Abstract
Background
A streamlined transition from inpatient to outpatient care can decrease 30-day readmissions. Outpatient parenteral antibiotic therapy (OPAT) programs have not reduced readmissions; an OPAT bundle has been suggested to improve outcomes. We implemented a transition-of-care (TOC) OPAT bundle and assessed the effects on all-cause, 30-day hospital readmission.
Methods
Retrospectively, patients receiving postdischarge intravenous antibiotics were evaluated before and after implementation of a TOC-OPAT program in Bronx, New York, between July, 2015 and February, 2016. Pearson’s χ2 test was used to compare 30-day readmissions between groups, and logistic regression was used to adjust for covariates. Time from discharge to readmission was analyzed to assess readmission risk, using log-rank test to compare survival curves and Cox proportional hazards model to adjust for covariates. Secondary outcomes, 30-day emergency department (ED) visits, and mortality were analyzed similarly.
Results
Compared with previous standard care (n = 184), the TOC-OPAT group (n = 146) had significantly lower 30-day readmissions before (13.0% vs 26.1%, P < .01) and after adjustment for covariates (odds ratio [OR] = 0.51; 95% confidence interval [CI], 0.27–0.94; P = .03). In time-dependent analyses, TOC-OPAT patients were at significantly lower risk for readmission (log-rank test, P < .01; hazard ratio = 0.56; 95% CI, 0.32–0.97; P = .04). Propensity-matched sensitivity analysis showed lower readmissions in the TOC-OPAT group (13.6% vs 24.6%, P = .04), which was attenuated after adjustment (OR = 0.51; 95% CI, 0.25–1.05; P = .07). Mortality and ED visits were similar in both groups.
Conclusions
Our TOC-OPAT patients had reduced 30-day readmissions compared with the previous standard of care. An effective TOC-OPAT bundle can successfully improve patient outcomes in an economically disadvantaged area.
Keywords: bundle, outpatient parenteral antibiotic therapy, readmission, transitional care model
Outpatient parenteral antibiotic therapy (OPAT), administered in the home or other outpatient settings, has been increasingly used since the 1970s as a cost-effective alternative to prolonged hospitalization for management of patients with infectious conditions [1–6]. The use of OPAT has made it possible to discharge patients from the hospital earlier with many complex conditions that require prolonged intravenous (IV) antimicrobial therapy (endocarditis, osteomyelitis, prosthetic joint infections, complicated skin and soft tissue infections, intra-abdominal abscesses, etc) [1]. However, readmissions due to treatment failure, antibiotic-related adverse events, and IV-access complications are common [7–13]. As a result, current guidelines recommend close outpatient monitoring of patients receiving OPAT by a multidisciplinary team [14, 15].
In 2013, Muldoon et al [16] proposed an OPAT bundle as an evaluation tool for “an active performance improvement program” outlined in the Infectious Diseases Society of America OPAT guidelines [14]. They recommended a 6-part bundle comprised of patient selection, infectious diseases (ID) consultation, patient/caregiver education, discharge planning, outpatient monitoring/tracking, and a program outcomes review for quality improvement and optimization of OPAT care [16]. To date, few studies have evaluated the use of the proposed bundle and effect on clinical outcomes, such as 30-day readmissions.
Since 2012, the Affordable Care Act has required the Center for Medicaid and Medicare Services to reduce payments to hospitals with excess 30-day readmissions under the Hospital Readmissions Reduction Program [17]. Several studies have demonstrated the benefits of inpatient ID consultation on patient outcomes including 30-day readmissions [18, 19]. However, limited available data have not demonstrated a significant reduction in readmissions for OPAT programs [20, 21]. In contrast, studies in primary care settings have shown that a transitional care model incorporating phone calls to patients after hospital discharge, early outpatient physician follow-up visits, and clear communication between the inpatient and outpatient treatment teams are associated with improved 30-day readmissions [22–25].
A study evaluating infection-related 30-day readmissions found that hospitals serving a high proportion of patients living in a federal poverty area had higher all-cause and infection-related readmission rates [26]. Among more than 300 acute care hospitals studied, those with higher all-cause readmissions had a higher proportion of nonwhite, noncommercially insured patients from crowded homes in poor zip codes. The study’s findings highlight the challenge of improving infection-related outcomes in disadvantaged patients in poverty-affected areas.
The Bronx is New York City’s (NYC) poorest borough, with a median household income of $33079 compared with $57255 in New York State (NYS) [27, 28]. Several Bronx neighborhoods are seated in the nation’s poorest Congressional district [28]. As of 2014, 30.7% of the 1.4 million Bronx County residents live below poverty level [27]. Approximately 80% of adult Bronx residents have health insurance and a regular healthcare provider, but mortality rate per 100000 exceeds that of all other counties in the city [27]. In addition, emergency department (ED) visit rate and hospitalization rate per 10000 exceed that of both NYC and NYS [27]. The Bronx is also the least healthy county in NYS with the highest proportion of adult patients who are diabetic, obese, actively smoke, and have poor mental health [29].
The Montefiore Health System is the largest healthcare provider network serving the Bronx, with 1512 inpatient beds and 296614 ED visits in 2015. In 2013, 51% of Bronx residents had Medicaid claims [30], and 2014 Medicare spending in Bronx County was $15209 per capita vs $9501 nationally [31]. In 2013, Montefiore ranked first among Bronx Hospitals in percentage of providers serving Medicaid beneficiaries [30].
To address the needs of our complex patient population, transitional care models and the proposed OPAT bundle were adapted to design and pilot a multidisciplinary OPAT service at Montefiore Medical Center. The Transition-of-Care Outpatient Parenteral Antibiotic Therapy (TOC-OPAT) Program was initiated in July 2015. We aimed to assess its impact on hospital readmissions, ED use, and mortality in an economically disadvantaged setting.
METHODS
Study Design
We conducted a retrospective study of patients who received inpatient ID consultation and IV antibiotics after hospital discharge. We compared those enrolled in the Montefiore TOC-OPAT program and discharged between July 2015 and February 2016 with those receiving the previous standard of care that were discharged from January 2015 to June 2015. Clinical data were extracted using the TOC-OPAT program registry and Montefiore’s electronic medical record system using healthcare surveillance software (Clinical Looking Glass [CLG]; Emerging Health Information Technology, Yonkers, NY) and chart review. The Institutional Review Board of Montefiore Medical Center approved the study.
Inclusion and Exclusion Criteria
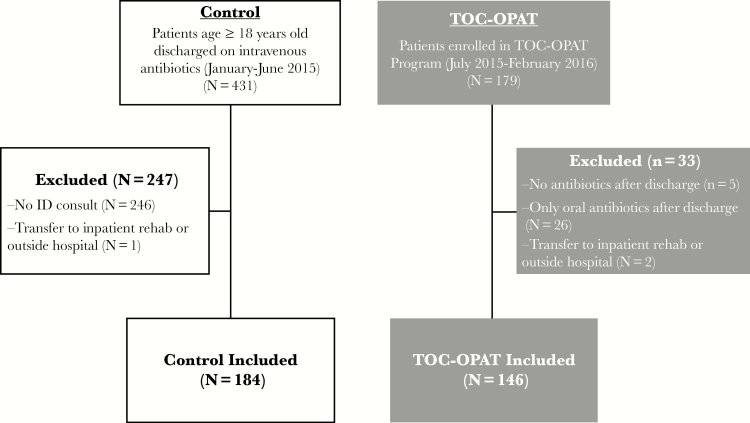
Patient selection and exclusions are summarized in Figure 1. Patients were included if they were aged 18 years and older and discharged from the hospital to either home or skilled-nursing facility after ID consultation with IV antibiotics. Patients were excluded if they expired before hospital discharge, were transferred to the inpatient rehabilitation unit or outside hospitals, did not receive inpatient ID consultation to assess the appropriateness of outpatient IV antibiotics, or did not ultimately receive IV antibiotics after hospital discharge.
Figure 1.
Patient selection and exclusions for control and Transition-of-Care Outpatient Parenteral Antibiotic Therapy (TOC-OPAT) groups. For the control group, 431 patients age 18 and older who were discharged from the hospital to their home or a skilled nursing facility from January 1, 2015 to June 30, 2015 and received intravenous (IV) antibiotics after discharge were identified retrospectively using Clinical Looking Glass software (Emerging Health Information Technology, Yonkers, NY). Of those, patients were excluded if they did not receive an Infectious Diseases (ID) consult before hospital discharge to assess the appropriateness of outpatient IV antibiotics (n = 246) or were transferred to an outside hospital or to the inpatient rehabilitation (rehab) unit (n = 1). The remaining 184 patients were included in the control group analysis. For the TOC-OPAT group, 179 patients (all age 18 or older) were referred to the program by inpatient ID providers and subsequently discharged from the hospital to their home or a skilled nursing facility between July 1, 2015 and February 29, 2016. Of those, patients were excluded (1) if they did not receive any antibiotics (n = 5) or exclusively oral antibiotics (n = 26) after discharge or (2) if they were transferred to an outside hospital or the inpatient rehabilitation unit (n = 2). The remaining 146 patients were included in the TOC-OPAT group analysis.
Baseline Assessment
We examined age, gender, race, ethnicity, insurance type, Charlson comorbidity score, admitting service, discharge disposition, discharge diagnosis based on International Classification of Diseases, Ninth Revision (ICD-9) or ICD-10 code (Supplementary Table 1), length of initial hospital stay (LOS), antibiotics prescribed, and prior hospitalizations in the preceding 12 months for both groups.
Transition-of-Care Outpatient Parenteral Antibiotic Therapy
Based on the OPAT bundle components proposed by Muldoon et al [16], we developed a TOC-OPAT model described in Table 1, using existing personnel and leveraging ID, antimicrobial stewardship, quality improvement, and programmatic expertise at the Montefiore Medical Center. Inpatient ID consultants identified adult patients requiring IV antibiotic therapy post-discharge, and referral was placed to our program. Our multidisciplinary team evaluated patients for optimal disposition plan (home versus skilled nursing facility). Patients and caregivers were educated on risks and benefits of therapy, line maintenance, and antibiotic infusion where appropriate.
Table 1.
Transition-of-Care Outpatient Parenteral Antibiotic Therapy Bundle
| Bundle Components | Description |
|---|---|
| Multidisciplinary OPAT team | • Inpatient social work team • ID physicians • OPAT nurse coordinator • Program administrator • Data manager • Homecare infusion liaisons • SNFs |
| Patient disposition screening | • Discussion of risks and benefits • Evaluation of home environment/support network • Insurance authorization of home-OPAT vs SNF • Patient engagement with plan |
| Patient and family education | • Home-OPAT patients: training on aseptic technique for IV antibiotic infusion and line maintenance • Medication side effects and potential complications • Trouble-shooting strategies • Contact numbers for medical providers and infusion nurses |
| Inpatient ID consultation | • Referral to OPAT program via email • Transition note in EMR: o Hospital course o Surgical procedures o Microbiology results o Radiology results o Duration of IV antibiotics o Frequency of lab monitoring o Time, date, and location of outpatient ID appointment o Outpatient ID provider phone number o OPAT fax number • Plan relayed to inpatient provider team |
| Care transition | • Inpatient providers coordinate discharge with inpatient social workers and order necessary monitoring laboratory tests based on IDSA OPAT guidelines [14] • OPAT plan documented in discharge summary and communicated to patient and outpatient providers |
| Outpatient care coordination | • OPAT nurse contacts patient or caregiver after hospital discharge to address concerns and provide appointment reminder • OPAT physicians evaluate patients in clinic at regular intervals and monitor laboratory results • OPAT physicians communicate with homecare agencies or SNFs with updates to treatment plan • OPAT physician arranges for IV line removal at the end of treatment • OPAT nurse continues patient outreach and elicits program feedback |
| OPAT program measures | • Patient data and outcomes recorded in TOC- OPAT program registry oReadmissions oED visits oAdverse events oTreatment failures oAppointment adherence • Quarterly performance improvement meetings |
Abbreviations: ED, emergency department; EMR, electronic medical record; ID, infectious diseases; IDSA, Infectious Diseases Society of America; IV, intravenous; OPAT, outpatient parenteral antibiotic therapy; SNF, skilled nursing facility; TOC, transition of care.
The program administrator scheduled an outpatient appointment within 2 weeks of the anticipated discharge and relayed details to referring provider. The inpatient ID consultant completed an electronic transition note with a comprehensive treatment plan accessible to all inpatient and outpatient providers. The treatment plan was also shared with the patient, receiving homecare agency, or nursing facility and included in the patient’s discharge summary.
Patients were contacted shortly after hospital discharge to discuss new concerns and to confirm the upcoming OPAT appointment. The OPAT physicians saw patients at regular intervals during their treatment course, and all changes in care plan were coordinated with the homecare agency or nursing facility. At the conclusion of IV antibiotic therapy, the OPAT physician arranged for IV line removal as appropriate. Attendance at TOC-OPAT appointments was not required for inclusion into the TOC-OPAT cohort; the OPAT team reviewed and acted on available test results and communicated with homecare agencies and nursing facilities for patients who were referred but did not attend appointments. The TOC-OPAT registry was created for surveillance and program auditing. Data were presented at quarterly OPAT performance improvement meetings.
Control Group
Under the previous standard of care, inpatient ID consultants evaluated patients in the hospital and recommended IV antimicrobial regimens after hospital discharge. Patients were referred for home infusion with contracted agencies or skilled nursing facilities based on insurance coverage, patient preference, living conditions, and caregiver support. A member of the primary team signed homecare orders and IV antimicrobial prescriptions upon hospital discharge. The primary team was often responsible for arranging ID outpatient follow up and documenting details in the discharge summary. Infectious diseases appointments were not guaranteed within 2 weeks of hospital discharge, and no specific outreach to patient was performed before ID appointment. Outpatient laboratory testing was inconsistently performed, and results were sent back to the inpatient attending of record. There was no consistent procedure for IV line removal at completion of therapy.
Clinical Outcomes
Primary outcome was all-cause, 30-day hospital readmission. Secondary outcomes were all-cause, 30-day ED visit and all-cause, 30-day mortality. We examined the reason for rehospitalization, including whether the admission was related to the infection or treatment and whether it was planned. We compared outpatient ID follow up before and after implementation of the TOC-OPAT program. We also examined adverse events and clinical failures during IV therapy for the TOC-OPAT group, including whether those events were associated with a readmission or ED visit. Adverse events and failures could not be accurately assessed for the control group due to poor clinical follow up and lack of documentation.
Statistical Methods
Baseline characteristics were compared between the TOC-OPAT and previous standard care groups using Wilcoxon-Mann-Whitney tests for continuous variables and Pearson’s χ2 tests or Fisher’s exact tests for categorical variables. Pearson’s χ2 test was used to compare the proportion of patients with a readmission within 30-days of index hospital discharge, and multivariate logistic regression was used to adjust for covariates (see below for the list of covariates). For time-dependent analysis, time to readmission was defined as the time from hospital discharge to the date of readmission; patients who were not readmitted within 30 days were censored on the date of death or at 30 days, whichever came first. Log-rank tests were used to compare Kaplan-Meier survival curves between the 2 groups, whereas Cox proportional hazards models were used to adjust for covariates. Emergency department visits and mortality within 30 days were analyzed similarly as 30-day readmission. For both logistic and cox regressions, we first assessed each of the following variables in univariable models: age, gender, race/ethnicity, language, insurance, admission service, disposition, diagnosis group, length of hospital stay, Charlson comorbidity score, and number of hospitalizations in the 12 months preceding the index admission. Charlson comorbidity score, and not individual comorbid conditions, was used to compare the 2 groups as an aggregate measure of comorbid disease to render the model more parsimonious. The final multivariate logistic regression model included variables with a P value of <.25 in the univariable model. As a sensitivity analysis, we used propensity score (PS) matching to match the 2 groups. The variables used in matching included age, gender, race/ethnicity, insurance, admission service, diagnosis group, length of hospital stay, disposition, Charlson score, and number of hospitalizations in the 12 months preceding the index admission. Matched datasets were then analyzed similarly as the full dataset.
RESULTS
There were 146 patients in the TOC-OPAT program and 184 patients in the control group (Table 2). The 2 groups were similar in age, gender, race/ethnicity, Spanish as primary language, insurance, length of hospital stay of the index visit, antibiotic drug class received, and number of prior hospitalizations. The OPAT group included a lower proportion of patients admitted to the medical service and a higher proportion admitted to a surgical service during the index hospital admission. There were differences in discharge diagnosis categories between the control and TOC-OPAT groups (P < .01) and a higher proportion of patients in the TOC-OPAT than the control group discharged to home (58% vs 45%, P = .02).
Table 2.
Baseline Characteristics for TOC-OPAT and Previous Standard of Care Groups at the Time of Index Hospital Discharge
| Baseline Patient Characteristics |
TOC-OPAT
(n = 146) |
Standard Care
(n = 184) |
P Value a |
|---|---|---|---|
| Age, mean (SD), years | 59.9 (14) | 61.7 (16.4) | .11 |
| Female, n (%) | 62 (42) | 81 (44) | .78 |
| Race/ethnicity, n (%) | .12 | ||
| Hispanic or Latino | 35 (24) | 47 (26) | |
| Non-Hispanic white | 29 (20) | 19 (10) | |
| Non-Hispanic black | 51 (35) | 65 (35) | |
| Non-Hispanic other | 9 (6) | 12 (7) | |
| Unknown | 22 (15) | 41 (22) | |
| Language n, (%) | .94 | ||
| English | 115 (79) | 147 (80) | |
| Spanish | 25 (17) | 32 (17) | |
| Other | 5 (3) | 5 (3) | |
| Insurance n, (%) | .40 | ||
| Public | 111 (76) | 147 (80) | |
| Private | 35 (24) | 37 (20) | |
| Admission service, n (%) | .01 | ||
| Medicine | 96 (66) | 144 (78) | |
| Surgical | 50 (34) | 40 (22) | |
| Antibiotic Class Received, n (%)b | |||
| Beta-Lactam | 106 (73) | 128 (70) | .55 |
| Glycopeptide | 51 (35) | 72 (39) | .43 |
| Lipopeptide | 4 (3) | 8 (4) | .56 |
| Aminoglycoside | 2 (1) | 9 (5) | .12 |
| Fluoroquinolone | 2 (1) | 0 (0) | .20 |
| Lincosamide | 1 (< 1) | 1 (< 1) | 1.00 |
| Azole | 1 (< 1) | 0 (0) | .44 |
| ICD groups, n (%) | <.01 | ||
| Bone and joint infection | 57 (39) | 16 (9) | |
| Cardiovascular infection | 4 (3) | 25 (14) | |
| Diabetes-related infection | 8 (6) | 13 (7) | |
| Septicemia | 42 (29) | 63 (34) | |
| Skin and soft tissue infection | 11 (8) | 6 (3) | |
| Other | 24 (16) | 61 (33) | |
| Hospital length of stay, days, median (IQR) |
10 (7,15) | 10 (7,15) | .87 |
| Disposition, n (%) | .02c | ||
| Home | 85 (58) | 82 (45) | |
| Facility | 61 (42) | 101 (55) | |
| Unknown | 0 | 1 (< 1) | |
| Charlson score, median (IQR) | 3 (1,5) | 5 (3,7) | <.01 |
| Individual Comorbidities, n (%) | |||
| MI or CHF | 31 (21) | 83 (45) | <.01 |
| PVD | 37 (25) | 64 (35) | .06 |
| CVD | 15 (10) | 44 (24) | <.01 |
| Dementia | 8 (5) | 13 (7) | .56 |
| Chronic pulmonary disease | 44 (30) | 78 (42) | .02 |
| Rheumatologic disease | 5 (3) | 7 (4) | 1.00 |
| PUD | 8 (5) | 11 (6) | .85 |
| Liver disease | 24 (16) | 47 (26) | .046 |
| DM | 85 (58) | 103 (56) | .68 |
| Hemi/paraplegia | 6 (4) | 24 (13) | <.01 |
| Renal disease | 42 (29) | 88 (48) | <.01 |
| Malignancy | 20 (14) | 35 (19) | .20 |
| HIV/AIDS | 3 (2) | 11 (6) | .10 |
| Number of prior hospitalizations, median (IQR) |
1 (0,3) | 1 (0,2) | .22 |
Abbreviations: AIDS, acquired immune deficiency syndrome; CHF, congestive heart failure; CVD, cerebrovascular disease; DM, diabetes mellitus; HIV, human immunodeficiency virus; ICD, International Classification of Diseases; IQR, interquartile range; MI, myocardial infarction; PUD, peptic ulcer disease; PVD, peripheral vascular disease; SD, standard deviation; TOC-OPAT, Transition-of-Care Outpatient Parenteral Antibiotic Therapy Program.
aWilcoxon-Mann-Whitney tests were used to compare continuous variables between the 2 groups, whereas Pearson’s χ2 tests or Fisher’s exact tests were used for comparing categorical variables.
bSome patients received more than 1 antibiotic. TOC-OPAT group: no patients received 3 antibiotics, 22 received 2 antibiotics, and 124 received 1 antibiotic. Control group: 5 patients received 3 antibiotics, 37 received 2 antibiotics, and 142 received 1 antibiotic.
cDisposition: P = .02 when excluding 1 patient with unknown disposition.
At baseline, Charlson comorbidity scores were lower in the TOC-OPAT group (median 3 [interquartile range {IQR}, 1–5] vs 5 [IQR, 3–7], P < .01). When comparing individual comorbidities, the TOC-OPAT group had a lower proportion of patients with myocardial infarction or congestive heart failure (P < .01), cerebrovascular disease (P < .01), chronic pulmonary disease (P = .02), liver disease (P = .046), hemi- or paraplegia (P < .01), and renal disease (P < .01). Other individual comorbidities were similar for the 2 groups.
Primary Outcome: 30-Day Readmission
The proportion of patients with 30-day readmissions was significantly lower in the TOC-OPAT group compared with the control group receiving the previous standard of care (13.0% vs 26.1%, P < .01) (Table 3). Adjusting for age, gender, Charlson comorbidity score, and prior hospitalizations, patients in the TOC-OPAT group were still significantly less likely to be readmitted as indicated by the following: adjusted odds ratio (OR) of 0.51 and 95% confidence interval (CI), 0.27–0.94 (P = .03) (Table 4).
Table 3.
All
-Cause 30-Day Readmission, All-Cause 30-Day Emergency Department Visit, and All-Cause 30-Day Mortality in the TOC-OPAT Group Compared With Previous Standard of Care Group
| Outcome | TOC-OPAT (n = 146) | Standard Care (n = 184) | P Value a |
|---|---|---|---|
| 30-day readmission, n (%) | 19 (13.0) | 48 (26.1) | <.01 |
| 30-day ED visit, n (%) | 35 (24.0) | 56 (30.4) | .19 |
| 30-day all-cause mortality, n (%) | 1 (0.7) | 3 (1.6) | .63 |
| Time to readmissionb, days, median (IQR) | 30 (30–30) | 30 (25–30) | <.01 |
| Time to ED visitc, days, median (IQR) | 30 (30–30) | 30 (20.5–30) | .18 |
| Time to deathd, days, median (IQR) | 30 (30–30) | 30 (30–30) | .43 |
Abbreviations: ED, emergency department; IQR, interquartile range; TOC-OPAT, Transition-of-Care Outpatient Parenteral Antibiotic Therapy Program.
aPearson’s χ2 tests or Fisher’s exact tests were used for comparing categorical variables between the 2 groups. Time-dependent variables were compared using log-rank test.
bTime to readmission was defined as the time from hospital discharge to the date of readmission; patients who were not readmitted within 30 days were censored on the date of death or at 30 days, whichever came first.
cTime to ED visit was defined similarly as time to readmission.
dTime to death was defined as the time from hospital discharge to the date of death; patients who had not died within 30 days were censored at 30 days.
Table 4.
Effects of TOC-OPAT Intervention Compared With Previous Standard of Care on All-Cause 30-Day Readmissions and Days to Readmission While Adjusting for Covariates
| Effect | Logistic Regression Results for 30-Day Readmission | Cox Regression Results for Days to Readmission | ||||||
|---|---|---|---|---|---|---|---|---|
| OR |
OR
95% CI |
P Value | HR | HR 95% CI | P Value | |||
| TOC-OPAT vs standard care | 0.51 | 0.27 | 0.94 | .03 | 0.56 | 0.32 | 0.97 | .04 |
| Age, every additional year of age | 1.02 | 1.00 | 1.04 | .11 | 1.01 | 1.00 | 1.03 | .14 |
| Female vs male | 0.49 | 0.27 | 0.89 | .02 | 0.55 | 0.33 | 0.92 | .02 |
| Charlson score, every additional point in the score | 1.04 | 0.95 | 1.14 | .38 | 1.03 | 0.96 | 1.11 | .40 |
| Prior hospitalizations, every additional hospitalization | 1.22 | 1.07 | 1.40 | <.01 | 1.21 | 1.09 | 1.34 | <.01 |
Abbreviations: CI, confidence intervals; HR, hazard ratio; OR, odds ratio; TOC-OPAT, Transition-of-Care Outpatient Parenteral Antibiotic Therapy Program.
Kaplan-Meier curves are plotted in Supplementary Figure 2. Time-dependent analysis results showed that the risk of readmission was also significantly lower in the TOC-OPAT group (log-rank test, P < .01). When adjusting for age, gender, comorbidity, and prior hospitalizations, TOC-OPAT patients were still at significantly lower risk for readmission as indicated by the following: adjusted hazard ratio (HR) of 0.56 and 95% CI, 0.32–0.97 (P = .04) (Table 4).
In the sensitivity analysis, the PS-matched datasets included 110 patients per group. Thirty-day readmission was significantly lower in the OPAT group, 13.6% vs 24.6% (P = .04). The risk for readmission was also lower in the OPAT group (log-rank P = .04). When adjusting for age, gender, Charlson score, and prior hospitalizations, these associations were attenuated: OR = 0.51 (95% CI, 0.25–1.05), P = .07 and HR = 0.57 (95% CI, 0.30–1.08), P = .09.
Secondary Outcomes: 30-Day Emergency Department Visit and Mortality
Mortality was low in both groups and not significantly different (Table 3). Emergency department visits were not different between the groups (Table 3). Kaplan-Meier curves were similar for both outcomes (Supplementary Figure 2).
Additional Outcomes: Reason for Readmission, Outpatient Infectious Diseases Follow-up Rates, Adverse Events, and Treatment Failures
Reason for readmission is summarized in Table 5. There were 2 planned readmissions in the TOC-OPAT group and 1 planned readmission in the control group, all of which were for staged surgical procedures related to the index infection. There were no significant differences in the causes for readmission between the TOC-OPAT group and standard of care group.
Table 5.
Reasons for Readmission in TOC-OPAT and Previous Standard of Care Groups
| Reason for Readmission a | TOC-OPAT (n = 19) | Standard Care (n = 48) | P Value |
|---|---|---|---|
| Related to index infection, n (%) | 7 (36.8%) | 19 (39.6%) | .84 |
| Complication of treatment, n (%) | 5 (26.3%) | 14 (29.2%) | 1.00 |
| Unrelated to index infection or treatment, n (%) | 7 (36.8%) | 17 (35.4%) | .91 |
| Planned admission, n (%) | 2 (10.5%) | 1 (2.1%) | .19 |
Abbreviation: TOC-OPAT, Transition-of-Care Outpatient Parenteral Antibiotic Therapy Program.
aTwo patients in the standard care group had a readmission that was both related to the index infection and due to complication of the treatment. All planned admissions in both groups were staged surgical procedures and considered related to the index infection.
In the TOC-OPAT group, 67% attended an ID follow-up visit within 30 days of discharge compared with 18% in the control group (P < .01). In the TOC-OPAT group, attendance of ID outpatient appointments improved from 70% in the first month of the intervention period to 81% in the last month of the intervention period. There were 31 reported adverse events occurring in 27 patients (18.5% of patients) in the TOC-OPAT group (Table 6). The most commonly reported adverse events were IV access-related problems (deep vein thrombosis, displacement, malfunction, or infection) and renal failure. Of the 31 adverse events, 9 (29%) required an ED visit and 7 (23%) required hospital admission for management. Only 1 patient developed Clostridium difficile colitis in the TOC-OPAT cohort. Additional operative management or a major change in antimicrobial management for treatment failure was required in 16 (11.0%) TOC-OPAT patients.
Table 6.
Adverse Events in TOC-OPAT Patients
| Adverse Events | Incidence N (% of All Adverse Events) | Required ED Visit N (% of All Adverse Events) | Required Readmission N (% of All Adverse Events) |
|---|---|---|---|
| Total | 31 (100) | 9 (29) | 7 (23) |
| IV access-related | 9 (29) | 6 (19) | 4 (13) |
| Renal failure | 8 (26) | 2 (7) | 2 (7) |
| GI | 4 (13) | 0 (0) | 0 (0) |
| Rash | 3 (10) | 0 (0) | 0 (0) |
| Cytopenia | 2 (7) | 0 (0) | 0 (0) |
| Transaminitis | 1 (3) | 0 (0) | 0 (0) |
| Drug fever | 1 (3) | 0 (0) | 0 (0) |
| Rhabdomyolysis | 1 (3) | 0 (0) | 0 (0) |
| Altered mental status | 1 (3) | 1 (3) | 1 (3) |
| Pruritis | 1 (3) | 0 (0) | 0 (0) |
Abbreviations: ED, emergency department; GI, gastrointestinal symptoms; IV, intravenous; TOC-OPAT, Transition-of-Care Outpatient Parenteral Antibiotic Therapy Program.
DISCUSSION
Patients in the TOC-OPAT program had significantly fewer all-cause, 30-day readmissions than those who received the previous standard of care, despite similar inpatient length of stay, ED use, and an adverse event rate of 18.5%. The TOC-OPAT patients also had a higher rate of outpatient ID follow-up. Readmission reduction using a TOC bundle approach for high-risk patients with infectious diseases has the potential for broad impact, considering that infection-related readmissions account for approximately one third of all readmissions, and other similarly socioeconomically disadvantaged patient populations have demonstrated a high risk for rehospitalization [26].
Antibiotic- or IV access-related adverse events were observed in the TOC-OPAT patient cohort at a rate similar to that described in previously published studies [5, 9]. Although 18.5% of patients experienced an adverse event in the TOC-OPAT group, less than one third of those events necessitated an ED visit, and less than one quarter of the events required a hospital readmission. Overall ED use was similar in both groups, but the TOC-OPAT patients were less likely to be readmitted to the hospital. We hypothesize that adverse events or problems in TOC-OPAT patients are detected sooner due to active surveillance and close follow-up and can be managed on an outpatient basis when detected early with support from a multidisciplinary team of providers, enabling safe ED discharge and avoiding readmission.
Our study is novel in demonstrating a significant reduction in readmission in OPAT patients using a TOC bundle. Our findings are particularly relevant given that OPAT patients have 30-day readmission as high as 50% in some populations [10]. A similar 2013 study evaluated the impact of an ID transition service on OPAT patient readmissions and ED visits. This study also evaluated secondary process measures such as antimicrobial prescribing errors, laboratory monitoring frequency, and completion of ID follow up within 60 days. However, the authors did not observe a significant difference in readmissions or ED visits, although an improvement in secondary process measures was observed [18]. Our data support implementation of a comprehensive OPAT bundle, as recommended by Muldoon et al [16], for enhanced quality of OPAT care. Our model is widely applicable to complex, urban, economically disadvantaged patient populations.
A TOC-OPAT model offers significant cost-savings potential by reducing hospital readmissions. Based on internal auditing, a total of 931 adult patients were discharged on IV antibiotics from Montefiore Medical Center in 2015. Based on our hazard ratio of 0.56, if 30-day readmissions among patients at our medical center are reduced by 44% using the OPAT transitional care model, 97 readmissions could be averted per year. Data from the Agency for Healthcare Research and Quality shows that the average cost of a nonmaternal adult 30-day readmission ranges across payers from $8600 to $15700 [32]. Therefore, the potential hospital-based cost savings of this program ranges from $834200 to $1522900 per year.
Our study has several limitations. Patient data extracted from CLG were limited to conditions documented in the electronic health record, which could underestimate the prevalence of comorbidities, although this presumably impacted the control and TOC-OPAT groups equally. Adverse events could not be evaluated for the control group due to lack of available data as a result of inadequate documentation and poor clinical follow-up; thus, it is unclear whether the adverse event rate was different in the control group and whether a bundle could have mitigated the risk of adverse event-related readmissions in this group. Duration of anticipated OPAT was often vague (a wide range or not well documented by clinicians) in both groups; thus, we were unable to evaluate the effect of this variable on readmission rates. There were clinical differences between the groups for admitting service, discharge diagnosis, disposition, and Charlson scores. Admitting service, discharge diagnosis, and disposition were not associated with 30-day readmission in the univariable logistic regression analysis (P = .82, P = .35, and P = .58, respectively) and therefore not considered confounders. Although Charlson score was associated with 30-day readmission in the univariable logistic regression (OR = 1.12; 95% CI, 1.04–1.21; P < .01), the adjusted analysis still shows fewer readmissions in the TOC-OPAT group. Although below the threshold for statistical significance, analysis of PS-matched data shows a similar trend to the unmatched adjusted analysis, and failure to meet statistical significance may indicate lack of power and small sample size rather than a lack of difference between outcomes. However, it is also possible that the lack of significance in the propensity-matching analysis is due to the fact that baseline differences between the intervention and control groups account for the difference in readmission rates. Review of previous studies examining factors associated with readmission in OPAT patients suggests that some characteristics, such as prior hospitalization and anticipated duration of OPAT, are consistently associated with readmission in different studies, whereas others, such as age and Charlson score, are variable, and different studies draw discordant conclusions. A prediction model for 30-day hospital readmissions in OPAT patients developed by Allison et al [33] found that age, aminoglycoside use, resistant organisms, and prior hospitalization in the preceding 12 months were associated with readmission, whereas Charlson score was not. Means et al [12] studied predictors of readmission in OPAT patients and found that prior hospitalization, longer planned OPAT duration, and history of lymphoma were associated with readmission. Huck et al [11] examined the impact of laboratory test result availability and readmission and found that nonavailability of laboratory tests, longer anticipated OPAT duration, and Charlson score were associated with readmission, whereas age was not. Further study of different OPAT populations could be helpful in understanding the impact of different contributing factors for rehospitalization and in identifying ways to improve the bundle to assist patients at high risk.
CONCLUSIONS
Overall, we observed significantly fewer 30-day hospital readmissions in a high-risk urban setting, using a transition-of-care OPAT bundle. We highlight the importance of a multidisciplinary OPAT team of social workers, ID physicians, a nurse coordinator, an administrator, home infusion teams, and skilled nursing facilities. More importantly, the program was developed using existing resources and personnel. Based on our early successes, we have implemented sustaining measures such as hiring of a full-time nurse coordinator and an additional ID physician to better accommodate the expanding needs of our patients. In the future, evaluation of TOC-OPAT patient experience and satisfaction will be incorporated into our care bundle to guide further programmatic improvements. Further study of high-risk OPAT patients is needed to guide readmission prevention efforts.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
We thank Dr. Michele Ritter for valuable insights into outpatient parenteral antibiotic therapy program development and outcome measures.
Financial support. Use of the REDCap database was funded by an institutional grant through the Albert Einstein College of Medicine (UL1TR001073).
Potential conflicts of interest. G. W. receives unrelated grant support from Allergan, A. E.’s spouse consults for Karus Therapeutics, and B. O. has applied for an unrelated grant supported by Allergan. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Antoniskis A, Anderson BC, Van Volkinburg EJ et al. . Feasibility of outpatient self-administration of parenteral antibiotics. West J Med 1978; 128:203–6. [PMC free article] [PubMed] [Google Scholar]
- 2. Balinsky W, Nesbitt S. Cost-effectiveness of outpatient parenteral antibiotics: a review of the literature. Am J Med 1989; 87:301–5. [DOI] [PubMed] [Google Scholar]
- 3. Grayson ML, Silvers J, Turnidge J. Home intravenous antibiotic therapy. A safe and effective alternative to inpatient care. Med J Aust 1995; 162:249–53. [PubMed] [Google Scholar]
- 4. Dalovisio JR, Juneau J, Baumgarten K, Kateiva J. Financial impact of a home intravenous antibiotic program on a medicare managed care program. Clin Infect Dis 2000; 30:639–42. [DOI] [PubMed] [Google Scholar]
- 5. Ruh CA, Parameswaran GI, Wojciechowski AL, Mergenhagen KA. Outcomes and pharmacoeconomic analysis of a home intravenous antibiotic infusion program in veterans. Clin Ther 2015; 37:2527–35. [DOI] [PubMed] [Google Scholar]
- 6. Rucker RW, Harrison GM. Outpatient intravenous medications in the management of cystic fibrosis. Pediatrics 1974; 54:358–60. [PubMed] [Google Scholar]
- 7. Berman SJ, Johnson EW. Out-patient parenteral antibiotic therapy (OPAT): clinical outcomes and adverse events. Hawaii Med J 2001; 60:31–3. [PubMed] [Google Scholar]
- 8. Cunha BA. Antibiotic side effects. Med Clin North Am 2001; 85:149–85. [DOI] [PubMed] [Google Scholar]
- 9. Hoffman-Terry ML, Fraimow HS, Fox TR et al. . Adverse effects of outpatient parenteral antibiotic therapy. Am J Med 1999; 106:44–9. [DOI] [PubMed] [Google Scholar]
- 10. Duggal A, Barsoum W, Schmitt SK. Patients with prosthetic joint infection on IV antibiotics are at high risk for readmission. Clin Orthop Relat Res 2009; 467:1727–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huck D, Ginsberg JP, Gordon SM et al. . Association of laboratory test result availability and rehospitalizations in an outpatient parenteral antimicrobial therapy programme. J Antimicrob Chemother 2014; 69:228–33. [DOI] [PubMed] [Google Scholar]
- 12. Means L, Bleasdale S, Sikka M, Gross AE. Predictors of hospital readmission in patients receiving outpatient parenteral antimicrobial therapy. Pharmacotherapy 2016; 36:934–9. [DOI] [PubMed] [Google Scholar]
- 13. Yan M, Elligsen M, Simor AE, Daneman N. Patient characteristics and outcomes of outpatient parenteral antimicrobial therapy: a retrospective study. Can J Infect Dis Med Microbiol 2016; 2016: 8435257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tice AD, Rehm SJ, Dalovisio JR et al. . Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis 2004; 38:1651–72. [DOI] [PubMed] [Google Scholar]
- 15. Williams DN, Rehm SJ, Tice AD et al. . Practice guidelines for community-based parenteral anti-infective therapy. ISDA practice guidelines committee. Clin Infect Dis 1997; 25:787–801. [DOI] [PubMed] [Google Scholar]
- 16. Muldoon EG, Snydman DR, Penland EC, Allison GM. Are we ready for an outpatient parenteral antimicrobial therapy bundle? A critical appraisal of the evidence. Clin Infect Dis 2013; 57:419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. The Patient Protection and Affordable Care Act (PPACA). 2010: 42 U. S. C. 3025. [Google Scholar]
- 18. Bai AD, Showler A, Burry L et al. . Impact of infectious disease consultation on quality of care, mortality, and length of stay in Staphylococcus aureus bacteremia: results from a large multicenter cohort study. Clin Infect Dis 2015; 60:1451–61. [DOI] [PubMed] [Google Scholar]
- 19. Schmitt S, McQuillen DP, Nahass R et al. . Infectious diseases specialty intervention is associated with decreased mortality and lower healthcare costs. Clin Infect Dis 2014; 58:22–8. [DOI] [PubMed] [Google Scholar]
- 20. Yang A, Fung R, Brunton J, Dresser L. Outpatient parenteral antimicrobial therapy for surgery patients: a comparison with previous standard of care. Can J Infect Dis Med Microbiol 2013; 24:74–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Keller SC, Ciuffetelli D, Bilker W et al. . The impact of an infectious diseases transition service on the care of outpatients on parenteral antimicrobial therapy. J Pharm Technol 2013; 29:205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med 2006; 166:1822–8. [DOI] [PubMed] [Google Scholar]
- 23. Dudas V, Bookwalter T, Kerr KM, Pantilat SZ. The impact of follow-up telephone calls to patients after hospitalization. Am J Med 2001; 111:26S–30S. [DOI] [PubMed] [Google Scholar]
- 24. Jack BW, Chetty VK, Anthony D et al. . A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med 2009; 150:178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Walraven C, Seth R, Austin PC, Laupacis A. Effect of discharge summary availability during post-discharge visits on hospital readmission. J Gen Intern Med 2002; 17:186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gohil SK, Datta R, Cao C et al. . Impact of hospital population case-mix, including poverty, on hospital all-cause and infection-related 30-day readmission rates. Clin Infect Dis 2015; 61:1235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Socio-Economic Status and General Health Indicators - Bronx County New York State Department of Health, 2011–2013. Available at: https://www.health.ny.gov/statistics/chac/chai/docs/ses_58.htm Accessed 15 September 2016. [Google Scholar]
- 28. Quick Facts Bronx County, New York United States Census Bureau, 2010–2015. Available at: http://www.census.gov/quickfacts/table/PST045215/36005 Accessed 15 September 2016. [Google Scholar]
- 29. County Health Rankings and Roadmaps The Robert Wood Johnson Foundation; 2016. Available at: http://www.countyhealthrankings.org/ Accessed 15 September 2016. [Google Scholar]
- 30. Research, Statistics, Data & Systems Centers for Medicaid and Medicare Services; 2013. Available at: https://www.cms.gov/Research-Statistics-Data-and-Systems/Research-Statistics-Data-and-Systems.html Accessed 15 September 2016. [Google Scholar]
- 31. Medicaid Regional Data Compendium The Medicaid Institute at United Hospital Fund; 2014. Available at: http://www.medicaidinstitute.org/publications/881021 Accessed 15 September 2016. [Google Scholar]
- 32. Statistical Brief #199. All-Cause Readmissions by Payer and Age, 2009–2013 Healthcare Cost and Utilization Project (HCUP) 2015. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/statbriefs.jsp. Accessed 4 February 2016. [Google Scholar]
- 33. Allison GM, Muldoon EG, Kent DM et al. . Prediction model for 30-day hospital readmissions among patients discharged receiving outpatient parenteral antibiotic therapy. Clin Infect Dis 2014; 58:812–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.