Glioblastoma (GBM) is the most common adult brain tumor and the most aggressive of all gliomas. Despite maximum surgical resection followed by chemoradiation, the median survival of patients with GBM remains dismal at 12–15 months. High-throughput pancancer initiatives such as The Cancer Genome Atlas have helped us gain an increased understanding of the evolution and molecular landscape of GBM. This knowledge must ultimately be put to clinical use in order to improve outcome in patients. In this issue, Wu et al demonstrate how data mined from publicly available datasets combined with molecular docking can be used to understand the biology and targeting of microRNA-155 (miR-155).
Noncoding RNAs, including miRNAs, piwiRNAs, and long noncoding RNAs (lncRNAs), are non-protein encoding transcripts that play essential functions in gene transcription, epigenetic regulation, and protein translation. Previous studies have demonstrated functional interactions between lncRNAs and miRNAs, wherein the stability of lncRNAs is reduced through interaction with miRNA, or lncRNAs act as decoys for miRNA binding. Alternatively, some lncRNAs themselves encode for miRNAs. Despite the high expression of lncRNAs in the central nervous system and their role in neural development, there are very few studies on the role of lncRNAs in gliomas.1–3 Previous studies involving miRNAs and lncRNAs from publicly available datasets have primarily used survival as the endpoint to identify miRNAs that associate with poor survival in GBM patients.4 Wu et al examined the expression of miR-155 and its host gene the lncRNA MIR155HG in gliomas.5 MiR-155 is a multifunctional miRNA with varied roles in immunity, inflammation, and cardiovascular diseases.6 It has also been shown to be overexpressed in numerous cancers, but the exact mechanism by which it promotes oncogenic functions is only beginning to be characterized.
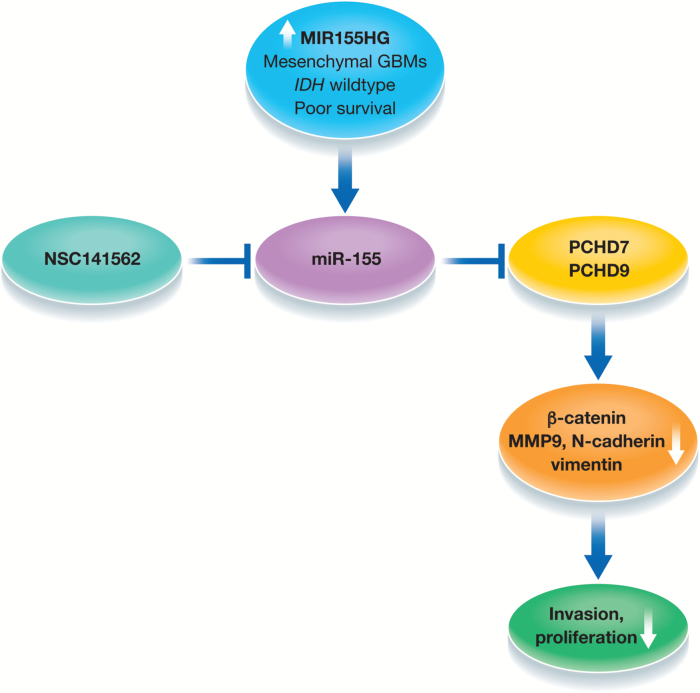
In this context, Wu et al first establish that MIR155HG is highly expressed in GBM compared with low-grade gliomas and normal brain using data mined from the Chinese Glioma Genome Atlas (CGGA).5 The higher expression of MIR155HG was verified in an independent manner using the Repository of Molecular Brain Neoplasia Data sets. Furthermore, MIR155HG expression was significantly associated with isocitrate dehydrogenase wild-type status, an unfavorable prognosis marker in glioma. Next, the authors correlated MIR155HG with gene expression patterns and found enrichment for genes involved in extracellular matrix and response to wounding in those with higher expression of MIR155HG. Analyses of mesenchymal genes (POST, MMP11, and FN1) by quantitative PCR confirmed the in silico findings that MIR155HG is upregulated in mesenchymal GBMs. Mechanistically, inhibition of MIR155HG using small interfering RNA approaches in established glioma cell lines as well as primary GBM cells suppressed invasion and proliferation and significantly improved survival by reducing tumor burden in xenograft-bearing mice. Importantly, silencing MIR155HG reduced expression of β-catenin, N-cadherin, vimentin, and matrix metalloproteinase 9, established markers of mesenchymal transition. Since MIR155HG is a primary precursor for miR-155, the authors analyzed the expression of miR-155 in the same datasets and found a positive correlation between MIR155HG and miR-155-5p/3p. Only miR-155-5p showed significant correlation with poor survival outcome. Overexpression of miR-155-5p/3p with concomitant inhibition of MIR155HG reversed the growth-promoting and invasive phenotypes, suggesting MIR155HG primarily executes its functions via miR-155-5p/3p. Several previous studies and the current study have now established miR-155-5p/3p as first executors of MIR155HG.6
Given the myriad of gene targets that miRNAs are known to suppress, the authors used bioinformatic approaches and found previously unrecognized mRNAs, protocadherin (PCDH) 9 and PCDH7, as targets of miR-155-5p/3p. PCDH7 and PCDH9 belong to a large subgroup within the cadherin superfamily, which are predominantly expressed in the nervous system.7 Previous studies have shown miR-155 targets numerous genes, such as FOXO3a, MXI1, and HBP1, but none of these have known roles in mesenchymal transition, which in essence makes the authors’ choice of PCDH7 and PCDH9 for further characterization interesting. Bioinformatic analysis in CGGA datasets showed a negative correlation between miR-155-5p/3p and PCDH9 and PCDH7 expression. Conversely, inhibition of miR-155-5p led to an increase in PCDH9 and PCDH7 at protein levels, further establishing PCDH9 and PCDH7 as primary targets of miR-155-5p/3p. Overexpression of PCDH9 rescued the miR-155-5p mediated cell proliferation, invasion, and migration phenotypes. Importantly, concomitant overexpression of miR-155-5p/3p and PCDH9/7 rescued the expression of β-catenin and cyclin D1, a known target of the β-catenin pathway. Collectively, the authors establish the importance of the miR-155–PCDH9/7–β-catenin axis in GBM (Figure 1).
Fig. 1.
Strategies for microRNA manipulation include anti-miRNA oligonucleotides (antagomirs), sponges, and anti-miR peptides, which have been shown to be effective in downregulating miRNA functions in animal models.8 However, many of these approaches have yet to reach the clinic, and the considerable costs associated with development of these agents and the potential side effects that can be induced by delivery of these agents remain major challenges. To overcome this, the authors take an interesting and alternative approach.5 Using high-throughput screening based on the 3D structure of the Dicer binding site on pre–miR-155, the authors screened for small molecules targeting miR-155. They conduct molecular docking studies on the National Cancer Institute’s Diversity Set, which consists of compounds with unique pharmacophores. The authors identified 3 compounds that had the highest predicted affinity for pre–miR-155, of which NSC141562 had the greatest inhibitory effects on cell growth, as well as causing induction of PCDH7/9 and downregulation of β-catenin.
The current study raises several interesting questions relevant to targeting MIR155HG in GBM. First, PCDHs have been demonstrated to have tumor suppressor functions.9 Are PCDH7 and 9 the prototypical PCDHs in GBM that play tumor suppressor functions and are under genetic regulation by miR-155? Second, does silencing MIR155 cause global alterations in mesenchymal signatures and does this involve establishing master regulators of the mesenchymal subtype, STAT3 (signal transducer and activator of transcription 3), C/EBP-β (CCAAT-enhancer binding homologous protein beta), and TAZ (transcriptional coactivator with PDZ-binding motif)?10 Third, can NSC141562 inhibit growth of gliomas in vivo by crossing the blood–brain barrier? And finally, does NSC141562 exert its growth inhibitory properties via inhibition of MIR155 or does it target other miRNAs or proteins? This cannot be ruled out completely given that NSC141562 was one of the 6 compounds recently shown to interact with H1N1 pandemic neuraminidase using similar docking approaches.11 Nevertheless, this study is an example of the power of integrating genomic data with biology. Like an increasing number of studies of lncRNAs and miRNAs in cancer, similar approaches to identify small molecule inhibitors against this class of RNAs may present unprecedented opportunities to develop effective therapies.
References
- 1. Briggs JA, Wolvetang EJ, Mattick JS, Rinn JL, Barry G. Mechanisms of long non-coding RNAs in mammalian nervous system development, plasticity, disease, and evolution. Neuron. 2015;88(5):861–877. [DOI] [PubMed] [Google Scholar]
- 2. Shi J, Dong B, Cao J et al. . Long non-coding RNA in glioma: signaling pathways. Oncotarget. 2017;8(16):27582–27592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gao YF, Wang ZB, Zhu T et al. . A critical overview of long non-coding RNA in glioma etiology 2016: an update. Tumour Biol. 2016;37(11):14403–14413. [DOI] [PubMed] [Google Scholar]
- 4. Srinivasan S, Patric IR, Somasundaram K. A ten-microRNA expression signature predicts survival in glioblastoma. PLoS One. 2011;6(3):e17438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu X, Wang Y, Yu T, et al. . Blocking MIR155HG/miR-155 axis inhibits mesenchymal transition in glioma. Neuro Oncol. 2017; 19(9): 1195–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elton TS, Selemon H, Elton SM, Parinandi NL. Regulation of the MIR155 host gene in physiological and pathological processes. Gene. 2013;532(1):1–12. [DOI] [PubMed] [Google Scholar]
- 7. Hirayama T, Yagi T. Clustered protocadherins and neuronal diversity. Prog Mol Biol Transl Sci. 2013;116:145–167. [DOI] [PubMed] [Google Scholar]
- 8. Lundin KE, Gissberg O, Smith CI. Oligonucleotide therapies: the past and the present. Hum Gene Ther. 2015;26(8):475–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang C, Tao B, Li S et al. . Characterizing the role of PCDH9 in the regulation of glioma cell apoptosis and invasion. J Mol Neurosci. 2014;52(2):250–260. [DOI] [PubMed] [Google Scholar]
- 10. Bhat KP, Balasubramaniyan V, Vaillant B et al. . Mesenchymal differentiation mediated by NF-κB promotes radiation resistance in glioblastoma. Cancer Cell. 2013;24(3):331–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mai BK, Viet MH, Li MS. Top leads for swine influenza A/H1N1 virus revealed by steered molecular dynamics approach. J Chem Inf Model. 2010;50(12):2236–2247. [DOI] [PubMed] [Google Scholar]