for this method was 5 ng/mL.
Flow Cytometry
At indicated timepoints, spleen, draining lymph node (dLN), and tumor were collected and processed. Cells were analyzed by flow cytometry using a Becton Dickinson FACS (fluorescence activated cell sorting) Canto flow cytometer running Diva software. Further analysis was conducted using FlowJo software. Supplementary Tables 1 and 2 contain cell population descriptions and antibody clones, respectively. The Supplementary materials contain additional details on cell staining.
Statistics
In cases where only 2 groups were compared, a t-test was used to calculate P-values. In cases where more than 2 groups were compared, a one-way ANOVA was used. Tumor growth data over time was analyzed by 2-way ANOVA. GraphPad Prism software was used for all statistical analyses. Statistical significance was defined as P < .05.
Results
Toca 511/5-FC Treatment Results in Modulation, over Time, of Tumor-Associated Immune Cell Populations
Three days after 5-FC treatment initiation, total T helper cell populations are reduced in the tumor, with the remaining T helper cells expressing an activated phenotype
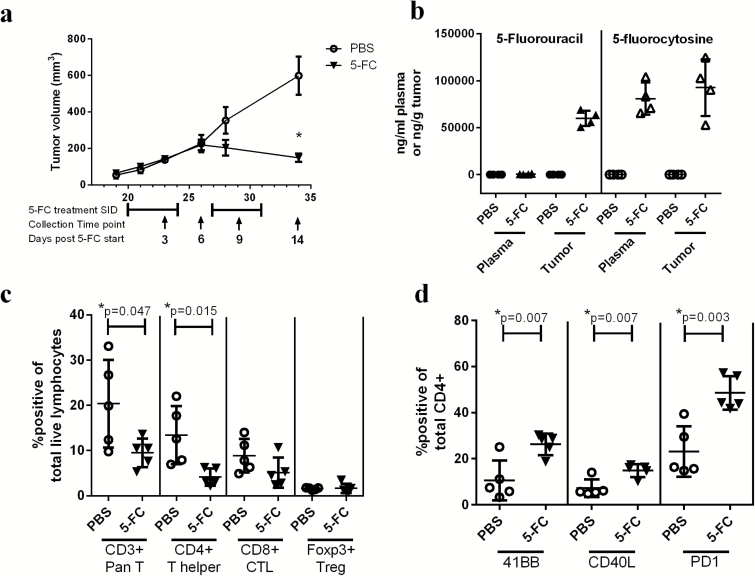
Subcutaneously implanted Tu-2449SC 2% Toca 511 tumors were allowed to grow until the average tumor size was 100 mm3 before 5-FC or PBS treatment was initiated. Tumor burden was significantly reduced by 14 days post 5-FC treatment initiation (Fig. 1A). Supplementary Fig. 1 shows that 5-FC does not effectively reduce tumor growth if Toca 511 is not present. Tumor and plasma from a subset of animals was collected on day 14 for pharmacokinetic analysis of 5-FC (prodrug) and 5-FU (active chemotherapeutic agent) one hour after the last dose of 5-FC. Figure 1B shows that while the exogenously administered prodrug, 5-FC, is detectable in plasma and tumor at high levels, 5-FU (the active chemotherapeutic agent which was generated endogenously through conversion of 5-FC by CD) is only detected in the tumor.
Fig. 1.
Toca 511 and 5-FC treatment concentrates 5-FU at the site of the tumor and reduces tumor burden; however, changes in immune cell subsets in the tumor 3 days after treatment initiation are minimal. (A) Tumor burden expressed as tumor volume (mm3) over time (days post tumor implant). 5-FC treatment cycles are shown below the graph and collection dates are indicated by black arrows and labeled as days post 5-FC start (n = 5). (B) 5-FC and 5-FU were assessed in plasma and tumor 1 hour after the last administration of 5-FC (n = 4). (C) Pan T cells (CD3+), CD4+, CD8+, or Tregs were analyzed as a percentage of total live lymphocytes in the tumor 3 days post 5-FC treatment initiation (n = 5). (D) 41BB, CD40L, and PD1 expressions on CD4+ T cells were analyzed 3 days after 5-FC treatment initiation. These data were repeated with similar results in at least one additional experiment. *Statistical significance was defined as P < .05.
Three days after treatment initiation, spleen and tumor from one group of mice were collected for immunophenotyping and determination of cytokine expression. Interestingly, total CD3+ T cells were reduced with 5-FC treatment, which was determined to be from the CD4+ T helper cell population (Fig. 1C). Although CD4+ T cells were reduced in tumors of animals treated with 5-FC, those CD4+ T cells that remained expressed surface markers indicative of an activated phenotype, including a greater percentage of CD4+ T cells expressing 41BB, cluster of differentiation 40 ligand (CD40L), and programmed cell death protein 1 (PD1) (Fig. 1D).
At the 3-day timepoint, tumors were analyzed for cytokine expression by intracellular staining using flow cytometry. Of those cytokines analyzed, including interferon gamma (IFNγ), interleukin 4 (IL-4), tumor necrosis factor alpha (TNFα), and interleukin 17 (IL-17), no changes were observed (data not shown). Additionally, splenocytes and dLN were analyzed for immunophenotype as well as cytokine expression and no changes were observed between the 2 treatment groups (data not shown).
Six and 9 days after 5-FC treatment initiation, TAMs, MDSCs, and tumor associated monocytes are significantly reduced in tumors treated with 5-FC compared with PBS controls
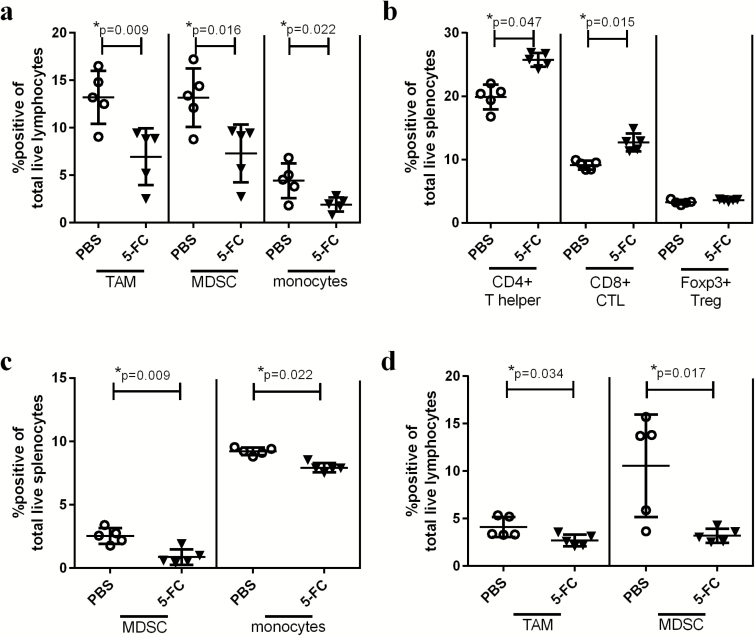
On day 6 post 5-FC start, total CD3+ T cells and, more specifically, CD4+ T cell numbers rebounded from the day 3 timepoint data (not shown). Notably, cells of myeloid origin were significantly reduced. Specifically, TAMs, MDSCs, and tumor associated monocytes were reduced in tumors treated with 5-FC (Fig. 2A).
Fig. 2.
Toca 511 and 5-FC treatment reduces immunosuppressive myeloid cells in the tumor 6 and 9 days after 5-FC treatment initiation. (A) TAMs, MDSCs, and monocytes were analyzed in the tumor 6 days after the initiation of 5-FC and expressed as a percentage of total live lymphocytes in the tumor. (B) CD4+, CD8+, or Tregs were analyzed as a percentage of total live splenocytes 6 days post 5-FC treatment initiation. (C) MDSCs and monocytes were analyzed in the spleen 6 days after the initiation of 5-FC and expressed as a percentage of total live splenocytes. (D) TAMs and MDSCs were analyzed in the tumor 9 days after the initiation of 5-FC and expressed as a percentage of total live lymphocytes in the tumor. These data, n = 5, were repeated with similar results in at least one additional experiment.
Six days after 5-FC initiation, splenocytes showed alterations in immunophenotype which included a significant increase in CD4+ and CD8+ T cells without a concomitant increase in regulatory T cells (Tregs) (Fig. 2B). Additionally, MDSC and monocytes were significantly reduced in spleens from mice treated with 5-FC at this timepoint (Fig. 2C). Interestingly, the only timepoint when any changes were observed in the spleen was 6 days post 5-FC treatment initiation. No changes were observed in the dLN.
In tumors collected on day 9 post 5-FC start, immunophenotyping data looked nearly identical to the data seen at the day 6 timepoint. T cell populations were unchanged but TAMs and MDSCs (Fig. 2D) remained significantly reduced in the presence of 5-FC. No changes were observed in the spleen or dLN.
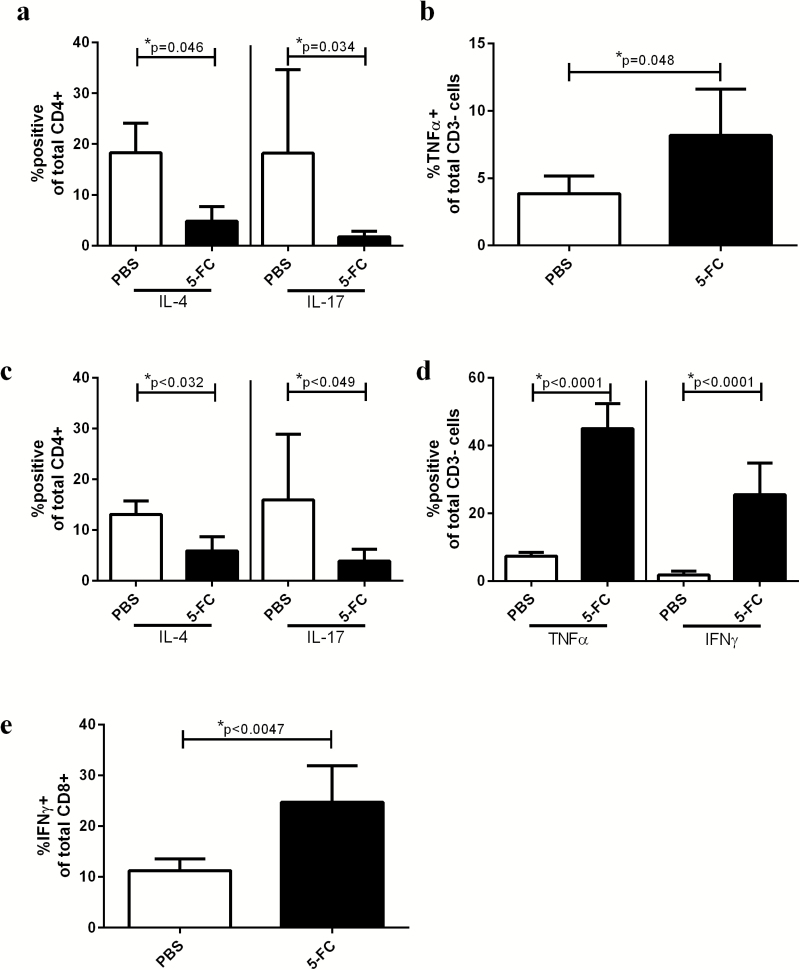
Six and 9 days after 5-FC treatment initiation, fewer T helper cells in tumors treated with 5-FC express IL-4 or IL-17 compared with PBS controls, and a larger proportion of cells in the non–T cell compartment express TNFα and IFNγ
While analysis at 3 days after the initiation of 5-FC showed very few immunological changes, by day 6 the CD4+ T helper cell population was recovered to baseline, and significantly fewer of those CD4+ T cells expressed IL-4 or IL-17 compared with PBS-treated tumors (Fig. 3A). Additionally, the non–T cell compartment of the tumor (CD3−) showed a significant increase in the percentage of cells expressing TNFα (Fig. 3B). Nine days post 5-FC treatment initiation, the percentage of CD4+ T helper cells expressing IL-4 or IL-17 continued to be reduced compared with PBS-treated tumors (Fig. 3C). Additionally, at 9 days post 5-FC start, the non–T cell (CD3−) compartment of the tumor continued to show a greater percentage of cells expressing TNFα but also revealed a greater percentage of non–T cells expressing IFNγ (Fig. 3D). This was complemented by a significant increase in the percentage of CD8 T cells that expressed IFNγ (Fig. 3E). No changes were observed in the spleen or dLN. Representative flow plots of these data are shown in Supplementary Fig. 2a–e.
Fig. 3.
Toca 511 and 5-FC treatment reduces Th2 and Th17 cells in the tumor 6 and 9 days after 5-FC treatment initiation while enhancing expression of TNFα at day 6 and TNFα and IFNγ at day 9. (A) Intracellular staining for IL-4 and IL-17 was conducted and expressed as a percentage of CD4+ T cells in the tumor 6 days after initiation of treatment with 5-FC. (B) Six days after 5-FC treatment initiation, intracellular staining for TNFα was conducted and expressed as a percentage of CD3−, or non–T cells in the tumor. (C) Intracellular staining for IL-4 and IL-17 was conducted and expressed as a percentage of CD4+ T cells in the tumor 9 days after initiation of treatment with 5-FC. (D) Nine days after 5-FC treatment initiation, intracellular staining for TNFα and IFNγ was conducted and expressed as a percentage of CD3−, or non–T cells in the tumor. (E) Intracellular staining for IFNγ was conducted and expressed as a percentage of CD8+ T cells in the tumor 9 days after initiation of treatment with 5-FC. These data, n = 5, were repeated with similar results in at least one additional experiment.
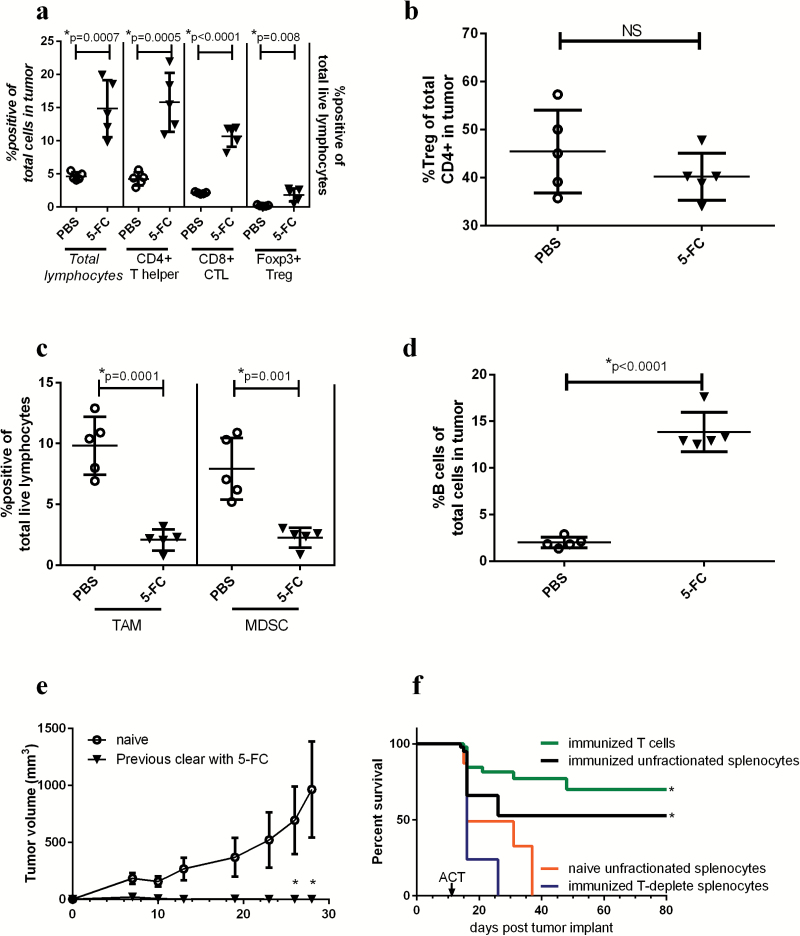
Fourteen days post 5-FC treatment initiation, tumor-associated T cell populations are dramatically augmented while immunosuppressive myeloid populations remain reduced
On day 14 post 5-FC start, tumor burden was greatly reduced in the 5-FC treatment group. Total live lymphocytes in 5-FC treated tumors were significantly increased, as were CD4+ and CD8+ T cells (Fig. 4A). Not surprisingly, CD4+ Tregs, as a population of total lymphocytes, were also increased in 5-FC treated tumors at the day 14 timepoint when the total T cell population was increased (Fig. 4A). Of note, when analyzing Tregs as a percentage of total CD4+ T cells, no increase was observed (Fig. 4B).
Fig. 4.
Toca 511 and 5-FC treatment continues to reduce immunosuppressive myeloid cells while increasing T cells in the tumor 14 days after treatment initiation. (A) Total lymphocytes were analyzed as a percentage of total cells in the tumor (data correspond to italicized axis on the left), CD4+, CD8+, or Tregs were analyzed as a percentage of total live lymphocytes in the tumor (data correspond to non-italicized axis on the treatment initiation). (B) Tregs were analyzed as a percentage of total CD4+ T cells in the tumor (right) 14 days post 5-FC treatment initiation. (C) TAMs and MDSCs were analyzed in the tumor 14 days after the initiation of 5-FC and expressed as a percentage of total live lymphocytes in the tumor. (D) Fourteen days after 5-FC treatment initiation, B cells were analyzed as a percentage of total live lymphocytes in the tumor. (E) Mice which had cleared tumors through treatment with Toca 511 and 5-FC and were tumor free for 9 weeks were rechallenged with Tu-2449 subcutaneous tumors and tumor burden was assessed over time (n = 5). Age-matched naïve controls were also challenged (n = 10). (F) Animals which had previously cleared Tu-2449 intracranial tumors through treatment with Toca 511 and 5-FC (termed “immunized”) were used for ACT into recipient mice bearing intracranial Tu-2449 tumors. Before transfer, immunized splenocytes were fractionated. Animals received either unfractionated splenocytes from immunized (black) or naïve mice (orange) or purified T cells from immunized spleens (green) or immunized splenocytes depleted of T cells (blue). These data were repeated with similar results in at least one additional experiment. *Statistical significance was defined as P < .05.
Myeloid populations continued to be reduced with 5-FC treatment 14 days after treatment initiation. TAMs as well as MDSCs were significantly reduced with 5-FC treatment as was seen at day 6 and day 9 post 5-FC start (Fig. 4C). Additionally, total B cells were dramatically increased with treatment compared with PBS controls (Fig. 4D).
No changes were observed in the spleen or dLN from these animals, and cytokine expression in tumors was not analyzed due to the limited amount of tumor tissue in the 5-FC treatment group 14 days after treatment initiation.
Animals that clear tumors through treatment with Toca 511 and 5-FC are protected from subsequent rechallenge with Tu-2449 subcutaneous tumors
A group of 10 mice bearing subcutaneous Tu-2449SC 2% Toca 511 were treated for 5 weeks with 5-FC (5 days/wk, s.i.d.). Once treatment was complete, the animals were observed for a period of 9 additional weeks without further treatment to confirm complete tumor clearance. Of the 10 animals treated, complete clearance of the tumor was confirmed in 5 mice 14 weeks after 5-FC treatment initiation. The 5 cured mice were then rechallenged on the opposite flank with Tu-2449SC cells and monitored for tumor growth. Naïve, age-matched control animals were also challenged. All animals that had previously cleared their tumors through treatment with 5-FC were able to completely clear tumor rechallenge within 2 weeks, whereas tumor grew unchecked in all naïve animals (Fig. 4E).
Adoptive transfer of immune cells from mice which have cleared Tu-2449 tumors through treatment with Toca 511 and 5-FC into recipient mice bearing orthotopic Tu-2449 intracranial implants results in improved survival and long-term cures
Mice were given intracranial implants of Tu-2449 cells and subsequently treated with 5-FC for 7 days followed by a 10-day treatment holiday. This was continued for 4 cycles and long-term survivors were monitored for 120 days before they were rechallenged subcutaneously. After 2 rounds of subcutaneous rechallenge/clearance, animals were termed “immunized” and splenocytes were harvested for ACT experiments. Naïve age-matched control mice were also used as donors for ACT experiments. Recipient mice were given intracranial implants with Tu-2449-luciferase on day 0; and 10 days later were myelo-ablated with cyclophosphamide (2 mg/mouse i.p.). On day 11, immunized and naïve donor splenocytes were harvested and fractionated for ACT. Recipient mice received one of 4 cell populations i.v.: (i) unfractionated splenocytes from immunized animals, (ii) purified T cells from immunized animals, (iii) splenocytes from immunized animals which were depleted of T cells prior to transfer, or unfractionated splenocytes from naïve age-matched donors. All cells were analyzed by flow cytometry before and after purification steps to ensure that each animal received the equivalent of 5 × 106 T cells during ACT. Therefore, mice that received unfractionated splenocytes from immunized animals were given an i.v. injection containing 13 × 106 splenocytes (5 × 106 of which were T cells). Mice that received purified immunized T cells received 5 × 106 total cells. Mice that received T-deplete immunized cells received 8 × 106 total cells. Animals that received naïve unfractionated splenocytes were given an i.v. injection containing 13 × 106 total cells. Recipient mice were then monitored for 80 days for survival. Animals were monitored weekly for tumor clearance by bioluminescence imaging. Figure 4F shows that survival was significantly improved, compared with naïve or T-deplete ACT recipients, in mice bearing orthotopic Tu-2449 gliomas which received ACT from immunized donors as long as T cells were present. Mice that received T-deplete splenocytes from immunized mice did not live significantly longer than those that received naïve unfractionated splenocytes. Upon imaging at 80 days post tumor inoculation, all surviving animals were tumor free.
Discussion
In the recent past there has been a revival in appreciation of the role of the immune system in both tumor progression and tumor clearance. It is now recognized that immunosuppressive factors such as MDSCs, TAMs, and Tregs utilize a number of mechanisms to promote tumor evasion from the immune system. It has also been shown that in some cases, just overcoming these suppressive factors through depletion or inhibition of function is enough to reactivate antitumor immune responses. Furthermore, while traditional chemotherapeutic approaches have been designed to directly kill tumor cells, it now appears that several of these agents rely on activation of antitumor immune responses for full efficacy. Immune activation by chemotherapeutic agents can take place through a number of mechanisms which have been outlined in a recent review.21 Of note, Bracci et al highlight that tumor cell death after treatment with select chemotherapeutic agents can reveal tumor antigens and promote epitope spread through ICD. Additionally, several chemotherapeutic agents have specificity for immunosuppressive cells in the tumor microenvironment which, upon clearance, leads to reactivation of immune effector function.7–11,21–23 The antineoplastic agent 5-FU has been reported to promote ICD, deplete immunosuppressive cells (specifically MDSCs) from the tumor microenvironment, and activate the adaptive arm of the immune system.7–11,24,25
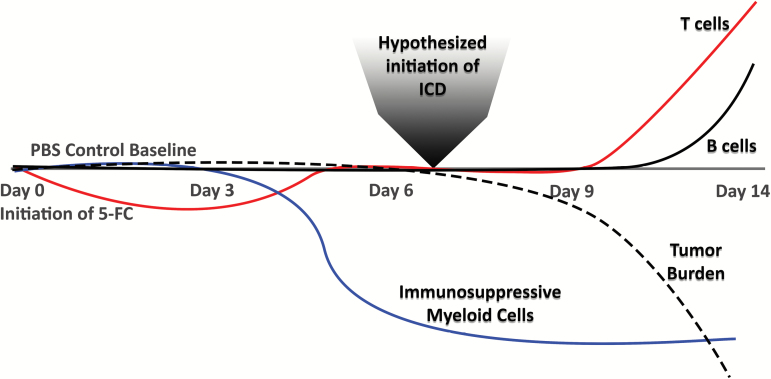
This work was conducted in parallel with Hiraoka et al (accompanying article), who show complementary immune responses in an orthotopic glioma model following treatment with Toca 511 and 5-FC. One goal of this work was to identify the immune cell populations that change over time following in vivo administration of 5-FC to mice bearing Toca 511–infected tumors. A summary of these findings is outlined in Fig. 5. As expected, tumor burden was significantly reduced in mice that received 5-FC compared with mice that received PBS control. Immunosuppressive cells of myeloid origin (MDSCs, TAMs, monocytes) were reduced in the tumor microenvironment starting at day 6 post 5-FC start and continued to be reduced throughout the remainder of the study. The myeloid cell depletion effect correlates with previous work with 5-FU which showed that 5-FU treatment results in depletion of MDSCs7–11,26 and that this effect is of translational importance as MDSCs are one immunosuppressive mechanism utilized by GBM to evade immune detection.26–28 Since TAMs and monocytes are of the same lineage as MDSCs, it is not surprising that a 5-FU–based treatment would also have some effect on those populations as well. At 14 days post 5-FC treatment initiation, both CD4+ and CD8+ T cells were significantly increased in tumors of treated animals. An increase in the ratio of effector T cells to tumor targets is favorable during promotion of an antitumor immune response, especially when Tregs remain unchanged as seen here.
Fig. 5.
Graphic representation of immune cell subset changes over time after initiation of 5-FC treatment.
Analysis of the cytokine expression profiles in tumors of 5-FC treated animals 6 and 9 days after treatment initiation showed differential expression of several key cytokines. Tumors treated with 5-FC had significantly fewer T helper cells expressing IL-4 (Th2) or IL-17 (Th17). A decrease in Th2 polarization is of clinical relevance, as Th2 skewing has been seen in patients with glioblastoma multiforme29 and correlates with poor prognosis in a number of solid tumors.30–32 Additionally, IL-4 secretion from Th2 cells has been shown to polarize macrophages toward an immunosuppressive/alternatively activated state (otherwise known as M2), and so reduction of Th2 cells is considered highly beneficial in the tumor microenvironment.33 Interestingly, the reduction in Th2 polarization with 5-FC treatment also correlates with our observation of a decrease in TAMs. While Th17 cells have been shown to play a clear role in inflammatory processes such as autoimmune disorders and antimicrobial immunity, their role in tumor immunity is less clear and is likely dependent on tumor type. It has been well established that IL-17 can promote angiogenesis in several tumor types, and for that reason IL-17 is not generally considered to be beneficial in the tumor microenvironment.34 Additionally, several mouse models have shown that IL-17 can potentiate MDSCs in the tumor microenvironment, which is further evidence for the benefits of reducing Th17 with Toca 511 and 5-FC.
The CD3, or non–T cell, compartment of the tumor showed an increase in the number of cells expressing TNFα at day 6, which continued into the final analysis at day 9 post treatment start and included an increase in IFNγ which suggested that the tumor microenvironment, in general, experienced a shift toward an inflammatory state. At day 9 post treatment initiation there was also a significant increase in the percentage of intratumoral CD8 T cells expressing IFNγ. There is substantial evidence that high levels of cytotoxic T cells expressing IFNγ are of great benefit to antitumor immune response.35
Toca 511 gene transfer of CD to tumors combined with successive delivery of the prodrug, 5-FC, results in subsequent conversion of 5-FC into 5-FU at the site of the tumor. The benefits of this approach are clear, as not only is the active agent concentrated at the site of the malignancy but the systemic effects are dramatically reduced.3 One major dose limiting toxicity of systemic 5-FU, as with most chemotherapeutic agents, is leukopenia. By reducing systemic exposure to 5-FU, this therapeutic platform can also spare many of the beneficial immune cells that are circulating in the blood and lymphatics, thus leaving them available for tumor infiltration and subsequent antitumor immune response. In fact, the work described herein showed that while 5-FC was detectable throughout the circulation, 5-FU was only present at detectable levels within the tumor tissue. Furthermore, very few immune effects were observed outside the tumor microenvironment in our studies, as spleen cells and draining lymph nodes were also analyzed at each timepoint and these tissues showed minimal immune subset changes.
Effects that were seen at the site of the tumor seem to track with what has been reported for 5-FU by others, including depletion of MDSCs with consequent expansion and activation of CD8 cytotoxic T cells. These alterations promote a long-term antitumor immune response, as evidenced by rapid clearance of subsequent challenges with the same tumor type. Of note, tumor rechallenge took place with cells that did not contain Toca 511, and therefore tumor rejection was due to antitumor and not antiviral immune response. Additionally, ACT of T cells which were harvested from mice which had previously cleared Tu-2449 tumors through treatment with Toca 511 and 5-FC were capable of clearing orthotopic gliomas in recipient mice. These data are further supported by work from Hiraoka et al (accompanying manuscript), who show that clearance of orthotopic gliomas results in protection from subsequent tumor challenge; an effect that was blunted when T cells were depleted during the rechallenge phase of the study. Therefore, treatment with Toca 511 and 5-FC results in complete cures in glioma-bearing animals with long-term protection from future recurrence through T cell mediated immune memory. While use of a subcutaneous tumor model is a caveat of these studies, this approach affords the opportunity to assess a multitude of immune endpoints simultaneously and therefore model the potential of this therapy. Ultimately, this type of preclinical assessment aims to serve as a guide for initiation of biomarker discovery efforts as well as identification of secondary endpoints for human clinical trials.
This work describes a novel approach to the treatment of cancer and validates the role that chemotherapeutics can have in cancer immunotherapy. The fact that direct administration of chemotherapeutic agents can have serendipitous immune “side” effects has been presented before; however, systemic administration is plagued by a narrow therapeutic index due to systemic dose limiting toxicities.20 Toca 511 is a retroviral replicating vector that selectively replicates and spreads in malignant cells, leading to tumor-selective expression of CD and subsequent local conversion of 5-FC into 5-FU. As a result, systemic effects are dramatically reduced and the benefits of 5-FU at the site of the tumor are preserved, including direct tumor cell killing and activation of a T cell mediated antitumor immune response.
Results from a recent phase I trial of treatment with Toca 511 and Toca FC (NCT01470794) in subjects with recurrent HGG show a favorable benefit-risk assessment and improvement in overall survival compared with an external control with durable tumor responses consistent with the antitumor immune response described here.36 Therapeutic efficacy of Toca 511 and Toca FC is currently being assessed in a randomized phase II/III trial (NCT02414165) in recurrent HGG and is being investigated in other solid tumors (NCT02576665), as well as in combination with other treatments (NCT02598011). Further, the findings from this work were directly applied to the design of the immune monitoring efforts in the randomized phase II/III trial (NCT02414165) in recurrent HGG.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
A.I., Y.K., K.H., and N.K. were supported in part by grants to N.K. from the National Institute of Neurological Disorders and Stroke (U01 NS059821) and the California Institute for Regenerative Medicine (TR2-01791).
Conflict of interest statement. L.A.M., F.L.E., D.M., N.K., H.E.G., D.J.J., and J.M.R. are employees and/or shareholders of Tocagen Inc. N.K. has received support from Tocagen Inc. for related nonclinical investigation.
Supplementary Material
Acknowledgments
We thank the ABC2 Foundation, the National Brain Tumor Society, the American Brain Tumor Association, the Musella Foundation, and Voices Against Brain Cancer for financial support.
References
- 1. Perez OD, Logg CR, Hiraoka K, et al. Design and selection of Toca 511 for clinical use: modified retroviral replicating vector with improved stability and gene expression. Mol Ther. 2012;20(9):1689–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang TT, Parab S, Burnett R, et al. Intravenous administration of retroviral replicating vector, Toca 511, demonstrates therapeutic efficacy in orthotopic immune-competent mouse glioma model. Hum Gene Ther. 2015;26(2):82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ostertag D, Amundson KK, Lopez Espinoza F, et al. Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neuro Oncol. 2012;14(2):145–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tai CK, Wang WJ, Chen TC, Kasahara N. Single-shot, multicycle suicide gene therapy by replication-competent retrovirus vectors achieves long-term survival benefit in experimental glioma. Mol Ther. 2005;12(5):842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Twitty CG, Diago OR, Hogan DJ, et al. Retroviral replicating vectors deliver cytosine deaminase leading to targeted 5-fluorouracil-mediated cytotoxicity in multiple human cancer types. Hum Gene Ther Methods. 2016;27(1):17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Logg CR, Robbins JM, Jolly DJ, Gruber HE, Kasahara N. Retroviral replicating vectors in cancer. Methods Enzymol. 2012;507:199–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghiringhelli F, Apetoh L. Chemotherapy and immunomodulation: from immunogenic chemotherapies to novel therapeutic strategies. Future Oncol. 2013;9(4):469–472. [DOI] [PubMed] [Google Scholar]
- 8. Ghiringhelli F, Apetoh L. The interplay between the immune system and chemotherapy: emerging methods for optimizing therapy. Expert Rev Clin Immunol. 2014;10(1):19–30. [DOI] [PubMed] [Google Scholar]
- 9. Ghiringhelli F, Apetoh L. Enhancing the anticancer effects of 5-fluorouracil: current challenges and future perspectives. Biomed J. 2015;38(2):111–116. [DOI] [PubMed] [Google Scholar]
- 10. Ghiringhelli F, Bruchard M, Apetoh L. Immune effects of 5-fluorouracil: ambivalence matters. Oncoimmunology. 2013;2(3):e23139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70(8):3052–3061. [DOI] [PubMed] [Google Scholar]
- 12. Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell. 2015;28(6):690–714. [DOI] [PubMed] [Google Scholar]
- 13. Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. [DOI] [PubMed] [Google Scholar]
- 14. Pol J, Vacchelli E, Aranda F, et al. Trial Watch: Immunogenic cell death inducers for anticancer chemotherapy. Oncoimmunology. 2015;4(4):e1008866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125(9):3356–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3(5):330–338. [DOI] [PubMed] [Google Scholar]
- 17. Peters GJ, van der Wilt CL. Thymidylate synthase as a target in cancer chemotherapy. Biochem Soc Trans. 1995;23(4):884–888. [DOI] [PubMed] [Google Scholar]
- 18. Peters GJ, van der Wilt CL, van Triest B, et al. Thymidylate synthase and drug resistance. Eur J Cancer. 1995;31A(7-8):1299–1305. [DOI] [PubMed] [Google Scholar]
- 19. van Kuilenburg AB, De Abreu RA, van Gennip AH. Pharmacogenetic and clinical aspects of dihydropyrimidine dehydrogenase deficiency. Ann Clin Biochem. 2003;40(Pt 1):41–45. [DOI] [PubMed] [Google Scholar]
- 20. Mathios D, Kim JE, Mangraviti A, et al. Anti-PD-1 antitumor immunity is enhanced by local and abrogated by systemic chemotherapy in GBM. Science Transl Med. 2016;8(370):370ra180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21(1):15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Emens LA, Middleton G. The interplay of immunotherapy and chemotherapy: harnessing potential synergies. Cancer Immunol Res. 2015;3(5):436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duffy AG, Greten TF. Immunological off-target effects of standard treatments in gastrointestinal cancers. Ann Oncol. 2014;25(1):24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Apetoh L, Ladoire S, Coukos G, Ghiringhelli F. Combining immunotherapy and anticancer agents: the right path to achieve cancer cure? Ann Oncol. 2015;26(9):1813–1823. [DOI] [PubMed] [Google Scholar]
- 25. Apetoh L, Tesniere A, Ghiringhelli F, Kroemer G, Zitvogel L. Molecular interactions between dying tumor cells and the innate immune system determine the efficacy of conventional anticancer therapies. Cancer Res. 2008;68(11):4026–4030. [DOI] [PubMed] [Google Scholar]
- 26. Otvos B, Silver DJ, Mulkearns-Hubert EE, et al. Cancer stem cell-secreted macrophage migration inhibitory factor stimulates myeloid derived suppressor cell function and facilitates glioblastoma immune evasion. Stem Cells. 2016;34(8):2026–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gielen PR, Schulte BM, Kers-Rebel ED, et al. Elevated levels of polymorphonuclear myeloid-derived suppressor cells in patients with glioblastoma highly express S100A8/9 and arginase and suppress T cell function. Neuro Oncol. 2016;18(9):1253–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raychaudhuri B, Rayman P, Ireland J, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13(6):591–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shimato S, Maier LM, Maier R, Bruce JN, Anderson RC, Anderson DE. Profound tumor-specific Th2 bias in patients with malignant glioma. BMC Cancer. 2012;12:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ruffell B, DeNardo DG, Affara NI, Coussens LM. Lymphocytes in cancer development: polarization towards pro-tumor immunity. Cytokine Growth Factor Rev. 2010;21(1):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DeNardo DG, Brennan DJ, Rexhepaj E, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1(1):54–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rolny C, Mazzone M, Tugues S, et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19(1):31–44. [DOI] [PubMed] [Google Scholar]
- 33. Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141(1):39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guéry L, Hugues S. Th17 cell plasticity and functions in cancer immunity. Biomed Res Int. 2015;2015:314620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14(10):1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cloughesy TF, Landolfi J, Hogan DJ, et al. Phase 1 trial of vocimagene amiretrorepvec and 5-fluorocytosine for recurrent high-grade glioma. Science Transl Med. 2016;8(341):341ra375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.