Abstract
Background
Although inactivation of the von Hippel–Lindau gene (VHL), located on chromosome 3p25, is considered to be a major cause of hemangioblastomas (HBs), the incidence of biallelic inactivation of VHL is reportedly low. The aim of this study was to determine the prevalence of VHL alterations in HBs, as well as to identify additional molecular aberrations.
Methods
Genetic and epigenetic alterations were comprehensively and comparatively analyzed in 11 VHL-related and 21 sporadic HBs.
Results
VHL alterations detected by sequencing and multiplex ligation-dependent probe amplification (MLPA) analysis were more frequent in VHL-related HBs than in sporadic HBs (100% vs 62%; P = 0.029). VHL alterations were found only in 4 sporadic HBs by direct sequencing; however, targeted deep sequencing detected 9 additional alterations. Loss of heterozygosity (LOH) on chromosome 3 was found in 64% and 57% of VHL-related and sporadic HBs, respectively, by single nucleotide polymorphism (SNP) array analysis. Among 19 tumors with chromosome 3 LOH, 5 were classified as copy-neutral LOH. VHL promoter hypermethylation was detected only in sporadic HBs (33%), indicating that epigenetic suppression of VHL is a common mechanism in sporadic HBs. The rate of biallelic VHL inactivation among VHL-related and sporadic HBs was 64% and 52%, respectively. LOH on either chromosome 6 or 10 was detected only in sporadic HBs (43%).
Conclusion
Although biallelic inactivation of VHL is a dominant mechanistic cause of the pathogenesis of HB, other unknown mechanisms may also be involved, and such mechanisms may be different between VHL-related and sporadic HB.
Keywords: DNA methylation, hemangioblastoma, loss of heterozygosity, mutation, von Hippel-Lindau disease
Importance of the study.
To reevaluate the significance of VHL alterations in HBs, we comprehensively and comparatively analyzed VHL-related HBs and sporadic HBs using recently advanced technologies such as next-generation sequencing, SNP array, MLPA analysis, and bisulfite sequencing. With these methods, we identified increased numbers of HBs with genetic and epigenetic inactivation of VHL than previously reported. In addition, our data suggest that other unknown mechanisms may also be involved in the pathogenesis of sporadic HBs. We believe that these findings will contribute to accurate understanding of the etiology of HBs and lead to further investigation of yet unknown mechanisms of HB pathogenesis, as well as to development of novel therapeutic strategies in the future.
Hemangioblastomas (HBs) are benign neoplasms of the central nervous system (World Health Organization [WHO] grade I) that have dense vascular networks and sometimes accompany large cysts.1,2 Histologically, HBs are composed of 2 main components: vascular cells and stromal cells with multiple vacuoles and granular eosinophilic cytoplasm,1 and tissue microdissection analysis verified that the stromal cells are the neoplastic cells harboring the genetic alterations.3 Among all diagnosed HBs in patients, 75% occur sporadically and 25% occur as a part of von Hippel–Lindau (VHL) disease,4 an autosomal dominant hereditary disease caused by a germline mutation of the VHL gene that predisposes individuals to multi-organ tumors, including clear cell renal cell carcinoma (ccRCC), pheochromocytomas, pancreatic islet tumors, retinal angiomas, as well as HBs.5 HBs occur most frequently in the cerebellum, followed by the brainstem and spinal cord, and the spinal HBs are more likely to be observed in patients with VHL disease than in patients with sporadic HBs.6 Surgical resection is the standard treatment of HBs, while stereotactic radiosurgery is reported to be an effective alternative for small HBs.7
In 1993, VHL, the tumor suppressor gene that causes VHL disease, was identified and mapped to chromosome (chr.) 3p25 via positional cloning.8 Then, somatic mutations of VHL were also found in sporadic HBs.9 The VHL protein is involved in degradation of hypoxia inducible factor (HIF)-1 and HIF-2, which promote transcription of vascular endothelial growth factor and platelet-derived growth factor in hypoxic conditions.10,11 When the normal VHL protein function is lost due to VHL mutation or deletion, HIF proteins do not undergo the VHL-mediated degradation and therefore activate transcription of these downstream growth factors that promote tumorigenesis in VHL disease.11
A previous report on integrated genome analysis of ccRCCs demonstrated that VHL intragenic mutation, deletions of chr. 3 involving VHL, and VHL promoter methylation were present in 82%, 94%, and 10% of sporadic ccRCCs, respectively.12 The rate of biallelic VHL inactivation in sporadic ccRCC was nearly 98% and indicated that biallelic VHL inactivation is an important mechanism during ccRCC tumorigenesis; this mechanism is consistent with Knudson’s 2-hit theory, which explains the importance of biallelic inactivation of tumor suppressor genes in inherited cancer susceptibility syndromes.13 In contrast, several studies reported that inactivation of VHL is less frequent in HB than in RCC, especially in sporadic HB.14,15 These studies reported that the rates of biallelic inactivation of VHL in sporadic HB were 10% to 33%. Even with next-generation sequencing, Shankar et al reported that the rates of VHL mutation and biallelic inactivation of VHL in sporadic HBs were 56% and 46%, respectively.16 Therefore, it is possible that the biallelic VHL inactivation is not essential in HB tumorigenesis. Alternatively, the lower incidence of biallelic inactivation of VHL and apparent absence of VHL promoter methylation in HB could be due to technological limitations of the analytical approaches used.
To reevaluate the contribution of VHL inactivation to HB tumorigenesis, we used multiple analytical genetic techniques to comparatively analyze VHL-related and sporadic HBs. Our findings suggested that in addition to well-known mechanisms such as mutation or deletion of VHL, epigenetic suppression of VHL as well as other unknown mechanisms may play roles in the pathogenesis of sporadic HB.
Materials and Methods
Patients and Tumor Specimens
Thirty-two surgically resected HB specimens from 28 patients treated at the University of Tokyo Hospital between 1995 and 2012 were analyzed retrospectively. Of the 32 specimens, 11 were VHL-related HBs from 7 patients, and 21 were sporadic HBs from 21 patients. The 7 patients with VHL disease included 2 pairs of siblings; therefore, 5 families were represented by VHL-related HBs. In the cases in which multiple HBs were resected from one patient, each tumor was resected on a separate date. For all tumor specimens, paired peripheral blood samples were also obtained to be used as patient-specific normal genetic controls. Histopathological diagnosis was made according to the 2007 World Health Organization classification.1 Diagnoses of VHL disease were made according to the clinical criteria.17 This study was approved by the Ethics Committee of the University of Tokyo (#G3512). All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments. Written informed consent was obtained from all individual participants included in the study.
DNA Extraction
The QIAamp DNA mini kit (Qiagen) was used to extract a genomic DNA sample from each frozen tumor sample and each paired blood sample, according to the manufacturer’s instructions.
Direct Sequencing
The 3 exons of VHL were amplified using 6 primer sets described by Weil et al.18 An aliquot of DNA was amplified by PCR using AmpliTaq Gold DNA polymerase (Applied Biosystems) with annealing temperature at 60°C. Direct sequencing was then performed with an ABI Genetic Analyzer 3130xL or an ABI DNA Analyzer 3730xL (Applied Biosystems). Sequence Scanner version 1.0 (Applied Biosystems) was used to analyze all sequencing data.
Targeted Deep Sequencing
A set of primer pairs to amplify all exons of VHL by multiplex PCR was designed using Ion AmpliSeq Designer software (https://www.ampliseq.com, accessed March 16, 2017, Life Technologies). Sequencing was performed using an Ion Torrent platform (Life Technologies) according to the manufacturer’s protocol. Briefly, multiplex PCRs were performed using an Ion AmpliSeq Library Kit 2.0. Emulsion PCR was performed with an Ion OneTouch Instrument and an Ion PI Hi-Q OT2 200 Kit, and a template-positive Ion sphere was subsequently retrieved by Ion OneTouch ES. Sequencing was run on an Ion Torrent Proton Sequencer using an Ion PI Hi-Q Sequencing 200 Kit and Ion PI chip v3. The obtained sequence data were mapped onto the hg19 human reference genome sequence by Ion Torrent Suite software. Mapped data were imported to the Ion Reporter v4.6, and sequence variations with frequencies of 5% or more were identified. Only those variations with a read depth of above 100× were considered.
According to the method used by Shankar et al,16 tumor purity (PT/target) was further estimated from the allele frequency of the VHL alterations (AFVHL) in the 3 following scenarios: (i) if loss of heterozygosity (LOH) of chr. 3 represents a copy neutral event, then the tumor purity (PT/target) is given by the following equation: PT/target = AFVHL; (ii) if LOH of chr. 3 is a result of haploidization, then the tumor purity is given by: PT/target = 2 × AFVHL/(1 + AFVHL); (iii) if LOH of chr. 3 was not detected, then the tumor purity is given by: PT/target = 2 × AFVHL.
Single Nucleotide Polymorphism Arrays
Single nucleotide polymorphism (SNP) array analyses were performed with pairs of tumor DNA and blood DNA samples; the Genome-Wide Human SNP Arrays 6.0 (Affymetrix) were used according to the manufacturer’s instructions for these analyses. The Genome Imbalance Map algorithm was used to calculate allelic and total genome copy numbers.19
Multiplex Ligation-Dependent Probe Amplification
SALSA P016-C2 VHL probe kits (MRC-Holland) were used for multiplex ligation-dependent probe amplification (MLPA) analyses according to the manufacturer’s instructions. The P016-C2 kit contains 9 probes for VHL (4 in exon 1, 3 in exon 2, and 2 in exon 3), 6 probes for genes located on chr. 3p close to VHL, 2 probes for loci on chr. 3p that are telomeric or centromeric relative to VHL, and 12 reference probes for sequences on other chromosomes. Data were generated using GeneMapper Software v4.0 (Applied Biosystems). We determined VHL gene dosage with an Excel template offered by the manufacturer (MRC-Holland).20 The manufacturer’s instructions stated that when allele copy numbers (ACNs) are 1.7 to 2.3 for individual probes, the region is “normal diploid,” and when ACNs are 0.7 to 1.3 for 2 or more adjacent probes, the region has “heterozygous deletion.” Additionally, when ACNs are 1.3 to 1.7 for 2 or more adjacent probes, the region has “suspected heterozygous deletion.”
Methylation-Specific PCR
EZ DNA Methylation Kits (Zymo Research) were used according to the manufacturer’s instructions along with genomic DNA samples (250 ng/reaction) for bisulfite modification. For methylation-specific PCR (MSP), the following primers were used as described by Herman et al.21 Unmethylated specific primers (amplicon length: 165 bp): forward: 5′-GTT GGAGGATTTTTTTGTGTATGT-3′, reverse: 5′-CCCAAACCAA ACACCACAAA-3′. Methylated specific primers (amplicon length: 158 bp): forward: 5′-TGGAGGATTTTTTTGCGTA CGC-3′, reverse: 5′-GAACCGAACGCCGCGAA-3′. PCR was carried out with the following program: 37 cycles of denaturation at 95°C for 30 s, annealing at 59°C for 30 s, and extension at 72°C for 30 s. PCR products were then analyzed by electrophoresis through 1.5% agarose gels. CpG Methylated Jurkat Genomic DNA and 5-Aza dc Jurkat Genomic DNA (New England Biolabs) were used as positive and negative controls, respectively.
Bisulfite Sequencing
For bisulfite sequencing, bisulfite modification was performed as described by Nickerson et al, and the following primers were then used to amplify both methylated and unmethylated allele sites along the VHL promoter.22,23 Initial PCR primers (amplicon length: 335 bp): forward: 5′-TTAYGG AGGTYGATTYGGGAG-3′, reverse: 5′-ACRATTACAAAAAAT AACCTA-3′; nested PCR primers (amplicon length: 226 bp); forward: 5′-YGGGTGGTTTGGATYG-3′, reverse: 5′-AATTCAC CRAACRCAACA-3′. Initial PCR was carried out with the touchdown program with an annealing temperature decreasing from 56°C to 50°C. Nested PCRs were then performed with 1 µL of a 1:10 dilution of the first-round reaction and the same PCR program used for first-round amplification. Sequencing reactions were then performed. Based on sequence chromatograms from bisulfite sequencing, the cytosine at several cytosine-phosphate-guanine (CpG) sites remained a cytosine after bisulfite conversion; these findings indicated that the cytosine residue was methylated at each of these sites. Samples with at least 4 out of a total of 11 analyzed CpG sites were considered to be methylated. Determination of methylation status was performed with the online analysis tool QUMA (Quantification tool for Methylation Analysis; http://quma.cdb.riken.jp/top/quma_main.html, accessed March 16, 2017). As with MSP, CpG Methylated Jurkat Genomic DNA and 5-Aza dc Jurkat Genomic DNA were used as positive and negative controls, respectively, for bisulfite sequencing.
Statistical Analysis
Student’s t-tests (for age) or Fisher’s exact tests (for other factors) were used to assess differences between groups. JMP 11 (SAS Institute) was used for statistical analysis. A probability value <0.05 was considered significant.
Results
Clinical Characteristics of Patients with HB
The clinical characteristics of the patients are shown in Table 1. Consistent with previous findings,24 the median age of patients with VHL-related HB was significantly younger than that of patients with sporadic HB (30.1 vs 47.5 y; P = 0.0004, Student’s t-test). The majority of tumors were located in the cerebellum, and others were located in the brainstem or spinal cord.
Table 1.
Clinical characteristics of patients who provided samples
| 1 | VHL | Sporadic |
|---|---|---|
| Samples (patients) (n) | 11 (7) | 21 (21) |
| Male:female (n) | 9:2 (5:2) | 9:12 (9:12) |
| Mean age, y, a ± SD | 31 ± 11 | 48 ± 16 |
| Tumor location | ||
| Cerebellum | 9 | 16 |
| Brainstem | 2 | 3 |
| Spinal cord | 0 | 2 |
VHL: VHL-related HBs.
Sporadic: sporadic HBs.
aPatient’s age when the tumor specimens were resected.
Sequence Analysis Detected VHL Mutations and Small Frameshift Deletions
Results of the genetic analysis are shown in Tables 2 and 3. For the 32 HBs, direct sequencing analysis of VHL revealed alterations in 11 HBs from 9 patients. The rate of VHL alterations detected by direct sequencing was significantly higher for VHL-related HBs (7/11, 64%) than for sporadic HBs (4/21, 19%) (P = 0.020, Fisher’s exact test). Targeted deep sequencing of VHL was performed for 17 sporadic HB specimens in which VHL mutation was not detected by direct sequencing, along with 2 samples (S13 and S14) that showed VHL mutation by direct sequencing as positive controls. Of these 17 sporadic HBs, VHL alterations were revealed in 9 of them. Two different VHL alterations were found in 3 HBs (S1, S13, and S20). However, whether 2 alterations in the same sample existed in a single tumor cell was not clear; in other words, whether these 2 alterations caused biallelic VHL inactivation was not verified.
Table 2.
Results of genetic analysis of VHL-related hemangioblastomas
| VHL (ID) |
Age | Sex | Tumor Location |
VHL Mutation/Deletion (direct sequence/MLPA) |
Chr. 3 LOH (MLPA/SNP array) |
VHL Methylation | Other Chr. Abnormality (SNP array) |
Biallelic Inactivation |
|---|---|---|---|---|---|---|---|---|
| V1-1 | 22 | M | Cerebellum | c.216delC | Chr. 3 LOH | — | — | Yes |
| −2 | 24 | M | Cerebellum | c.216delC | — | — | — | |
| V2-1 | 30 | M | Brainstem | c.216delC | Chr. 3p cnLOH | — | — | Yes |
| V3-1 | 29 | F | Cerebellum | exon 3 deletion a | Chr. 3 LOH | — | 22− | Yes |
| V4-1 | 23 | M | Brainstem | exon 3 deletion a | Chr. 3p cnLOH | — | — | Yes |
| −2 | 23 | M | Cerebellum | exon 3 deletion a | Chr. 3 cnLOH | — | — | Yes |
| −3 | 27 | M | Cerebellum | exon 3 deletion a | Chr. 3p LOH | — | — | Yes |
| V5-1 | 35 | M | Cerebellum | c.329A>C | — | — | — | |
| −2 | 47 | M | Cerebellum | c.329A>C | — | — | — | |
| V6-1 | 52 | M | Cerebellum | IVS2-1G>T | — | — | — | |
| V7-1 | 19 | F | Cerebellum | c.194C>T | Chr. 3 LOH | — | — | Yes |
cnLOH: copy-neutral LOH, p: short arm of chromosome
VHL (ID): sample identification, for example, “V1” refers to patient 1 who provided 2 samples, V1-1 and V1-2.
Sample V1 and V2, as well as V3 and V4, each represent specimens from pairs of siblings that belonged to one family.
a The sample with a deletion in exon 3 of VHL detected by MLPA analysis.
Table 3.
Results of genetic analysis of sporadic hemangioblastomas
| Sporadic (ID) |
Age | Sex | Tumor Location |
VHL Mutation/Deletion (direct sequence/targeted deep sequence) |
Chr. 3 LOH (MLPA/SNP array) |
VHL Methylation | Other chr. abnormality (SNP array) |
Biallelic inactivation |
|---|---|---|---|---|---|---|---|---|
| S1 | 50 | M | Cerebellum | c.309_310insGGCACGGGCC a & c.497T>G a |
— | — | 6− | |
| S2 | 40 | F | Spinal cord | — | Chr. 3 LOH | — | — | |
| S3 | 68 | F | Cerebellum | c.331A>G a | — | — | 4 + 6− 10− | |
| S4 | 26 | F | Cerebellum | c.326T>G a | Chr. 3 LOH | — | 6− 18− | Yes |
| S5 | 57 | M | Cerebellum | c.499C>T a | — | M | 6− 10− | Yes |
| S6 | 20 | M | Brainstem | — | Chr. 3 cnLOH | M | — | Yes |
| S7 | 65 | M | Cerebellum | c.402delA | — | — | 6− 9− 10− | |
| S8 | 60 | F | Cerebellum | — | — | — | 5 + 6+ 18+ | |
| S9 | 52 | M | Cerebellum | c.444delT | — | M d | 6− | Yes |
| S10 | 57 | F | Cerebellum | — | Chr. 3 LOH c | — | — | |
| S11 | 50 | F | Cerebellum | — | Chr. 3 LOH | — | 13− 18− | |
| S12 | 42 | M | Brainstem | c.286C>T a | Chr. 3 LOH | — | — | Yes |
| S13 | 57 | F | Cerebellum | c.326T>G a & c.370-371ACdel b | — | M | — | Yes |
| S14 | 73 | F | Cerebellum | c.551T>C b | Chr. 3 LOH c | — | Multiple e | Yes |
| S15 | 45 | F | Cerebellum | c.341G>C a | Chr. 3 LOH c | M | — | Yes |
| S16 | 37 | M | Cerebellum | — | Chr. 3 LOH | — | 4 + 6− | |
| S17 | 35 | F | Spinal cord | — | Chr. 3p cnLOH | M d | — | Yes |
| S18 | 32 | M | Cerebellum | — | Chr. 3 LOH c | — | — | |
| S19 | 38 | F | Brainstem | c.463 + 1C>G a | Chr. 3 LOH c | — | — | Yes |
| S20 | 23 | M | Cerebellum | c.194C>A & c.458T>G a | — | M | — | Yes |
| S21 | 70 | F | Cerebellum | c.525C>G a | — | — | 10q− |
cnLOH: copy-neutral LOH, p: short arm of chromosome, q: long arm of chromosome.
a VHL mutation/deletion detected only by targeted deep sequencing.
b VHL mutation/deletion detected by both direct sequencing and targeted deep sequencing.
c The samples whose chr. 3 LOH were detected by MLPA analysis as well as by SNP array analysis.
d The samples with VHL promoter hypermethylation detected also by MSP, while all promoter hypermethylation were consistently detected by bisulfite sequencing.
e Sample S14 had multiple LOHs (1p−, 6−, and 9−) and gains (5+, 7+, 13+, and 20+).
In the “other chr. abnormality” column, “6−” means LOH of chromosome 6, “4+” means gain of chromosome 4.
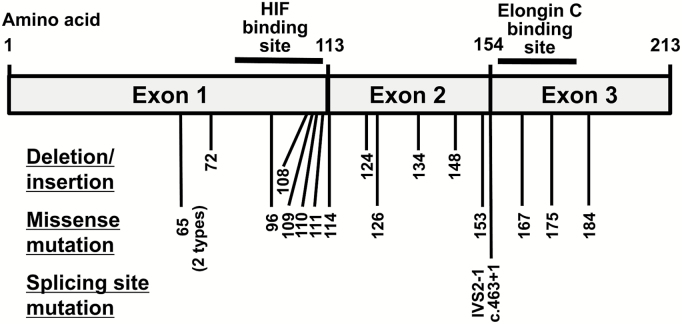
The positions of VHL alterations identified in our study are summarized in Fig. 1. All identified VHL alterations were previously reported in the Catalogue of Somatic Mutations in Cancer (COSMIC, http://cancer.sanger.ac.uk/cosmic, accessed March 16, 2017). Of the 11 VHL-related HBs, 7 (67%) had VHL sequence alterations: 2 types of missense alterations (c.329A>C or c.194C>T) in 3 samples, a splice site mutation (IVS2-1G>T) in 1, and c.216delC deletions in 3. Two HBs from one patient with VHL disease had the same c.329A>C mutation. Identical single base-pair deletions were found in 3 HBs from 2 patients with VHL disease from the same family, indicating that this small frameshift deletion was a germline mutation. Of the 21 sporadic HBs, 13 (62%) had VHL sequence alterations; 3 types of small deletions (c.402delA, c.444delT, or c.370-371ACdel) in 3 samples, a 10-bp insertion (c.309_310insGGCACGGGCC) in 1, a splice site mutation in 1, and 10 types of missense alterations in 10.
Fig. 1.
The positions of VHL mutations and deletions identified by sequencing in our HBs.
Results of tumor purity calculated by targeted deep sequencing are shown in Supplementary Table S1. The mean read depth of VHL by targeted deep sequencing was 64794×. The mean allele frequency of VHL alterations was 21.5% (range, 11.82%‒38.98%), and the estimated mean tumor purity calculated based on AFVHL and the status of chr. 3 LOH was 39.4% (range, 23.64%‒71.14%). For VHL alterations detected by both direct sequencing and targeted deep sequencing, AFVHL values were 14.45% and 38.98% in S13 and S14, respectively.
SNP Array Detected a Difference Between VHL-Related and Sporadic HBs with Regard to Copy Number Abnormalities
All results of the SNP array analysis are shown in Supplementary Figure S1. Among the 32 HBs, loss of 1 of 2 copies of chr. 3 or 3p was found in 14 (44%) samples (Tables 2 and 3). In addition, copy-neutral LOHs that denote LOH without copy number loss on chr. 3 or 3p were identified in 5 other cases (Tables 2 and 3). In total, 19 (59%) HBs had LOH of chr. 3 or 3p.The incidences of chr. 3 and 3p LOH were similar between VHL-related and sporadic HBs: in VHL-related HBs, 7 of 11 (64%) samples had LOH on chr. 3 or 3p, and in sporadic HBs, 12 of 21 (57%) samples had LOH on chr. 3 or 3p (Table 4). In contrast, incidences of chr. 6 or 10 LOH were significantly higher in sporadic HB than in VHL-related HB (43% vs 0%, P = 0.013, Fisher’s exact test) (Tables 2 and 3).
Table 4.
Frequency of VHL alterations and chromosome 3 LOH
| VHL | Sporadic | P-value | |
|---|---|---|---|
| VHL mutation/small deletion | 11/11 (100%) | 13/21 (62%) | .029a |
| Chr. 3 LOH | 7/11 (64%) | 12/21 (57%) | 1 |
| VHL promoter methylation | 0/11 (0%) | 7/21 (33%) | .066 |
| VHL biallelic inactivation | 7/11 (64%) | 11/21 (52%) | .71 |
VHL: VHL-related HBs. Sporadic: sporadic HBs.
a P < 0.05: Fisher’s exact test was used for statistical analysis.
MLPA Analysis Detected VHL Intragenic Deletions and Losses of Chromosome 3
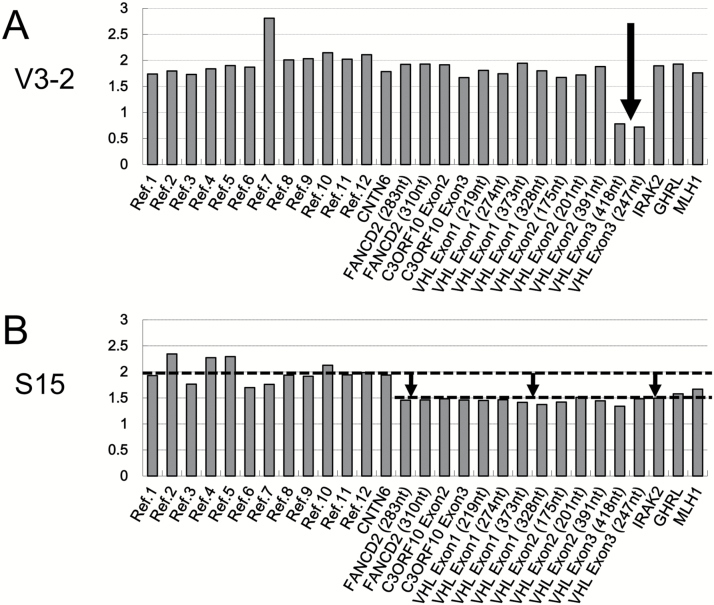
Of the 11 VHL-related HBs, we could not find any VHL mutations or small deletions by direct sequencing in 4 specimens. However, MLPA analysis revealed that these 4 had intragenic deletions within VHL exon 3 (Fig. 2A). These intragenic deletions could not be detected by our SNP array analysis because no corresponding SNP probe was present on the arrays for this region. These 4 HBs were from 2 patients from the same family, and therefore the deletion was a germline alteration. In summary, the sequencing and MLPA analysis detected VHL mutation/deletion more frequently in VHL-related HBs than in sporadic HBs (100% vs 62%; P = 0.029, Fisher’s exact test) (Table 4).
Fig. 2.
Representative results of MLPA analysis. Each probe is represented on each x-axis. On the right side of each x-axis, the probes for VHL sites and adjacent regions (eg, FANCD, IRAK2) are arranged according to their order on chr. 3. Reference probes (eg, Ref. [1], which target sites expected to be present in 2 copies, are located on the left side of each x-axis. Each y-axis represents the estimated copy number. (A) A representative sample with a deletion in exon 3 of VHL. (B) A representative sample that had an ambiguous lesion, including a possible deletion within VHL.
Based on SNP array data, more than half of all HBs (sporadic or disease related) exhibited chr. 3 LOH (19/32, 59%). However, MLPA analysis could detect the chr. 3 loss in only 5 HBs (5/32, 16%, Tables 2 and 3). Furthermore, MLPA data regarding chr. 3 losses were ambiguous; specifically, the average measured copy number for probes on chr. 3 involving VHL was approximately 1.5 based on MLPA, but did not approach 1.0, which is the expected value for chromosome segments reduced to 1 copy from 2 copies due to chromosomal loss. A representative MLPA result indicating chr. 3 loss is shown in Fig. 2B. Overall, the MLPA analysis was not sufficient to detect the various patterns of LOH in chr. 3 (Tables 2 and 3). Only 26% of samples with chr. 3 LOH detected with SNP arrays were determined to have LOH with MLPA analysis.
VHL Promoter Hypermethylation Was Detected Only in Sporadic HBs
In VHL-related HBs, no case with VHL promoter methylation was detected (Table 2). In contrast, VHL promoter hypermethylation was detected by MSP in 2 of the 21 sporadic HBs (6.3%) (Fig. 3A); while on bisulfite sequencing, 7 (33%) sporadic HBs were demonstrated to have VHL promoter hypermethylation, including the 2 cases (samples S9 and S17) also detected by MSP technique (Fig. 3B) (Table 3). Therefore, the bisulfite sequencing is a more sensitive method to detect promoter methylation. VHL promoter hypermethylation was more frequent in sporadic HBs, although no statistically significant difference between these groups was observed (Table 4).
Fig. 3.
The methylation status of the VHL promoter in HBs. (A) Results of electrophoresis following MSP. A band representing the PCR product of methylated-specific primers was detected in samples S9 and S17. (B) The results of the analysis of methylation at all analyzed CpG sites. Black ovals represent methylated CpG sites, and white ovals represent unmethylated CpG sites. Methylation was identified in 7 samples. *These samples exhibited methylation of the VHL promoter based on MSP data.
Biallelic Inactivation of VHL
Here, we defined biallelic inactivation of VHL (2 VHL hits) as the presence of at least 2 of these 3 alterations (intragenic mutation of VHL, chr. 3 LOH involving VHL, or VHL promoter methylation) (Tables 2 and 3). As shown in Table 4, the rates of biallelic VHL inactivation were similar between VHL-related and sporadic HBs (64% vs 52%, P = 0.71, Fisher’s exact test). Of the 21 sporadic HBs, 5 had chr. 3p LOH with neither VHL alterations nor VHL promoter methylation.
Discussion
In this study, the rates of verified biallelic VHL inactivation in VHL-related HBs and sporadic HBs were 64% and 52%, respectively, confirming that VHL inactivation is probably the most important mechanism for tumorigenesis of both VHL-related and sporadic HBs, as previously reported for VHL-related and sporadic ccRCC, although the frequency of biallelic VHL inactivation among HBs was not as high as that among ccRCC.12,25 In addition, we observed notable differences between VHL-related and sporadic HB with regard to the patterns of genetic or epigenetic alterations, although there were statistical limitations in comparing these 2 groups due to the potential similarity among VHL-related HBs, since some of the samples were collected from the same patient or siblings. In VHL-related HBs, all samples had mutations or intragenic deletions of VHL. In contrast, sporadic HBs had VHL mutation/deletion at an apparently lower frequency. On the other hand, VHL promoter hypermethylation was observed only in sporadic HBs, and epigenetic suppression of VHL expression may contribute to VHL inactivation in the sporadic tumors. In addition, the higher frequencies of LOH on chr. 6 and 10 in sporadic HBs suggested that HB-causative genes besides VHL reside on these chromosomes.
By direct sequencing, VHL alterations were detected in only 4 of 21 sporadic HBs, a rate similar to that in the previous studies.14,15 Here, we could detect VHL alterations in 9 additional sporadic HBs by targeted deep sequencing. Shankar et al16 also reported that exome sequence analysis and target sequence analysis could detect additional VHL alterations at a similar rate. One possible reason for such a low mutation detection rate with direct sequencing may be the low tumor cell content of HBs, which typically have dense vascular networks containing numerous vasculature and blood cells. However, mutant allele frequencies of VHL were not necessarily lower in samples in which VHL alteration was detected only by targeted deep sequencing in our analysis.
The sensitivity of the SNP array to detect copy number abnormalities in HBs was higher than that of MLPA; this difference may be due to the fact that SNP arrays have more probes than MLPA, and because the computational statistical analysis of more probes on chr. 3 with SNP arrays could detect small copy number differences between tumor samples and normal cells. The actual copy number of each LOH region within a tumor was prone to be obscured by the diploid copy number of contaminating normal cells, and the measured ratios would be higher than 1.0 for these mixed cell populations.
By using SNP array analysis that can measure allele-specific copy numbers, we could identify copy-neutral LOH in HB. Because the copy number is normal (2 copies) at these sites of copy-neutral LOH, neither MLPA nor comparative genomic hybridization analysis, which were the methods used for most previous studies, could be used to identify this type of copy number abnormality. Copy-neutral LOH has been shown to occur frequently in various types of tumors.26–28 Recently, Shankar et al reported that copy-neutral LOH of chr. 3 was present in 2 of 10 sporadic HBs.16 Our result is concordant with this finding, and together these findings suggest that copy-neutral LOH, along with LOH with accompanying copy number loss, contributes prominently to the biallelic inactivation of VHL. Although we found additional cases with copy-neutral 3p LOH, the overall frequency of chr. 3p LOH was approximately 60% for both VHL-related and sporadic HBs; this rate is lower than that for ccRCCs, which reportedly have rates of chr. 3p LOH between 80% and 90%.12,25 To be noted is that the findings from SNP array analysis of different tumors from the same VHL disease patient were not always identical. For example, samples V4-1, V4-2, and V4-3 were from the same patient, and SNP array results for these samples were (i) copy-neutral LOH of chr. 3p, (ii) copy-neutral LOH of chr. 3, and (iii) copy number loss of chr. 3, respectively. These findings confirmed that the second VHL hits are different between tumors from the same patient with VHL disease.
Notably, VHL promoter methylation was identified only in sporadic HBs (7/21 cases; 33%), suggesting that epigenetic suppression of VHL may be relatively common in sporadic HBs. However, the frequency and functional consequences of these promoter methylation events, especially those detected by bisulfite sequencing, have not been clearly documented. Consistent with our results, findings from Prowse et al indicate VHL promoter hypermethylation in 2 of 5 HBs based on methylation-sensitive restriction enzyme analysis.29 However, Gläsker et al used the same method as Prowse and colleagues and did not find any VHL promoter hypermethylation in 41 HB samples.14 Muscarella et al also reported that none of the 18 samples they examined had VHL promoter hypermethylation based on MSP.15 The reason for such inconsistency in these results is unclear. The inconsistency between our findings from MSP and bisulfite sequencing may have been caused by differences in the locations of the targeted CpG sites; as shown at the top of Fig. 3B, the target CpG sites of bisulfite sequencing were located on the 3′ side of the minimal promoter. Although the target CpG sites of MSP primers included the established minimal promoter,30 whether the CpG sites recognized by each type of analysis technology are really related to suppression of the VHL promoter has not been well studied. Besides promoter methylation, VHL expression can be downregulated by other mechanisms; for example, microRNA may bind to VHL and decrease its expression, as reported in a leukemia cell line.31
Another possible explanation for less frequent mutation or deletion of VHL alterations in sporadic HBs is the existence of causative gene alterations other than VHL in these sporadic tumors. Several recent studies with whole-genome and whole-exome sequencing analysis have identified several novel candidate genes that may be causative for ccRCCs. These candidates include polybromo 1 (PBRM1), BRCA-1 associated protein 1 (BAP1), and SET domain containing 2 (SETD2),32–34 which are all located on chr. 3p, indicating that several novel putative HB-causative genes are located on chr. 3p. However, few reports have yet to comprehensively analyze genomic alterations of HB.35–38 SNP array data also demonstrated that 43% of sporadic HBs had LOH on chr. 6 or 10. Four of the 21 sporadic HBs in this study (Table 3) had LOH on chr. 10 or 10q; and a gene encoding a well-known tumor suppressor, phosphatase and tensin homolog (PTEN), resides on chr. 10q. Frew et al report that combined conditional inactivation of VHL and PTEN in the mouse kidney facilitates formations of kidney cysts, which are thought to be precancerous lesions.39 Therefore, PTEN may also play some role in HB tumorigenesis. Notably, of the 9 sporadic HBs that lacked chr. 3 LOH, 6 (67%) had LOH on chr. 6, chr. 10, or both. These results were in accordance with a previous study on 16 sporadic HBs and 7 VHL-related HBs that were screened for copy number abnormalities via comparative genomic hybridization.36 This study showed that 10 sporadic HBs (63%) had chr. 3 LOH, 7 (44%) had chr. 6 LOH, 3 (19%) had chr. 10 LOH, and none of the samples with LOH on chr. 6 or 10 were VHL-related HBs. These previous results together with our findings may indicate that chr. 6 or chr. 10 LOH is characteristic of sporadic HBs and that tumorigenesis of sporadic HBs may be through an alternative pathway involving tumor suppressor genes other than VHL that reside on these chromosomes.
In conclusion, the results of the present study indicated that alternative pathways may exist for tumorigenesis of sporadic HB. Whether such difference in the tumorigenic mechanism among hemangioblastomas would require a different approach in the management of the affected patients is an interesting question that should be answered in the future studies.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was supported by the Japan Society for the Promotion of Science KAKENHI [grant numbers JP15K19950 to S.T. and JP26670636 to A.M].
Supplementary Material
Acknowledgments
The authors thank Reiko Matsuura for extracting DNA from blood samples and Hiroko Meguro for assisting with SNP array analyses. A part of the study was presented as a poster at the 21st Annual Meeting of the Society for Neuro-Oncology.
Conflict of interest statement. Koichi Ichimura has received research funding from Chugai Pharmaceutical Co., Ltd. and EPS Co., Ltd.
References
- 1. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, ed WHO Classification of Tumours of the Central Nervous System. 4th ed Lyon: International Agency for Research on Cancer; 2007. [Google Scholar]
- 2. Neumann HP, Eggert HR, Weigel K, Friedburg H, Wiestler OD, Schollmeyer P. Hemangioblastomas of the central nervous system. A 10-year study with special reference to von Hippel–Lindau syndrome. J Neurosurg. 1989;70(1):24–30. [DOI] [PubMed] [Google Scholar]
- 3. Vortmeyer AO, Gnarra JR, Emmert-Buck MR, et al. von Hippel–Lindau gene deletion detected in the stromal cell component of a cerebellar hemangioblastoma associated with von Hippel–Lindau disease. Hum Pathol. 1997;28(5):540–543. [DOI] [PubMed] [Google Scholar]
- 4. Neumann HP, Bender BU. Genotype-phenotype correlations in von Hippel–Lindau disease. J Intern Med. 1998;243(6):541–545. [DOI] [PubMed] [Google Scholar]
- 5. Maher ER, Kaelin WG., Jr Von Hippel–Lindau disease. Medicine (Baltimore). 1997;76(6):381–391. [DOI] [PubMed] [Google Scholar]
- 6. Takai K, Taniguchi M, Takahashi H, Usui M, Saito N. Comparative analysis of spinal hemangioblastomas in sporadic disease and von Hippel–Lindau syndrome. Neurol Med Chir (Tokyo). 2010;50(7):560–567. [DOI] [PubMed] [Google Scholar]
- 7. Hanakita S, Koga T, Shin M, et al. The long-term outcomes of radiosurgery for intracranial hemangioblastomas. Neuro Oncol. 2014;16(3):429–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel–Lindau disease tumor suppressor gene. Science. 1993;260(5112):1317–1320. [DOI] [PubMed] [Google Scholar]
- 9. Kanno H, Kondo K, Ito S, et al. Somatic mutations of the von Hippel–Lindau tumor suppressor gene in sporadic central nervous system hemangioblastomas. Cancer Res. 1994;54(18):4845–4847. [PubMed] [Google Scholar]
- 10. Iliopoulos O, Levy AP, Jiang C, Kaelin WG, Jr, Goldberg MA. Negative regulation of hypoxia-inducible genes by the von Hippel–Lindau protein. Proc Natl Acad Sci U S A. 1996;93(20):10595–10599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maxwell PH, Wiesener MS, Chang GW, et al. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399(6733):271–275. [DOI] [PubMed] [Google Scholar]
- 12. Sato Y, Yoshizato T, Shiraishi Y, et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet. 2013;45(8):860–867. [DOI] [PubMed] [Google Scholar]
- 13. Knudson AG.Jr. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68(4):820–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gläsker S, Bender BU, Apel TW, et al. Reconsideration of biallelic inactivation of the VHL tumour suppressor gene in hemangioblastomas of the central nervous system. J Neurol Neurosurg Psychiatry. 2001;70(5):644–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muscarella LA, la Torre A, Faienza A, et al. Molecular dissection of the VHL gene in solitary capillary hemangioblastoma of the central nervous system. J Neuropathol Exp Neurol. 2014;73(1):50–58. [DOI] [PubMed] [Google Scholar]
- 16. Shankar GM, Taylor-Weiner A, Lelic N, et al. Sporadic hemangioblastomas are characterized by cryptic VHL inactivation. Acta Neuropathol Commun. 2014;2:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lonser RR, Glenn GM, Walther M, et al. Von Hippel–Lindau disease. Lancet. 2003;361(9374):2059–2067. [DOI] [PubMed] [Google Scholar]
- 18. Weil RJ, Vortmeyer AO, Zhuang Z, et al. Clinical and molecular analysis of disseminated hemangioblastomatosis of the central nervous system in patients without von Hippel–Lindau disease. Report of four cases. J Neurosurg. 2002;96(4):775–787. [DOI] [PubMed] [Google Scholar]
- 19. Komura D, Shen F, Ishikawa S, et al. Genome-wide detection of human copy number variations using high-density DNA oligonucleotide arrays. Genome Res. 2006;16(12):1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cho HJ, Ki CS, Kim JW. Improved detection of germline mutations in Korean VHL patients by multiple ligation-dependent probe amplification analysis. J Korean Med Sci. 2009;24(1):77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herman JG, Graff JR, Myöhänen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A. 1996;93(18):9821–9826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nickerson ML, Jaeger E, Shi Y, et al. Improved identification of von Hippel–Lindau gene alterations in clear cell renal tumors. Clin Cancer Res. 2008;14(15):4726–4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moore LE, Nickerson ML, Brennan P, et al. Von Hippel–Lindau (VHL) inactivation in sporadic clear cell renal cancer: associations with germline VHL polymorphisms and etiologic risk factors. PLoS Genet. 2011;7(10):e1002312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wanebo JE, Lonser RR, Glenn GM, Oldfield EH. The natural history of hemangioblastomas of the central nervous system in patients with von Hippel–Lindau disease. J Neurosurg. 2003;98(1):82–94. [DOI] [PubMed] [Google Scholar]
- 25. Shuib S, Wei W, Sur H, et al. Copy number profiling in von Hippel–Lindau disease renal cell carcinoma. Genes Chromosomes Cancer. 2011;50(7):479–488. [DOI] [PubMed] [Google Scholar]
- 26. Fitzgibbon J, Smith LL, Raghavan M, et al. Association between acquired uniparental disomy and homozygous gene mutation in acute myeloid leukemias. Cancer Res. 2005;65(20):9152–9154. [DOI] [PubMed] [Google Scholar]
- 27. Kuga D, Mizoguchi M, Guan Y, et al. Prevalence of copy-number neutral LOH in glioblastomas revealed by genomewide analysis of laser-microdissected tissues. Neuro Oncol. 2008;10(6):995–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tuna M, Knuutila S, Mills GB. Uniparental disomy in cancer. Trends Mol Med. 2009;15(3):120–128. [DOI] [PubMed] [Google Scholar]
- 29. Prowse AH, Webster AR, Richards FM, et al. Somatic inactivation of the VHL gene in von Hippel–Lindau disease tumors. Am J Hum Genet. 1997;60(4):765–771. [PMC free article] [PubMed] [Google Scholar]
- 30. Kuzmin I, Duh FM, Latif F, Geil L, Zbar B, Lerman MI. Identification of the promoter of the human von Hippel–Lindau disease tumor suppressor gene. Oncogene. 1995;10(11):2185–2194. [PubMed] [Google Scholar]
- 31. Ghosh AK, Shanafelt TD, Cimmino A, et al. Aberrant regulation of pVHL levels by microRNA promotes the HIF/VEGF axis in CLL B cells. Blood. 2009;113(22):5568–5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Varela I, Tarpey P, Raine K, et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature. 2011;469(7331):539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peña-Llopis S, Vega-Rubín-de-Celis S, Liao A, et al. BAP1 loss defines a new class of renal cell carcinoma. Nat Genet. 2012;44(7):751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dalgliesh GL, Furge K, Greenman C, et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature. 2010;463(7279):360–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sprenger SH, Gijtenbeek JM, Wesseling P, et al. Characteristic chromosomal aberrations in sporadic cerebellar hemangioblastomas revealed by comparative genomic hybridization. J Neurooncol. 2001;52(3):241–247. [DOI] [PubMed] [Google Scholar]
- 36. Gijtenbeek JM, Jacobs B, Sprenger SH, et al. Analysis of von Hippel-Lindau mutations with comparative genomic hybridization in sporadic and hereditary hemangioblastomas: possible genetic heterogeneity. J Neurosurg. 2002;97(4):977–982. [DOI] [PubMed] [Google Scholar]
- 37. Lemeta S, Aalto Y, Niemelä M, et al. Recurrent DNA sequence copy losses on chromosomal arm 6q in capillary hemangioblastoma. Cancer Genet Cytogenet. 2002;133(2):174–178. [DOI] [PubMed] [Google Scholar]
- 38. Lemeta S, Pylkkänen L, Sainio M, et al. Loss of heterozygosity at 6q is frequent and concurrent with 3p loss in sporadic and familial capillary hemangioblastomas. J Neuropathol Exp Neurol. 2004;63(10):1072–1079. [DOI] [PubMed] [Google Scholar]
- 39. Frew IJ, Thoma CR, Georgiev S, et al. pVHL and PTEN tumour suppressor proteins cooperatively suppress kidney cyst formation. EMBO J. 2008;27(12):1747–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.