Abstract
Background.
Glioma comprises a heterogeneous group of mostly malignant brain tumors, whereof glioblastoma (GBM) represents the largest and most lethal subgroup. Body height and body mass index (BMI) are risk factors for other cancers, but no previous study has examined anthropometric data in relation to different glioma subgroups.
Methods.
This prospective cohort study includes 1.8 million Norwegian women and men between ages 14 and 80 years at baseline. Body weight and height were measured, and incident cases of glioma were identified by linkage to the National Cancer Registry. Cox regression analyses were performed to evaluate risk for different glioma subgroups in relation to anthropometric measures.
Results.
During 54 million person-years of follow-up, 4,382 gliomas were identified. Overweight and obesity were not associated with risk for any glioma subgroup. Height was positively associated with risk for GBM and all other gliomas (hazard ratio [HR] per 10 cm increase: 1.24; 95% confidence interval [CI], 1.17–1.31 and 1.18; 95% CI, 1.09–1.29) but not with the proxy for isocitrate dehydrogenase (IDH)-mutant glioma (HR, 1.09; 95% CI, 0.98–1.21). In further subgroup analyses, the effect of height on glioma risk varied significantly with positive associations for oligoastrocytoma (HR, 1.74; 95% CI, 1.20–2.53) and malignant glioma not otherwise specified (NOS) (HR, 1.42; 95% CI, 1.16–1.76, but not with diffuse astrocytoma (WHO grades II and III) or oligodendroglioma.
Conclusion.
This epidemiologic study consolidates height as a risk factor for GBM and other gliomas. It further indicates that this association is not universal for gliomas but may differ between different glioma subgroups.
Keywords: body mass index, cohort study, glioma, height, risk factor
Importance of the study
Glioblastoma (GBM) represents the largest subgroup of gliomas and is the most common malignant brain tumor in adults. The etiology of glioma is largely unknown, and no previous study has assessed overweight, obesity, or height in regard to risk for different glioma subgroups. This study reports the association between anthropometric measures and risk for the 5 most common glioma subgroups: GBM, diffuse astrocytoma (WHO grades II and III), oligodendroglioma, oligoastrocytoma, and malignant glioma not otherwise specified (NOS). While overweight and obesity were not associated with either of the glioma subgroups, there was heterogeneity in the association between height and different glioma subgroups. This epidemiologic study consolidates height as a risk factor for GBM but raises doubt that this association is universal for all gliomas.
Intracranial gliomas represent a heterogeneous group of primary brain tumors that arise from glial or precursor cells, and the most common histological subgroups in adults include GBM, diffuse astrocytoma, oligodendroglioma, and malignant glioma NOS.1 GBM is the most common malignant brain tumor in adults and represents >50% of all intracranial gliomas.1 The prognosis of GBM is devastating in spite of all treatment efforts, with a median overall survival of 10–15 months.1–4 Understanding the genetic basis of brain tumorigenesis has clearly improved over the last decades. A paradigm change was recently introduced in the updated 2016 WHO diagnostic criteria for brain tumors.5 For the first time, molecular factors such as the isocitrate dehydrogenase (IDH) mutation status in gliomas were incorporated into the classification of CNS tumor entities with the aim of achieving more homogenous diagnostic entities and improving diagnostic accuracy. In contrast, the etiology of glioma is still poorly understood, and evidence is mostly derived from epidemiologic studies that have explored associations between potential risk factors and the heterogeneous group of all gliomas without considering different glioma subtypes. As such, height has been demonstrated to be associated with glioma risk in several studies,6–13 but whether or not this association is consistent across the different glioma subgroups is unclear.
The aim of this prospective population-based cohort study was to assess whether body mass index (BMI) or body height are associated with risk for GBM and other glioma subgroups, including subdivision into proxies for IDH mutation status as well as the other 4 most common glioma subgroups of intracranial diffuse astrocytoma WHO grades II and III, oligodendroglioma, oligoastrocytoma, and malignant glioma NOS in adolescent and adult women and men.
Materials and Methods
Ethical Statement
This study was approved by the Regional Committee for Ethics in Medical Research and by the Norwegian Data Inspectorate.
Study Population
This study cohort consisted of adolescent and adult women and men between ages14 and 80 years who participated in a nationwide Norwegian health survey performed by the National Mass Radiography Service between 1963 and 1975. The health survey, which had a participation rate of 83%, was part of the last national screening campaign for tuberculosis that was carried out in 17 of the 19 counties in Norway.14 Trained personnel measured the body height and weight of participants at baseline. Participants with incomplete anthropometric data (3,380; 0.2%), ongoing pregnancy (5,855; 0.3%), or age >80 years (24,469; 1.3%) were excluded from the analyses. Also, participants with a prevalent cancer diagnosis (including benign and malignant CNS tumors) prior to the baseline screening were excluded (22,283; 1.2%).
Linkage of Databases
In 1961, a unique 11-digit ID number was introduced in Norway for every resident, which has since been used universally for personal identification. Since 1951, reporting to the Norwegian Cancer Registry has been mandatory for clinicians and pathology departments. Subsequent intracranial glioma was identified by linkage of the study cohort to the Norwegian Cancer Registry using participants’ ID numbers.
Outcome Characteristics
Based on the International Classification of Diseases for Oncology, Third Edition (ICD-O-3), glioma subgroups were defined as follows: glioblastoma: 9440–9442 (also as proxy for IDH-wildtype); glioma other than GBM: 9380–9384, 9391–9460, exclusive of 9440–9442; diffuse astrocytoma (WHO grade II and III): 9400, 9401, 9410, 9411, 9420; oligodendroglioma (WHO grades II and III): 9450, 9451, 9460; oligoastrocytoma: 9382; diffuse astrocytoma (WHO grades II and III), oligodendroglioma, and oligoastrocytoma as proxy for IDH mutation status positive: 9382, 9400, 9401, 9410, 9411, 9420, 9450, 9451, 9460; and malignant gliomaNOS: 9380. To define intracranial location, morphology codes were combined with topography codes 193.0–193.2 and 195.3–195.5 based on the International Classification of Diseases, Seventh Revision (ICD-7).
Categorization of Independent Variables
Height and weight of study participants were measured by trained personnel at baseline. BMI was calculated as weight divided by height squared (kg/m2) and categorized as <20, 20–24.9, 25–29.9, and ≥30 kg/m2 as well as per 5 kg/m2 increase in BMI. Overweight was defined as BMI 25–29.9 kg/m2, obesity as BMI ≥ 30 kg/m2, underweight as BMI <20 kg/m2, and BMI 20–24.9 kg/m2 was used as the reference category in the statistical analyses. Participants’ birth years were used for categorization into 4 cohorts: before 1911; 1911–1925, 1926–1941, and after 1941. Participants were further categorized into the following age groups: <30, 30–44, 45–59, and ≥60 years. Body height was categorized according to quartiles for men and women, and per 10 cm increase.
Definition of Follow-up Time
Follow-up time was calculated as person-years from the time of the study’s baseline (the date of height and weight measurement) until the date of glioma diagnosis, any other cancer diagnosis, date of emigration, date of death from any cause, or the end of follow-up at December 15, 2011, whichever occurred first.
Statistical Analysis
Cox proportional hazard regression, using attained age at study baseline as the time axis, adjusted for birth cohort and sex, was performed to calculate hazard ratios (HRs) with 95% confidence intervals (CIs). Stratified analyses were performed by sex and in different age groups. Sensitivity analysis was performed to minimize the likelihood of reverse causality of BMI by excluding participants with ≤ 5 years of follow-up. The proportional hazard assumptions were tested by plotting the logarithm of the integrated hazards (log-log survival plots) and by Schoenfeld tests and were found to be satisfied. The difference between HR estimates was assessed by test of interaction, as previously described.15 Power calculations for Cox proportional hazard regression models were performed for each tumor subgroup by utilizing the individual probability of failure as well as the standard deviation and squared multiple correlation coefficients of height and BMI, respectively.16 Two-sided probability with a significance level of 0.05 was used throughout. Statistical analyses were performed with STATA/SE statistics software Version 14.0 (StataCorp).
Results
Characteristics of the Study Population
In total, 1,855,333 women and men between ages14 and 80 years were eligible for analysis. Forty-eight percent of the study cohort were men, and 28% were < 30 years of age at baseline. Median follow-up time was 33 years (interquartile range: 19–42). During follow-up of > 54 million person-years, 3,102 GBMs and 1,280 gliomas other than GBM were identified. Further division into glioma subgroups identified 485 diffuse astrocytomas (WHO grades II and III), 269 oligodendrogliomas, 73 oligoastrocytomas, and 234 malignant gliomas NOS. The proxy group for IDH mutation status positive, including diffuse astrocytoma (WHO grades II and III), oligodendroglioma, and oligoastrocytoma comprised 827 cases. Details of the baseline characteristics are provided in Table 1.
Table 1.
Baseline characteristics
| Population at risk | GBMs | Gliomas other than GBMs | Diffuse astrocytomas (WHO II/III), oligodendro gliomas, oligoastro cytomas | Diffuse astrocytomas (WHO grades II and III) | Oligodendrogliomas | Oligoastrocytomas | Malignant gliomas, NOS | |
|---|---|---|---|---|---|---|---|---|
| Number of participants | 1,855,333 | 3102 | 1280 | 827 | 522 | 269 | 73 | 234 |
| Women; No (%) | 959,798 (52) | 1331 (43) | 586 (46) | 369 (45) | 226 (43) | 123 (46) | 38 (52) | 112 (48) |
| Mean age at entry (SD) | 43.4 (17.8) | 40.0 (13.0) | 33.0 (13.2) | 32.2 (12.9) | 32.3 (13.1) | 32.8 (12.8) | 26.5 (9.5) | 37.4 (13.7) |
| Mean height in cm (SD); women/men | 162 (6)/175 (7) | 163 (6)/176 (7) | 164 (6)/176 (7) | 164 (6)/176 (7) | 164 (5)/176 (7) | 163 (6)/176 (8) | 165 (6)/180 (6) | 164 (5)/177 (7) |
| Mean BMI in kg/m2 (SD); women / men | 25 (4) / 24 (3) | 25 (4) / 24 (3) | 23 (4) / 24 (3) | 23 (4) / 24 (3) | 24 (4) / 24 (3) | 23 (4) / 24 (3) | 22 (2) / 23 (3) | 24 (4) / 24 (3) |
Abbreviations: BMI, body mass index; SD, standard deviation; NOS, not otherwise specified.
Body Mass Index and Risk for Glioma Subgroups
Overweight and obesity were not associated with risk for GBM or glioma other than GBM in the total population or in stratified analyses by sex (Tables 2 and 3). In further subgroup analyses, there was no association between overweight or obesity and risk for the proxy group for IDH mutation status positive (Table 5) or the other glioma subgroups (Table 4). For underweight, there was an increased risk for oligodendroglioma (HR, 1.53; 95% CI, 1.08–2.15) and a decreased risk for malignant glioma NOS (HR, 0.58; 95% CI, 0.34–1.00) (Table 4). BMI as continuous variable and per 5 kg/m2 increase in BMI were :not associated with risk for any of the glioma subgroups (Tables 2–5).
Table 2.
Hazard ratios (95% confidence intervals) for body mass index, height, and glioblastoma risk
| Gender | ||||
|---|---|---|---|---|
| Women and men | ||||
| Time at risk (years) | 54,869,912 | |||
| GBM No | 3102 | |||
| BMI per 5 kg/m2 increase | 1.00 (0.95–1.06); P = 96 | |||
| Height per 10 cm increase | 1.24 (1.17–1.31); P < 001 | |||
| BMI category (kg/m2) | <20 | 20–24.9 | 25–29.9 | ≥30 |
| HR (95% CIs) | 0.90 (0.78–1.04) | Ref | 0.98 (0.90–1.06) | 0.96 (0.82–1.11) |
| Women | ||||
| Time at risk (years) | 29,539,250 | |||
| GBMs No | 1331 | |||
| BMI per 5 kg/m2 increase | 0.98 (0.91–1.05); P =.52 | |||
| Height per 10 cm increase | 1.27 (1.15–1.40); P < 001 | |||
| BMI category (kg/m2) | <20 | 20–24.9 | 25–29.9 | ≥30 |
| HR (95% CIs) | 0.89 (0.73–1.08) | Ref | 0.89 (0.78–1.01) | 0.92 (0.76–1.11) |
| Height in quartiles | 1. | 2. | 3. | 4. |
| HR (95% CIs) | Ref | 1.03 (0.87–1.21) | 1.20 (1.02–1.40) | 1.34 (1.14–1.57) |
| Men | ||||
| Time at risk (years) | 25,330,662 | |||
| GBM No | 1771 | |||
| BMI per 5 kg/m2 increase | 1.04 (0.96–1.13); P =.38 | |||
| Height per 10 cm increase | 1.22 (1.14–1.32); P < 001 | |||
| BMI category (kg/m2) | <20 | 20–24.9 | 25–29.9 | ≥30 |
| HR (95% CIs) | 0.90 (0.73–1.11) | Ref | 1.04 (0.94–1.15) | 0.96 (0.75–1.24) |
| Height in quartiles | 1. | 2. | 3. | 4. |
| HR (95% CIs) | Ref | 1.34 (1.16–1.54) | 1.36 (1.17–1.58) | 1.48 (1.28–1.70) |
Cox regression model with age as the time axis, including sex (where not stratified), height, BMI and birth year cohort; all P values derived from BMI or height as continuous variables.
Abbreviations: CI s, confidence intervals; HR, hazard ratio; Ref, reference group.
Table 3.
Hazard ratios (95% confidence intervals) for body mass index, height, and risk for gliomas other than glioblastomas
| Gender | ||||
|---|---|---|---|---|
| Women and Men | ||||
| Time at risk (y) | 54,826,767 | |||
| Glioma No | 1,280 | |||
| BMI per 5 kg/m2 increase | 0.96 (0.88–1.05); P = 38 | |||
| Height per 10 cm increase | 1.18 (1.09–1.29); P <.001 | |||
| BMI category (kg/m2) | <20 | 20–24.9 | 25–29.9 | ≥30 |
| HRs (95% CIs) | 1.10 (0.92–1.30) | Ref | 0.99 (0.87–1.14) | 0.92 (0.70–1.21) |
| Women | ||||
| Time at risk (years) | 29,520,716 | |||
| Glioma No | 586 | |||
| BMI per 5 kg/m2 increase | 0.95 (0.85–1.07); P =.41 | |||
| Height per 10 cm increase | 1.20 (1.04–1.38); P =.013 | |||
| BMI category (kg/m2) | <20 | 20–24.9 | 25–29.9 | ≥30 |
| HR (95% CIs) | 0.97 (0.76–1.24) | Ref | 0.96 (0.77–1.18) | 0.83 (0.58–1.18) |
| Height in quartiles | 1. | 2. | 3. | 4. |
| HR (95% CIs) | Ref | 1.14 (0.88–1.48) | 1.16 (0.89–1.50) | 1.34 (1.04–1.71) |
| Men | ||||
| Time at risk (years) | 25,306,052 | |||
| Glioma No | 694 | |||
| BMI per 5 kg/m2 increase | 1.01 (0.88–1.15); P =.91 | |||
| Height per 10 cm increase | 1.17 (1.05–1.31); P =.004 | |||
| BMI category (kg/m2) | <20 | 20–24.9 | 25–29.9 | ≥30 |
| HR (95% CIs) | 1.21 (0.94–1.55) | Ref | 1.04 (0.87–1.24) | 1.18 (0.78–1.78) |
| Height in quartiles | 1. | 2. | 3. | 4. |
| HR (95% CIs) | Ref | 1.16 (0.92–1.46) | 1.26 (0.99–1.59) | 1.33 (1.06–1.66) |
Cox regression model with age as the time axis, including sex (where not stratified), height, BMI, and birth year cohort; all P values derived from BMI or height as continuous variables.
Abbreviations: BMI, body mass index; CIs, confidence intervals; HR, hazard ratio; Ref, reference group.
Table 5.
Hazard ratios (95% confidence intervals) for body mass index, height, and risk for different subgroups of glioma in women and men
| Subgroup of glioma | ||||
|---|---|---|---|---|
| Diffuse astrocytoma (WHO grade II and III) | ||||
| Time at risk (years) | 54,810,267 | |||
| Astrocytoma No | 485 | |||
| BMI per 5 kg/m2 increase | 0.98 (0.85–1.13); P =.78 | |||
| Height per 10 cm increase | 1.07 (0.93–1.23); P =.35 | |||
| BMI category (kg/m2) | <20 | 20–24.9 | 25–29.9 | ≥30 |
| HR (95% CIs) | 1.08 (0.82–1.44) | Ref | 1.05 (0.84–1.31) | 1.02 (0.66–1.57) |
| Oligodendroglioma | ||||
| Time at risk (years) | 54,804,528 | |||
| Oligodendroglioma No | 269 | |||
| BMI per 5 kg/m2 increase | 0.86 (0.71–1.04); P =.13 | |||
| Height per 10 cm increase | 1.00 (0.83–1.20); P =.99 | |||
| BMI category (kg/m2) | <20 | 20–24.9 | 25–29.9 | ≥30 |
| HR (95% CIs) | 1.53 (1.08–2.15) | Ref | 1.02 (0.75–1.38) | 0.95 (0.52–1.73) |
| Oligoastrocytoma | ||||
| Time at risk (years) | 54,801,928 | |||
| Oligoastrocytoma No | 73 | |||
| BMI per 5 kg/m2 increase | 0.87 (0.58–1.30); P =.49 | |||
| Height per 10 cm increase | 1.74 (1.20–2.53); P =.003 | |||
| BMI category (kg/m2) | <20 | 20–24.9 | 25–29.9 | ≥30 |
| HR (95% CIs) | 0.75 (0.37–1.55) | Ref | 0.83 (0.44–1.55) | 0.39 (0.05–2.89) |
| Malignant glioma NOS | ||||
| Time at risk (years) | 54,804,131 | |||
| Glioma NOS No | 234 | |||
| BMI per 5 kg/m2 increase | 1.07 (0.88–1.29); P =.50 | |||
| Height per 10 cm increase | 1.42 (1.16–1.76); P =.001 | |||
| BMI category (kg/m2) | <20 | 20–24.9 | 25–29.9 | ≥30 |
| HR (95% CIs) | 0.58 (0.34–1.00) | Ref | 0.92 (0.68–1.26) | 1.07 (0.63–1.83) |
Cox regression model with age as the time axis, including sex, height, body mass index, and birth year cohort; all P values derived from BMI or height as continuous variables.
Abbreviations: BMI, body mass index; CIs, confidence intervals; HR, hazard ratio; Ref = reference group.
Table 4.
Hazard ratios (95% confidence intervals) for body mass index, height, and risk for diffuse astrocytoma (WHO grades II and III), oligodendroglioma, and oligoastrocytoma as proxy for IDH-mutant glioma in women and men
| Diffuse astrocytoma (WHO grade II and III), oligodendroglioma, oligoastrocytoma | ||||
|---|---|---|---|---|
| Time at risk (years) | 54,817,758 | |||
| Glioma No | 827 | |||
| BMI per 5 kg/m2 increase | 0.93 (0.83–1.04); P =.20 | |||
| Height per 10 cm increase | 1.09 (0.98–1.21); P =.10 | |||
| BMI category (kg/m2) | <20 | 20–24.9 | 25–29.9 | ≥30 |
| HR (95% CIs) | 1.18 (0.96–1.45) | Ref | 1.02 (0.86–1.21) | 0.94 (0.67–1.33) |
Cox regression model with age as the time axis, including sex, height,
BMI and birth year cohort; all P values derived from BMI or height as continuous variables.
Abbreviations: CIs, confidence intervals; HR, hazard ratio; Ref = reference group.
Height and Risk for Glioma Subgroups
Height per 10 cm increase and as a continuous variable was associated with risk for GBM and gliomas other than GBM in the total population (HR, 1.24; 95% CI, 1.17–1.31; P = .001 and HR, 1.18; 95% CI, 1.09–1.29; P < 001, respectively). In separate analyses for women and men, height per 10 cm increase was significantly associated with risk for GBM and glioma other than GBM in both sexes without a difference in effect size (GBM: HR, 1.27; 95% CI, 1.15–1.40 for women; HR, 1.22; 95% CI, 1.14–1.32 for men; test of interaction P =.52; glioma other than GBM: HR, 1.20; 95% CI, 1.04–1.38 for women; HR, 1.17; 95% CI, 1.05–1.31 for men; test of interaction P =.39). When comparing the highest to the lowest quartile in height, the risk for GBM increased by 34% in women and 48% in men (Table 2) and for gliomas other than GBM by 34% in women and 33% in men (Table 3).
Height per 10 cm increase or as continuous variable was not associated with risk for the proxy group of IDH mutation status positive glioma, comprising diffuse astrocytoma (WHO grades II and III), oligodendroglioma, or oligoastrocytoma in the total population (HR, 1.09; 95% CI, 0.98–1.21) (Table 4). Furthermore, in separate analyses for the 4 most common histology subgroups of intracranial glioma, there was no association between height and risk for diffuse astrocytoma (WHO grades II and III) and oligodendroglioma, either plotted as a continuous variable or per 10 cm increases in height (Table 5). Yet, height was associated with risk for malignant gliomas NOS and oligoastrocytomas (HR per 10 cm increase, 1.41; 95% CI, 1.16–1.76 and 1.74; 95% CI, 1.20–2.53, respectively) (Table 5).
Power Calculation
Power calculations for Cox proportional hazard regression models (performed for each tumor subgroup) confirmed a power of at least 80% for assessment of BMI per 5 kg/m2 in association with tumor risk (assuming a hazard ratio of 1.3with the exception of oligoastrocytoma [power 37%]). For height per 10 cm increase and an assumed hazard ratio of 1.3, the power was >99% for GBM, >99% for gliomas other than GBM, >99% for the proxy group of IDH mutation status positive gliomas, 95% for diffuse astrocytomas (WHO grades II and III), 79% for oligodendrogliomas, 73% for malignant gliomas NOS, and 30% for oligoastrocytomas.
Body Mass Index, Height, and GBM Risk in Different Age Groups
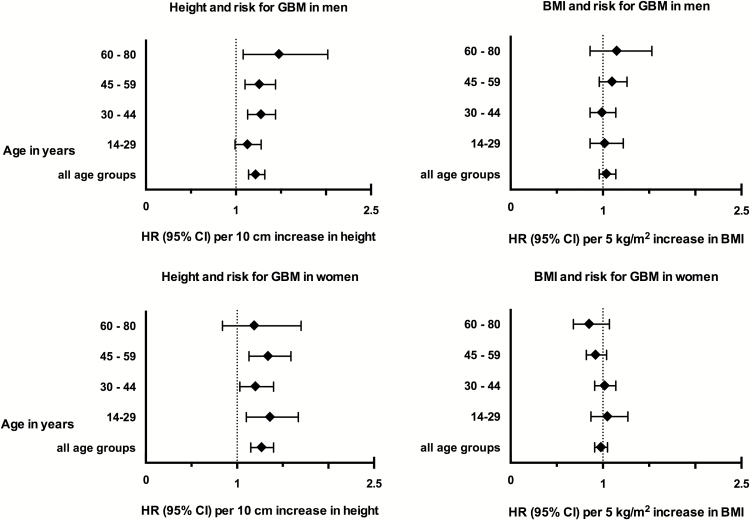
GBM risk was not associated with either overweight and obesity or underweight in any of the different age groups but was consistently associated with height (see supplementary table S1). Furthermore, separate analyses for women and men in different age groups demonstrated consistency of the association between height and GBM risk throughout all subgroups, as demonstrated by forest plots in Figure 1.
Fig. 1.
Glioblastoma risk in relation to body mass index and height, stratified by sex and different age groups. Hazard ratio (HR) and 95% confidence intervals (CI).
Sensitivity Analysis
Sensitivity analyses, excluding participants with ≤5 years of follow-up, did not change the risk estimates for BMI in association with the different tumor subgroups (data not shown).
Discussion
Height and Glioma Risk
In this prospective cohort study, body height in women and men was positively associated with risk for GBM and gliomas other than GBM, but this association differed significantly when assessing different subgroups of gliomas more specifically.
Other studies have reported a positive association between height and risk for glioma6–8,11–13,17,18 and recently confirmed by results based on this study cohort study (HR per 10 cm increase, 1.22; 95% CI, 1.17–1.28) (M.K.H. Wiedmann, unpublished manuscript). However, glioma comprises a heterogeneous group of pathologies, and consistency of the association across glioma subgroups is uncertain. To our knowledge, only 4 other studies have assessed the association of anthropometric measures and GBM risk in particular but not for other glioma subgroups.7,13,17,18 Of those, Helseth et al. reported data from a large case-control study with measured height and weight at baseline and found a significant association between each 15 cm increase in height and GBM risk in males (HR, 1.36; 95% CI, 1.10–1.69) but not in females (HR, 1.18; 95% CI, 0.90–1.54).17 In another case-control study including women and men between ages 50 and 71 years, current height and weight were self-reported in addition to height and weight at ages 18, 35, and 50 years.7 In this study, an association for each 10 cm increase in height and glioma risk in men and women (HR, 1.18; 95% CI, 1.02–1.36 and HR, 1.30; 95% CI, 1.02–1.67, respectively) was reported, which remained evident for GBM (data not presented).7 Kitahara et al. presented results from a pooled case-control study based on 15 different databases, of which only 3 included measured height and weight, while this information was self-reported in the other studies.13 Overall, GBM risk was associated with each 5 cm increase in height in men and women in this study (odds ratio, 1.06; 95% CI, 1.00–1.14).13 Furthermore, a recent prospective cohort study with measured weight and height of school children between ages 7 and 13 years demonstrated a significant association between increased height in boys (HR at age 13, 1.28; 95% CI, 1.12–1.47 per one standard deviation difference) but not in girls (HR, 0.87; 95% CI, 0.73–1.03).18
In our study, height was significantly associated with GBM risk in women and men, without differences in risk estimates. It has been previously hypothesized that height in childhood, rather than adult height, may be a better proxy for early exposures that may influence glioma development.18 In our study, data for participants aged < 14 years were not available, but associations between height and GBM risk were consistent in all 4 age groups including further exploratory analysis for a young age group between ages 14 and 20 years (data not shown). Thus, we did not find that height in young age altered the association with GBM risk, which likely reflected the correlation of ultimate adult height with height in childhood and young adulthood.7,19 To our knowledge, no previous study has reported height in relation to risk for other glioma subgroups. Overall, risk for all gliomas other than GBM was associated with height in our study. However, the more homogeneously defined subgroup of diffuse astrocytomas (WHO grades II and III), oligodendrogliomas, and oligoastrocytomas, which we thought would be more in keeping with the updated 2016 CNS WHO definition5 and could thus be considered a proxy for positive IDH mutation status, was not associated with height. Also, assessing different glioma subgroups defined by phenotypic criteria, as was standard practice until recently,5,20 indicated significant differences in the association between height and tumor risk.
Plausible Mechanisms
Although the biological mechanisms underlying the differing associations between height and glioma subgroups remain unknown, the insulin-like growth factor (IGF) pathway may represent a link between height and glioma risk. IGFs are important determinants of body height attained during childhood and adolescence and have previously been linked to cancer development.21,22 Growth hormone (GH) is a key factor in IGF expression in postnatal life and is influenced by the supply of dietary energy and protein.23 Circulating levels of IGFs are highest during puberty and decrease rapidly in the third decade of life but seem to stay consistently higher in taller adults.22,24 Insufficiency in GH and IGFs during childhood and adolescence leads to reductions in body height and bone mineral density and increases in body fat mass.25
Expression of insulin-like growth factor binding protein-2 (IGFBP-2) in glial cells is important for brain development in utero but decreases significantly after birth.26 IGFBP-2 has been shown to be overexpressed in > 80% of GBMs and to be one of the strongest biomarkers of aggressive behavior.27,28 Recent data from animal studies further indicate that IGFBP-2 inhibition leads to a significant decrease in tumor progression and prolonged survival and thus may represent a target for treatment.28 In contrast, IGFBP-2 has largely been undetectable in low-grade astrocytic and oligodendroglial tumors,29 while upregulation of IGFBP-2 was found to be a consistent and distinct gene expression change in different classes of gliomas.30 Furthermore, IGFBP-2 overexpression appeared when tumor progression in astrocytomas and oligodendrogliomas occurred and was shown to be a key oncogenic signal in tumorigenesis.31
The IGF pathway may thus present a link between height and glioma risk, and its level of engagement may explain differences in the association between height and glioma subgroups. Information about the IGFBP-2 expression (shown to be an important oncogene defining more aggressive glioma phenotypes) may help to elucidate this hypothetical pathway in future studies.28
Definition of Glioma Subgroups
The definition of glioma subgroups requires some consideration. Until recently, the definition of glioma and its subgroups has been based on concepts of histogenesis including microscopic features, immunostaining, and ultrastructure characterization.5 Thus, tumors with an astrocytic phenotype were grouped separately from those with an oligodendroglial phenotype, and mixed glioma or oligoastrocytoma constituted a diagnostic category that was difficult to define and had significant interobserver variability.5 The 2016 update of the CNS WHO classification system has refined the definition of glioma subgroups including genotypic parameters such as IDH mutation or 1p/19q codeletion status, leading to a more objective definition of diffuse glioma subgroups and regrouping astrocytomas and oligodendrogliomas in regard to different growth patterns and prognosis.5 Thus, about 90% of all GBMs are IDH-wildtype, corresponding largely with the clinical diagnosis of primary or de novo GBMs, while IDH-mutant GBM corresponds closely to secondary GBM arising from lower WHO grade diffuse glioma.5 Furthermore, diffusely infiltrating gliomas are now grouped together, whether they are of astrocytic or oligodendroglial origin, based on shared driver mutations in IDH genes and their growth pattern. As this new classification system has not yet been applied to our study cohort, we defined 2 proxy groups in which the first consisted of GBMs (the majority being IDH-wildtype) and the second of diffuse astrocytomas, oligodendrogliomas, or oligoastrocytomas (WHO grades II and III), of which the majority can be considered to be IDH-mutation status positive. In comparing the 2 groups, height was significantly associated with the proxy group for IDH-wildtype, but not with the proxy group for IDH mutation status positive. As of the recent 2016 CNS WHO update including the new concept of glioma classification, (which has not yet been applied to epidemiological studies), we also considered the 5 largest glioma subgroups based on the established phenotypical criteria comprising GBMs, diffuse astrocytomas (WHO grades II and III), oligodendrogliomas, oligoastrocytomas, and malignant gliomas NOS.20 There was significant heterogeneity in the association between height and risk for the different glioma subgroups, with GBMs, malignant gliomas NOS, and oligoastrocytomas being associated with height but not diffuse astrocytomas (WHO grades II and III) or oligodendrogliomas. However, a type II error could not be ruled out with high certainty for oligodendrogliomas if the effect size of height on tumor risk was much less than a HR of 1.3. The subgroup of malignant gliomas NOS also needs to be considered critically. Obviously, it does not represent a homogenous subgroup of gliomas but, according to data from the Cancer Registry of Norway, comprises a variety of diagnoses such as malignant gliomas with high cellularity from limited biopsy material as well as gliosarcomas. However, the prognosis of malignant gliomas NOS was comparably poor, as was the group of GBMs, in contrast to diffuse glioma WHO grades II and III (unpublished data from the Cancer Registry of Norway).
Body Mass Index and Glioma Risk
Overweight or obesity was not associated with risk for GBMs or other glioma subgroups in this study.
A recent meta-analysis did not report an association between overweight, obesity, or BMI per 5 kg/m2 increase and glioma risk32 and has been further confirmed for glioma by results from this study cohort (M.K.H. Wiedmann, unpublished manuscript). In regard to GBMs, 2 other studies have reported no association between tumor risk and overweight or obesity.17,18 However, Moore et al. found a > 3-fold increase of GBM risk in study participants who were obese at the age of 18 years (RR, 3.53; 95% CI, 1.72–7.24).7 This could not be confirmed in our study when assessing young age groups only, including participants <aged 30 years, or in exploratory analysis in a subgroup of participants aged ≤ 20 years (data not shown). In the study by Moore et al., participants aged 50–70 years recalled their BMI at the age of 18 years. Recall bias may thus have contributed to the association found.7
Underweight was associated with a decreased risk for gliomas in a recent meta-analysis (HR, 0.71; 95% CI, 0.58–0.88).32 Interestingly, our study found a decreased risk for malignant gliomas NOS and an increased risk for oligodendrogliomas but without a dose response relation between BMI and tumor risk otherwise. This result,therefore, may have been due to chance.
External Validity
At the time of recruitment, 99% of the eligible study population was Caucasian. This study included > half of the Norwegian women and men in the age group > 13 years and may therefore be considered representative for similar populations (but may differ for other ethnicities due to known differences in glioma incidences).33 However, a large study comparing Australasian (Caucasian) and Asian populations in regard to body height and risk for a large number of different cancers did not find differing associations for height and cancer risk in the 2 populations of different ethnicities.34 This remains to be confirmed for height and risk for glioma subgroups in populations of different ethnicities.
Study Limitations
This study has several limitations:
Other potential confounding factors could not be included in the analyses. This applies most significantly to allergic conditions, which is the only factor besides radiation that is consistently associated with glioma risk.35–37
The socio-economic status for participants of our study was not known. Socio-economic status is associated with height and body weight38 and may thus confound the effect on glioma risk, yet previous cohort studies based on the Norwegian population never indicated an association between socio-economic status and risk for CNS tumor,11,39 (consistent with other studies,6,7,13 but not all).40
BMI usually increases slightly with aging, and BMI in adolescence and young adulthood is only moderately associated with BMI at higher ages.41 As for the long follow-up time, changes in BMI could influence glioma risk but remain unnoticed if the effect size is small.
No recent pathology review of the study cohort was performed to formally apply the latest update of the 2016 WHO CNS criteria for glioma diagnosis.
Study Strengths
The main strengths of this study are:
its large size, including women and men in a wide range of ages (14–80 years)
measurement of height and weight at baseline
virtually complete follow-up by linkage to the Cancer Registry of Norway
the high quality of incidence data from a national cancer registry.42
Conclusion
This is the first epidemiologic study to assess height and BMI in relation to different glioma subgroups. Height was positively associated with risk for GBMs and gliomas other than GBM. However, risk for the proxy of IDH-mutant glioma was not associated with height, and there was significant heterogeneity in the association between tumor risk and height among the 5 largest intracranial glioma subgroups. This provides novel epidemiologic evidence indicating that height may not be universally associated with glioma risk. Genotypic parameters should be included in future studies for defining glioma subgroups, which may contribute to a more clear indication of the association with molecular pathways.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
Markus Wiedmann has received research grants from the South-Eastern Norway Regional Health Authority (grant number 2014060). The other authors have declared no conflict of interest.
Conflict of interest statement. None declared.
Supplementary Material
Acknowledgements
This study used data from the Cancer Registry of Norway. The interpretation and reporting of these data were the sole responsibility of the authors.
References
- 1. Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15Suppl 2:ii1–ii56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 4. Helseth R, Helseth E, Johannesen TB, et al. Overall survival, prognostic factors, and repeated surgery in a consecutive series of 516 patients with glioblastoma multiforme. Acta Neurol Scand. 2010;122(3):159–167. [DOI] [PubMed] [Google Scholar]
- 5. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 6. Benson VS, Pirie K, Green J, Casabonne D, Beral V; Million Women Study Collaborators Lifestyle factors and primary glioma and meningioma tumours in the million women study cohort. Br J Cancer. 2008;99(1):185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moore SC, Rajaraman P, Dubrow R, et al. Height, body mass index, and physical activity in relation to glioma risk. Cancer Res. 2009;69(21):8349–8355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Michaud DS, Bové G, Gallo V, et al. Anthropometric measures, physical activity, and risk of glioma and meningioma in a large prospective cohort study. Cancer Prev Res (Phila). 2011;4(9):1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Edlinger M, Strohmaier S, Jonsson H, et al. Blood pressure and other metabolic syndrome factors and risk of brain tumour in the large population-based Me-Can cohort study. J Hypertens. 2012;30(2):290–296. [DOI] [PubMed] [Google Scholar]
- 10. Schoemaker MJ, Swerdlow AJ. Risk factors for pituitary tumors: a case-control study. Cancer Epidemiol Biomarkers Prev. 2009;18(5):1492–1500. [DOI] [PubMed] [Google Scholar]
- 11. Wiedmann M, Brunborg C, Lindemann K, et al. Body mass index and the risk of meningioma, glioma and schwannoma in a large prospective cohort study (The HUNT Study). Br J Cancer. 2013;109(1):289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Little RB, Madden MH, Thompson RC, et al. Anthropometric factors in relation to risk of glioma. Cancer Causes Control. 2013;24(5):1025–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kitahara CM, Wang SS, Melin BS, et al. Association between adult height, genetic susceptibility and risk of glioma. Int J Epidemiol. 2012;41(4):1075–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Helseth A, Mørk SJ, Johansen A, Tretli S. Neoplasms of the central nervous system in Norway. IV. A population-based epidemiological study of meningiomas. APMIS. 1989;97(7):646–654. [DOI] [PubMed] [Google Scholar]
- 15. Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326(7382):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsieh FY, Lavori PW. Sample-size calculations for the Cox proportional hazards regression model with nonbinary covariates. Control Clin Trials. 2000;21(6):552–560. [DOI] [PubMed] [Google Scholar]
- 17. Helseth A, Tretli S. Pre-morbid height and weight as risk factors for development of central nervous system neoplasms. Neuroepidemiology. 1989;8(6):277–282. [DOI] [PubMed] [Google Scholar]
- 18. Kitahara CM, Gamborg M, Rajaraman P, Sørensen TI, Baker JL. A prospective study of height and body mass index in childhood, birth weight, and risk of adult glioma over 40 years of follow-up. Am J Epidemiol. 2014;180(8):821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Healy MJ, Lockhart RD, Mackenzie JD, Tanner JM, Whitehouse RH. Aberdeen growth study. I. The prediction of adult body measurements from measurements taken each year from birth to 5 years. Arch Dis Child. 1956;31(159):372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Roberts DL, Dive C, Renehan AG. Biological mechanisms linking obesity and cancer risk: new perspectives. Annu Rev Med. 2010;61:301–316. [DOI] [PubMed] [Google Scholar]
- 22. Crowe FL, Key TJ, Allen NE, et al. A cross-sectional analysis of the associations between adult height, BMI and serum concentrations of IGF-I and IGFBP-1 -2 and -3 in the European prospective investigation into cancer and nutrition (EPIC). Ann Hum Biol. 2011;38(2):194–202. [DOI] [PubMed] [Google Scholar]
- 23. Chard T. Hormonal control of growth in the human fetus. J Endocrinol. 1989;123(1):3–9. [DOI] [PubMed] [Google Scholar]
- 24. Juul A, Bang P, Hertel NT, et al. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin Endocrinol Metab. 1994;78(3):744–752. [DOI] [PubMed] [Google Scholar]
- 25. Thankamony A, Capalbo D, Jonsson PJ, Simpson HL, Dunger DB. Predictors of insulin-like growth factor-i responses to growth hormone replacement in young adults with growth hormone deficiency. Horm Res Paediatr. 2016;85(6):379–388. [DOI] [PubMed] [Google Scholar]
- 26. Lee WH, Michels KM, Bondy CA. Localization of insulin-like growth factor binding protein-2 messenger RNA during postnatal brain development: correlation with insulin-like growth factors I and II. Neuroscience. 1993;53(1):251–265. [DOI] [PubMed] [Google Scholar]
- 27. Patil SS, Railkar R, Swain M, Atreya HS, Dighe RR, Kondaiah P. Novel anti IGFBP2 single chain variable fragment inhibits glioma cell migration and invasion. J Neurooncol. 2015;123(2):225–235. [DOI] [PubMed] [Google Scholar]
- 28. Phillips LM, Zhou X, Cogdell DE, et al. Glioma progression is mediated by an addiction to aberrant IGFBP2 expression and can be blocked using anti-IGFBP2 strategies. J Pathol. 2016;239(3):355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zheng S, Houseman EA, Morrison Z, et al. DNA hypermethylation profiles associated with glioma subtypes and EZH2 and IGFBP2 mRNA expression. Neuro Oncol. 2011;13(3):280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang W, Wang H, Song SW, Fuller GN. Insulin-like growth factor binding protein 2: gene expression microarrays and the hypothesis-generation paradigm. Brain Pathol. 2002;12(1):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dunlap SM, Celestino J, Wang H, et al. Insulin-like growth factor binding protein 2 promotes glioma development and progression. Proc Natl Acad Sci U S A. 2007;104(28):11736–11741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang D, Chen J, Wang J, et al. Body mass index and risk of brain tumors: a systematic review and dose-response meta-analysis. Eur J Clin Nutr. 2016;70(7):757–765. [DOI] [PubMed] [Google Scholar]
- 33. Inskip PD, Linet MS, Heineman EF. Etiology of brain tumors in adults. Epidemiol Rev. 1995;17(2):382–414. [DOI] [PubMed] [Google Scholar]
- 34. Batty GD, Barzi F, Woodward M, et al. ; Asia Pacific Cohort Studies Collaboration. Adult height and cancer mortality in Asia: the Asia Pacific cohort studies collaboration. Ann Oncol. 2010;21(3):646–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen C, Xu T, Chen J, et al. Allergy and risk of glioma: a meta-analysis. Eur J Neurol. 2011;18(3):387–395. [DOI] [PubMed] [Google Scholar]
- 36. Ostrom QT, Bauchet L, Davis FG, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16(7):896–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schwartzbaum J, Ding B, Johannesen TB, et al. Association between prediagnostic IgE levels and risk of glioma. J Natl Cancer Inst. 2012;104(16):1251–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tyrrell J, Jones SE, Beaumont R, et al. Height, body mass index, and socioeconomic status: mendelian randomisation study in UK Biobank. BMJ. 2016;352:i582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wiedmann M, Brunborg C, Lindemann K, et al. Smoking, obesity and the risk of pituitary adenoma: a large prospective cohort study (The HUNT Study). Eur J Epidemiol. 2016;31(1):95–98. [DOI] [PubMed] [Google Scholar]
- 40. Porter AB, Lachance DH, Johnson DR. Socioeconomic status and glioblastoma risk: a population-based analysis. Cancer Causes Control. 2015;26(2):179–185. [DOI] [PubMed] [Google Scholar]
- 41. Aarestrup J, Bjerregaard LG, Gamborg M, et al. Tracking of body mass index from 7 to 69 years of age. Int J Obes (Lond). 2016;40(9):1376–1383. [DOI] [PubMed] [Google Scholar]
- 42. Larsen IK, Småstuen M, Johannesen TB, et al. Data quality at the cancer registry of Norway: an overview of comparability, completeness, validity and timeliness. Eur J Cancer. 2009;45(7):1218–1231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.