Abstract
Background.
Clinical genomics platforms are needed to identify targetable alterations, but implementation of these technologies and best practices in routine clinical pediatric oncology practice are not yet well established.
Methods.
Profile is an institution-wide prospective clinical research initiative that uses targeted sequencing to identify targetable alterations in tumors. OncoPanel, a multiplexed targeted exome-sequencing platform that includes 300 cancer-causing genes, was used to assess single nucleotide variants and rearrangements/indels. Alterations were annotated (Tiers 1–4) based on clinical significance, with Tier 1 alterations having well-established clinical utility. OncoCopy, a clinical genome-wide array comparative genomic hybridization (aCGH) assay, was also performed to evaluate copy number alterations and better define rearrangement breakpoints.
Results.
Cancer genomes of 203 pediatric brain tumors were profiled across histological subtypes, including 117 samples analyzed by OncoPanel, 146 by OncoCopy, and 60 tumors subjected to both methodologies. OncoPanel revealed clinically relevant alterations in 56% of patients (44 cancer mutations and 20 rearrangements), including BRAF alterations that directed the use of targeted inhibitors. Rearrangements in MYB-QKI, MYBL1, BRAF, and FGFR1 were also detected. Furthermore, while copy number profiles differed across histologies, the combined use of OncoPanel and OncoCopy identified subgroup-specific alterations in 89% (17/19) of medulloblastomas.
Conclusion.
The combination of OncoPanel and OncoCopy multiplex genomic assays can identify critical diagnostic, prognostic, and treatment-relevant alterations and represents an effective precision medicine approach for clinical evaluation of pediatric brain tumors.
Keywords: array CGH, brain tumor, clinical sequencing, pediatric neuro-oncology, precision medicine
Importance of the study
Clinically validated genomics platforms are required for the implementation of precision medicine, including in pediatric neuro-oncology. In this study, we demonstrate the utility of a targeted exome-sequencing platform in combination with genome-wide copy number profiling using aCGH in a clinical setting. These platforms detected clinically relevant alterations in 55% of all patients, including identification of driver genomic mutations. In addition, we identified alterations that confer diagnostic and prognostic significance, such as those that allow subtyping of medulloblastoma. Our results indicate that genomic profiling of pediatric brain tumors is feasible and clinically useful.
Over the last 30 years, childhood mortality from cancer has declined significantly across most cancer types, with an average decrease of more than 50%. By contrast, however, pediatric brain cancers have demonstrated only a 30% decrease in mortality, thus highlighting the pressing clinical necessity to improve diagnostics and disease management. Furthermore, from 2008 to 2012, brain tumors accounted for 25% of all pediatric cancer deaths.1 Although mortality has decreased, many patients suffer long-lasting and debilitating morbidities as a result of aggressive surgical resection and high doses of radiation therapy and cytotoxic chemotherapy.2
Across a variety of cancer types, the identification of subtype-specific mutations has often represented a critical initiating step in the path toward targeted therapies.3,4 Recent work in pediatric brain tumors has demonstrated key differences between these tumors compared with adult counterparts. Tumors from both these cohorts show not only different incidence rates across their respective populations, but substantial variation in identified driver events.5–8
In order to translate these advances into clinical practice, physicians require a means by which to obtain accurate clinically relevant genomic data for these tumors. Patients require that this type of testing be affordable and widely accessible. Implementation of such platforms has been well described in the adult neuro-oncology population.9–12 Herein, we report our experience of performing genomic profiling of pediatric neuro-oncology patients using a targeted exome approach in conjunction with genome-wide copy number analysis in a setting certified by the Clinical Laboratory Improvement Amendments (CLIA).
Methods
Ethics Statement
Ethics approval was granted by the human institutional review board committees of the Dana-Farber/Harvard Cancer Center. Informed consent was obtained from all patients and/or their guardians. Targeted exome sequencing was performed on tumors from patients enrolled on the Profile clinical study.
Clinical Data and Review of Histology
Clinical data were extracted from clinical charts and de-identified. A histological diagnosis for each patient was rendered for the study by at least one pediatric neuropathologist for all cases (S.H.R.) and most cases were reviewed by a second neuropathologist.
Clinical Array Comparative Genomic Hybridization
High resolution array comparative genomic hybridization (aCGH) (Agilent Sure Print G3 Human 1 × 1M feature array) was performed on clinical biopsy samples from formalin-fixed paraffin embedded material in a CLIA-certified laboratory as part of clinical care using a methodology previously described.13,14 Two micrograms of patient and reference DNA (Promega) were fragmented and hybridized to each microarray. The average resolution of the aCGH platform across Reference Sequence genes is 1.8 kb, and across the genome 2.1 kb.
Analysis was performed as previously described14 and clinical reports were generated by teams of board-certified clinical cytogeneticists, neuropathologists, and molecular pathologists.
Targeted Next-Generation Sequencing—OncoPanel
For patients enrolled on the Profile clinical trial, detection of mutations and gene rearrangements in tumors was achieved using targeted next-generation sequencing (OncoPanel).15,16 This assay (Agilent SureSelect for target capture and Illumina HiSeq sequencing) surveys exonic DNA sequences of 300 cancer genes, including 91 introns across 30 genes (Supplementary Table 1) for mutation and rearrangement detection in tumor-derived DNA (minimum 50 ng, formalin-fixed paraffin embedded–derived tissue). Neuropathologists review each tumor specimen and estimate the number of neoplastic cells in the submitted sample (tumor purity). OncoPanel results were interpreted and reported by molecular pathologists, and alterations were classified into 4 tiers (Tier 1 to Tier 4) to provide therapeutic and prognostic significance16 (Supplementary Table 1), with Tier 1 alterations having well-established published evidence confirming clinical utility. BreaKmer, an algorithm that predicts insertions, deletions, tandem duplications, and gene rearrangements, was implemented in April 2014 and detected aberrations in 70/120 (58%) patients.17
Whole-Genome Sequencing
Tumor and normal control were sequenced at the Broad Institute of MIT and Harvard. DNA was randomly fragmented, and libraries prepared for paired-end sequencing on an Illumina HiSeq 2000. Read pairs were aligned to reference genome hg19 (Build 37) using the Burrows-Wheeler Aligner with options −q 5 −l 32 −k 2 −o 1.18 Reads were sorted by coordinates, normalized, and cleaned and duplicates were marked using SAMtools and Picard. Base quality score assignments were recalibrated to control for biases due to flow cell, lane, dinucleotide context, and machine cycle using the Genome Analysis Toolkit.19 Rearrangements were detected using Snowman, an assembly-based method (Wala et al, manuscript in preparation).
Exploratory Analysis of Copy Number Profiles
Copy number profiles can be inferred from OncoPanel profiling; however, the resolution of genome-wide aCGH is higher. Copy number profiles presented here were generated from aCGH analysis. A circular binary segmentation algorithm was used to segment copy number data for research purposes using parameters (α = 0.01, undo.splits = none, minimum width = 5). Focal versus broad somatic copy number alteration events were defined with a cutoff of 0.5× chromosome arm length and gene confidence level of 0.99. Segmented data were visually presented using Integrative Genomics Viewer 2.320 in heatmap format.
Unsupervised hierarchical clustering of arm-level copy number profiles was performed in GenePattern,21 using Euclidean distance as the column distance measurement and pairwise complete-linkage as the clustering method. Nonnegative matrix factorization (NMF) clustering was performed in GenePattern, with 20 clusterings per K and divergence error function.
Support vector machine (SVM) multiclass analysis was performed within MatLab, using the fitcecoc module, and a one-versus-one coding design. Tumors were assigned one of the following labels: glial, embryonal, meningiomas, or not otherwise specified (NOS). The model was trained with 136 of the 146 samples; the remaining 10 samples were then used to assess the model’s ability to predict classes. This method was repeated 15 times, leaving out a different set of 10 samples each time so that the accuracy of the model was tested using every sample within the aCGH cohort.
Medulloblastoma Subtyping
DNA-based molecular subtyping was performed for medulloblastoma samples through analysis of the molecular aberrations (including both single nucleotide variants and copy number aberrations) identified through microarray and sequencing studies. Previously reported findings in large cohort series22–24 were used to guide subtyping according to criteria in Supplementary Table 4. For example, the Wingless (WNT) subgroup tumors were identified by monosomy 6 and/or CTNNB1 mutations. Sonic hedgehog (SHH)–activated tumors were categorized by the presence of at least one of the following: (i) any direct alteration in genes involved in SHH signaling, including PTCH1, SMO, SUFU, GLI1, GLI2, or (ii) more than one of the following: 9q deletion, which includes PTCH1 single copy loss, the co-occurrence of chromothripsis, and TP53 mutations. Group 3 tumors were classified by the presence of MYC amplification in the absence of isodicentric chromosome 17. Group 4 tumors were identified by the presence of isodicentric chromosome 17p11.2. Group 3/4 was assigned if tumors lacked diagnostic Group 3, 4, or WNT/SHH features or contained Groups 3 and 4 features in the same tumor.
Statistical Analysis
Unpaired 2-sided t-tests were used to determine differences in percentage of genome afflicted by genomic disruption across subtypes. P-values of <.05 were deemed to be significant.
Results
Assay Overview
Our institutions implemented a precision medicine program for pediatric patients with brain tumors that included next-generation sequencing and copy number profiling. The goals of this testing included determining diagnosis, clarifying prognoses, and identifying possible actionable molecular changes that could be used for assigning patients to clinical trials. For the purposes of this study we analyzed the OncoPanel data for single nucleotide variations (SNVs), insertions/deletions (indels), and rearrangements only and analyzed the OncoCopy (aCGH) data for copy number changes.
Cohort Demographics and Sample Characteristics
Between January 2013 and June 2015, OncoPanel testing was requested on 142 pediatric brain tumor specimens. All histological subtypes of pediatric brain tumors were represented (Supplementary Table 2). The median age of children enrolled was 8 years and the range 0.15 to 25.5 years. At the time of our data analysis, clinical reports were generated for 120 patients (82% of requested); 3 specimens could not be evaluated because of technical limitations of the material submitted (2.5%). In addition, aCGH copy number testing was requested and reporting completed on 146 pediatric brain tumor specimens. Data from both assays were available for 60 patients during this time frame.
The mean reported tumor purity of samples profiled by OncoPanel was 70% (range 20%–95%), the mean coverage was 181× (range 75–634), and the mean percentage of exons with greater than 30 reads across the cohort was 96% (range 91%–99%).
Copy Number Profiles Are Predictive of Histology in Pediatric Brain Tumors
Embryonal tumors (medulloblastomas, primitive neuroectodermal tumors [PNETs], and atypical teratoid rhabdoid tumors [AT/RTs]) are known to exhibit distinct copy number profiles compared with glial tumors (gliomas, ependymomas, astrocytomas, and glioblastomas) and to carry specific diagnostic or prognostic implications.25
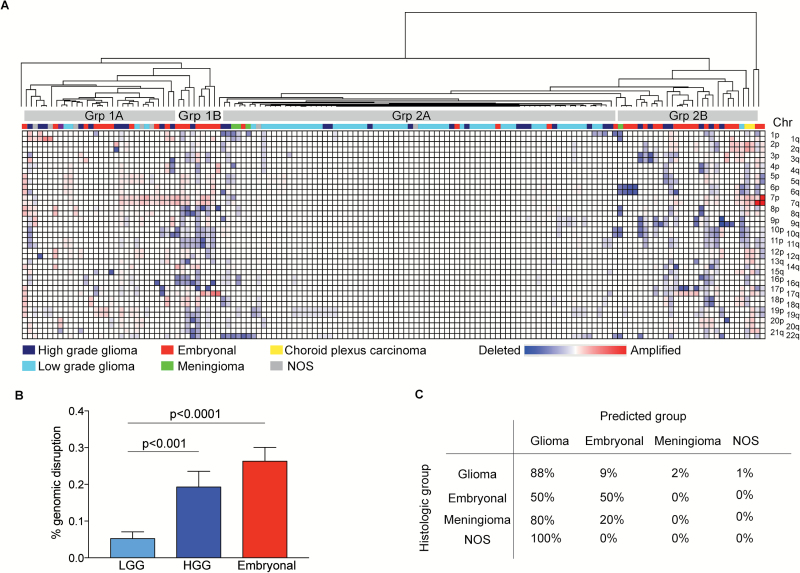
To determine the degree to which the profiles from the clinical aCGH assay differed across histological subtypes, we performed unsupervised hierarchical clustering of genome-wide copy number profiles of 146 pediatric brain tumors (Fig. 1A). Unsupervised hierarchical clustering revealed the tumors to separate into 2 dominant clusters of tumors, each with at least 2 further subclusters within each branch (Fig. 1A). Embryonal tumors were the most common tumor in cluster 1 (18 of 39 tumors, 46%) with high-grade gliomas (HGGs) being the next most frequent (11 of 39, 28%). Cluster 2 was enriched with glial tumors (76 of 107, 71%). Pediatric low-grade gliomas (PLGGs) clustered separately to HGGs, with PLGGs tending to cluster in Group 2A (20 of 28 gliomas, 71%) and HGGs clustering with embryonal tumors in Group 1 and Group 2B. WNT-positive medulloblastomas segregated into cluster 2B while meningiomas clustered with glial tumors in cluster 2. The 2 choroid plexus carcinomas clustered together with the embryonal tumors in cluster 2B. An independent analysis with NMF clustering suggested at least 8 copy number clusters.
Fig. 1.
Pediatric brain tumors can be distinguished by copy number profiles. (A) Unsupervised hierarchical clustering of 146 pediatric copy number profiles (arm-level events). (B) Percent of genome disruption in glial tumors (low-grade and high-grade) and embryonal tumors. Values represent mean level of disruption (± SEM). (C) Support vector machine classification of pediatric brain tumors.
To a large degree, these results were due to differing burdens of genomic alterations. In embryonal tumors, 26% of the genome was affected by arm-level copy number alterations, a significant increase compared with the frequency observed in glial tumors (10%). HGGs exhibited greater genomic disruption than LGGs (Fig. 1B). Indeed, 37 tumors had no discernible copy number alterations. Comparative marker selection revealed 13 arm-level events that were significantly different between glial and embryonal tumors (q < 0.25) (Supplementary Table 3); of these 10p, 17q, 7q, 19p, 19q, and 5p were the most differentially altered (q < 0.05).
We sought to harness a machine-learning algorithm (ie, SVMs) to quantify further how well copy number profiles could differentiate between the 5 histological subgroups (glioma, embryonal tumor, meningioma, choroid plexus carcinoma, and NOS). We anticipated that there would be significant error due to the limited number of tumors in some subgroups and significant levels of heterogeneity within these broadly defined subgroups. However, there was interest in using this large cross-cancer dataset to explore the extent to which lineage identification by copy number might be possible. We trained an SVM classifier on 136 tumors spanning these subgroups. The in-sample classification error was 15%; glial tumors were predicted as glial in 88% of instances and embryonal tumors as embryonal in 50% of instances. The remaining 50% of embryonal tumors were classified as glial (Fig. 1C). Cross-validation of the classification using 10-fold validation revealed an out-of-sample classification error of 33%. By contrast, random histological assignment revealed an out-of-sample classification error of 86%. SVM classification was unable to accurately distinguish HGGs from LGGs, classifying 58% of HGGs as LGGs and 26% as embryonal tumors.
Recurrent Activating Mutations and Genomic Rearrangements Are Detected by Targeted Exome Sequencing
OncoPanel data obtained from 117 brain tumors were analyzed to assess for both gene mutations and rearrangements. As part of the clinical reporting workflow, variants were included in reports based on the presence of both >5 variant reads and an allelic fraction greater than 1%. OncoPanel does not include matched normal DNA controls. Thus, private germline alterations could not be excluded and were often reported as Tier 4 variants, representing SNVs not previously reported to be pathogenic in cancer.
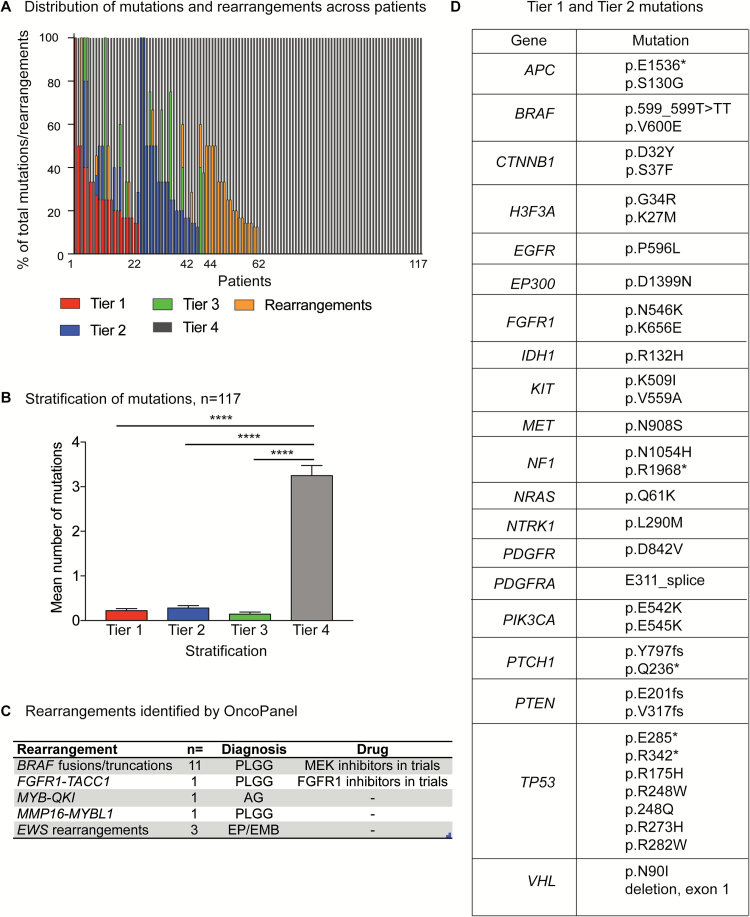
Excluding 2 glioblastoma (GBM) patients with a hypermutator phenotype, a mean of 4 SNVs (range 1–13) were detected in each patient. OncoPanel revealed clinically relevant alterations in 56% of patients (64/115). Across the 115 OncoPanel patients (excluding the 2 GBM patients with a hypermutator phenotype), 44 (38%) were found to have Tiers 1–3 mutations (42 of these patients had Tier 1 or Tier 2 mutations) and an additional 20 (17%) were found to have clinically actionable rearrangements. The remaining patients were observed to harbor Tier 4 alterations (Fig. 2A); this was the most frequent tier of alteration detected across the entire cohort (Fig. 2B).
Fig. 2.
Targeted exome sequencing with OncoPanel detects clinically relevant genomic alterations in pediatric brain tumors. (A) Mutations and rearrangements identified in 117 tumors profiled by OncoPanel. Tier classification of mutations is shown. (B) Incidence of Tier 1, 2, 3, or 4 mutations in 117 tumors profiled by OncoPanel. (C) Clinically relevant rearrangements detected by OncoPanel. (D) Tiers 1 and 2 mutations detected by OncoPanel.
Mutations were observed in a number of genes of direct relevance to pediatric brain tumor diagnosis and treatment, including BRAF, FGFR1, NTRK1, ATRX, TP53, and IDH1 in glial tumors, and CTNNB1 and PTCH1 mutations in medulloblastoma (Fig. 2D). We also observed driver mutations previously reported: KIT in germinoma,26VHL mutations in hemangioblastomas27; and the single case of primary CNS melanoma in association with neurocutaneous melanocytosis was driven by a mutation of NRAS (p.Q61K) as previously described.28
The identification of alterations for which small molecule inhibitors exist influenced clinical management of the patients. Across the entire cohort of patients profiled with OncoPanel, 37 had tumors with an alteration for which a small molecule inhibitor is in early phase clinical investigation (Supplementary Table 2). Of these 37 patients, 8 children (22%) were treated with a targeted small molecule inhibitor based on the mutation identified by OncoPanel, and more patients are likely to be treated in the future upon tumor progression.
OncoPanel Detects Clinically Relevant Rearrangements
OncoPanel detected rearrangements in 25 of 115 pediatric brain tumors analyzed and reported for rearrangements. These included rearrangements involving BRAF, FGFR1-TACC1, and MYB family members previously reported in PLGGs.5,29,30 The most frequent rearrangement/indel detected was the BRAF-KIAA1549 rearrangement found in PLGGs (Fig. 2C).5,29,31,32 We also detected EWSR1 rearrangements in 2 tumors: an integrase interactor 1–deficient AT/RT with EWSR1-PLAGL1 fusion and a spinal ependymoma with EWSR1-BEND2 fusion. We further validated the presence of the EWSR1-PLAGL1 rearrangement in the first tumor using whole-genome sequencing (Supplementary Figure 2). Rearrangements involving EWSR1 have recently been suggested as defining a subclass of central PNETs,25 and PLAGL genes have been previously implicated as a driver in gliomas.33 This alteration raises the question whether EWSR1 fusions occur in multiple tumor types or whether there may be potential overlap between central PNET and “classic” AT/RT.
Medulloblastoma Subtyping Achieved Through Integrated Mutation and Copy Number Analysis
Medulloblastoma has been categorized by the most recent World Health Organization (WHO) 2016 CNS tumor classification update34,35 as having 4 biological subtypes: WNT, SHH, Group 3, and Group 4 tumors.25,36–38 These subtypes have been defined most commonly using expression profiling and most recently methylation profiling.25,36–38 While these technologies have significantly advanced the understanding of the biology of medulloblastoma, the most appropriate approach to subtyping medulloblastoma in the clinical setting remains unclear.
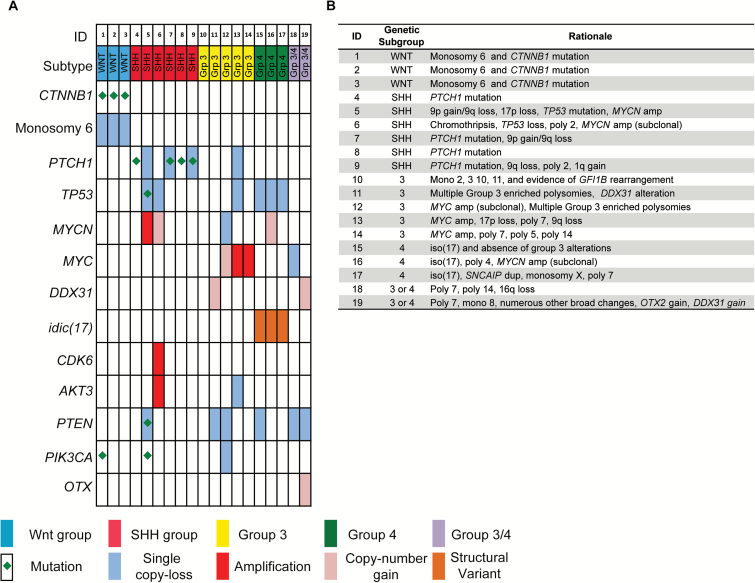
The combination of OncoPanel and aCGH led to the identification of genomic changes previously associated with transcriptome-defined subtypes (WNT, SHH, Group 3, and Group 4) in 89% (17/19) of medulloblastomas (Fig. 3A and 3B, Supplementary Table 4). Of the 31 medulloblastoma tumors in our study cohort, 30 were analyzed by aCGH and 19 were assessed by both OncoPanel and aCGH. We focused on the integrated analysis of somatic copy number alteration and SNV data across these 19 tumors in order to identify recurrent alterations and genomic subtyping performance. To categorize these tumors, we defined a classification scheme of “genetic WNT,” “genetic SHH,” “genetic 3,” “genetic 4,” or “genetic 3 or 4” based upon the presence of genetic features previously associated with these subtypes (see Methods, Fig. 3A, and Supplementary Table 4). For example, we classified tumors with CTNNB1 mutations or monosomy 6 as genetic WNT; SHH pathway alterations (Patched 1 [PTCH1] mutations, or 9q loss, or chromothripsis combined with tumor protein 53 [TP53] loss) as genetic SHH; 2 or more of MYC amplification, Group 3 associated polysomies, DEAD (Asp-Glu-Ala-Asp) box 31, or growth factor independent 1–family alterations as genetic 3; and structural alterations, including isochromosome 17q, as genetic 4. Criteria used to subtype each individual tumor are included in Fig. 3B.
Fig. 3.
Comprehensive genomic analysis with OncoPanel and aCGH aids genetic subgrouping of medulloblastoma. (A) Genomic landscape of 19 medulloblastomas profiled with combined OncoPanel and aCGH data. Focal copy number gains were reported to be likely subclonal amplifications based on patterns of occurrence in the literature and prior cases with fluorescence in situ hybridization validation. (B) Subgroup assignment with specific copy or mutation criteria used for individual tumor assignment. Single events and combined events were used to call genetic subgroup according to rules in Supplementary Table 4.
CTNNB1 mutations and monosomy 6 co-occurred in all tumors with mutations in either gene and were present in 16% (3/19) of tumors, consistent with genetic WNT (Fig. 3A). Four tumors harbored PTCH1 mutations, defining them as genetic SHH. MYC amplification was detected in 60% (3/5) of tumors, consistent with genetic 3, and Idic(17)(p11.2) was detected in 3 tumors, classified as genetic 4. Two tumors (10%) could not be definitively assigned to a subclass. However, these tumors lacked definitive WNT or SHH features and therefore likely belonged to Group 3 or Group 4 tumors. One exhibited focal gain/subclonal amplification of OTX2, favoring a Group 3 tumor.
Clinically Actionable Alterations in PLGGs with Implications for Targeted Therapy
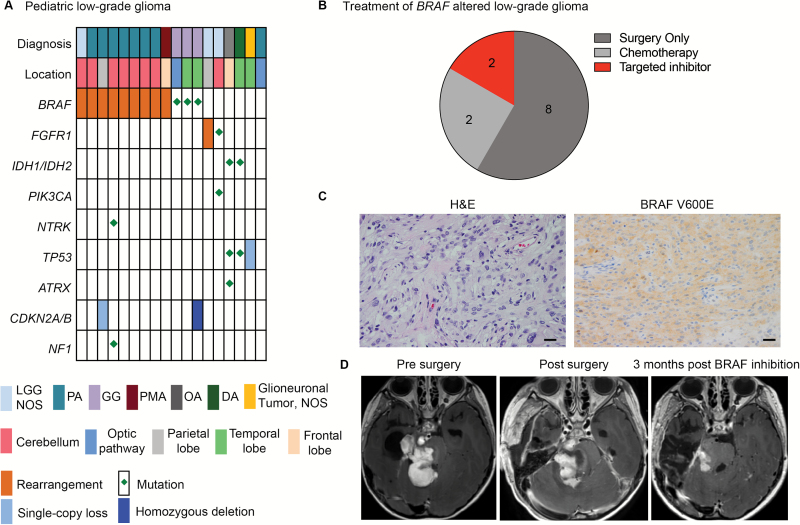
We performed comprehensive genomic profiling (CGP) of 18 PLGGs using both aCGH and OncoPanel and identified diagnosis-relevant alterations in 55% of PLGGs (Fig. 4A). Among pilocytic astrocytomas (PAs), 88% (7/8) harbored KIAA1549-BRAF rearrangements, including all cerebellar (6/6) and cerebral (1/1) PAs. BRAF V600E mutations were detected in all gangliogliomas and in no other tumors (3/3). CGP identified driver events in all 3 tumors designated LGG (NOS), including 2 FGFR1 events (rearrangement and mutation) and a BRAF-KIAA1549 rearrangement. An optic pathway PA showed no detectable alterations, including in BRAF or NF1, suggesting the need for broader analysis via whole-exome or whole-genome sequencing.
Fig. 4.
OncoPanel guides the use of targeted inhibitors in PLGG. (A) Landscape of genomic alterations identified in 18 PLGGs profiled with both OncoPanel and aCGH. (B) Treatment of patients with BRAF-altered PLGG. (C) Hematoxylin and eosin BRAFV600E stains of ganglioglioma with BRAFV600E mutation. Scale bar represents 1.2 µm. (D) Axial postcontrast MRI of tumor at diagnosis, postsurgery, and following treatment with a BRAF inhibitor.
Genomic Profiling-Aided Treatment Planning with Targeted Therapies
A key goal of precision medicine and genomic profiling is to better guide the use of targeted therapies in patients. While no targeted therapies are currently FDA approved for pediatric brain tumors, several ongoing clinical trials are evaluating such treatments. In our cohort the identification of BRAF alterations guided the use of targeted inhibitors in the clinical setting (Fig. 4B). Of the 12 patients with BRAF-altered PLGGs, 8 were treated with surgery alone and did not require any other tumor-directed therapy. Four required further therapy, 2 of whom received treatment with a targeted inhibitor (BRAF inhibitor for one patient with a BRAF V600E mutation, and an inhibitor of mammalian target of rapamycin for a patient with a BRAF-KIAA1549 rearrangement). The patient with the BRAF V600E mutation was a 7-year-old girl who presented with seizures and was subsequently diagnosed with disseminated ganglioglioma. Pathology revealed a glioneuronal neoplasm, for which immunohistochemistry was used to identify the BRAF V600E mutant protein (Fig. 4C). OncoPanel subsequently validated the presence of the BRAF V600E mutation. The patient was started on treatment with a BRAF inhibitor (dabrafenib), and a response to treatment was observed within 3 months of initiation of therapy (Fig. 4D).
In addition to BRAF alterations, OncoPanel detected previously reported driver alterations in PLGGs,5,29,30 including those affecting FGFR, NTRK, MYB family members, and IDH1. While IDH1/2 mutations are less common in pediatric compared with adult brain tumors, we identified IDH1 (R132H) variants in diffuse astrocytoma (2/3), oligoastrocytoma (1/1), oligodendroglioma (1/1), and anaplastic astrocytoma (1/5). The co-occurrence of IDH1/2, TP53, and ATRX mutations in PLGGs aligns with the signature previously reported in adult lower-grade astrocytomas.39 These findings highlight the necessity of broad multiplexed assays to genomically profile tumors, particularly in the adolescent age group, to distinguish adult versus pediatric diffuse astrocytoma types, which have dramatically different clinical outcomes and prognoses. Adult type astrocytomas are diagnosed in late adolescence, harboring genomic alterations more consistent with those seen in the adult disease (IDH, ATRX, TP53). Thus, such tumors should be regarded as young adult astrocytomas, analogous to those arising in older adults.
Profiling of High-Grade Gliomas Reveal Alterations with Diagnostic and Therapeutic Implications
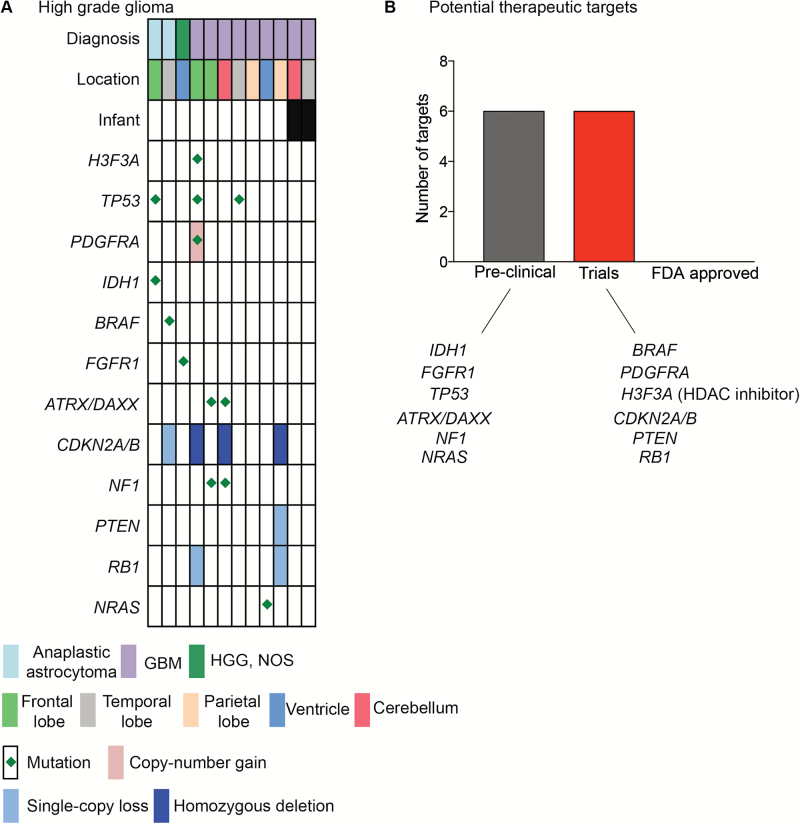
We examined a cohort of 12 pediatric HGGs, defined as WHO grades III and IV tumors, and observed alterations in genes previously reported to be associated with pediatric HGG in 9 of 12 patients (Fig. 5). Two of the 3 tumors for which we did not identify driver alterations were infant HGGs. TP53 mutations were detected in 40% of high-grade astrocytomas, including 2/5 anaplastic astrocytomas and 4/10 GBM tumors. H3F3A mutations were detected in 2/10 cases of GBM and included p.G34R (aka p.G35R) and p.K27M variants. NF1 and ATRX alterations were detected in 30% of GBM with 2 tumors harboring both an ATRX and an NF1 mutation. The hypermutator phenotype was detected in 2 untreated GBM patients with an average of 147 variants per tumor, often with multiple alterations per gene.
Fig. 5.
Genomic alterations identified in 12 pediatric HGGs profiled with OncoPanel and aCGH. (A) Landscape of genomic alterations identified in 12 HGGs profiled with both OncoPanel and aCGH. (B) Genomic alterations for that for which either small molecule inhibitors exist or where they guide enrollment onto clinical trials.
Comprehensive genomic analysis revealed clinically targetable mutations (Fig. 5B). Small molecule inhibitors for 6 targets (BRAF, PDGFRA H3F3A [histone deacetylase inhibitors], cell cycle inhibitors, and phosphatidylinositol-3 kinase inhibitors) have entered early phase clinical trials in pediatrics. In addition, brain-penetrant small molecules directed specifically against IDH1 (R132H) are currently in clinical trials for adults and likely to enter the pediatric arena in the near future, while pediatric clinical trials are currently being designed for FGFR inhibitors. These data suggest that CGP provides meaningful data that can guide precision medicine for children with HGGs.
Discussion
We present the largest cohort of pediatric brain tumors that have undergone genomic profiling in a CLIA-certified setting. In this cohort we observe clinically relevant alterations (Tiers 1–3 mutations and rearrangements) in 56% of patients. The genomic profiles also had diagnostic relevance. Embryonal tumors were found to harbor distinct copy number profiles compared with glial tumors. The combination of targeted sequencing with genome-wide copy number profiling revealed subtype-relevant alterations in 89% of all medulloblastoma.
We found that CGP was useful for improved clinical care of the patient. Histological diagnoses of pediatric brain tumors based on the current WHO CNS classification40 present diagnostic challenges, with a number of tumors that cannot be reliably distinguished. The classification of pediatric brain tumors based on genomic profiling provides both diagnostic and prognostic insight into many disease types.5,25,29,30,36,37,41,42 Thus, the ability to diagnose pediatric brain tumors based on genomic profiles is likely to be of great clinical utility. Our finding that copy number profiles broadly differ between embryonal and glial lineages holds the potential to further improve accuracy and reproducibility of diagnostics, particularly as increased numbers of tumors are profiled.
Recent large-scale efforts to profile the somatic cancer genomes of pediatric brain tumors have heralded the arrival of precision medicine in pediatric neuro-oncology. Our CGP revealed alterations in a number of genes for which inhibitors are currently in early phase pediatric clinical trials, including BRAF, IDH1, PIK3CA, PDGFRA, and KIT, as well as those for which inhibitors have entered, or are about to enter, early phase pediatric clinical trials including FGFR1, NTRK 2/3/4, MYC, or MYCN. Targeted therapeutics are likely to be most effective when deployed against tumors that have been characterized at a genomic level. Thus it is important that clinical practices in pediatric neuro-oncology incorporate comprehensive genomic analysis that will allow the identification of optimal patients for each targeted therapeutic agent.
Medulloblastomas have been consistently suggested to comprise at least 4 distinct biological subgroups with specific clinical and prognostic significance.36,37,41,43 These groups are robust across independent platforms, including gene-expression profiling and methylation profiling. However, these studies have been performed in a research setting, and the best methodology to translate these findings to the clinic remains unclear. In particular, RNA and methylation profiling are poorly suited to identifying therapeutic targets in specific tumors. In contrast, DNA-based genetic analysis of targeted gene panels is cost-effective, provides clinically actionable results, and tends to be more consistent across sites and experimental conditions.
Previous efforts have identified a strong concordance between genomic alterations and WHO subtype in medulloblastoma.36,37,41 We believe that leveraging these findings provides a more clinically useful route to tumor classification. Using our genomic approach of targeted exome sequencing combined with genome-wide copy number profiling, we identified subgroup-specific genomic alterations in almost 90% of medulloblastomas. Subgroup-specific clinical trials are now open to accrual (ClinicalTrials.gov IDs NCT01878617, NCT01601184, and NCT02212574). In addition, targeted therapeutics for specific genomic alterations in medulloblastoma are likely to have an increasing role in the treatment of children with medulloblastoma, highlighting the necessity of implementing technologies that allow the subtyping of medulloblastoma in the clinical setting. It will be useful to determine from these trials the relative value of genetic, transcriptomic, and methylation-based profiling in classifying patients. A limitation to our study is that subtyping of tumors in this cohort was not correlated to matching transcriptomic subtyping from the same tumors; future work will be needed to complete this effort.
Three questions merit particular focus in further study. First, to what extent do transcriptomic and epigenetic profiling add to the clinical utility of genetic profiles? Subclassification of pediatric brain tumors based upon these modalities has been performed in the research setting25,29,36–38 and is now beginning to be implemented clinically (ClinicalTrials.gov IDs NCT02285439, NCT02212574, and NCT01878617). Second, what is the predictive and prognostic value of any of these assays? Formal evaluation of the relative impact of each assay on clinically relevant metrics is necessary. Third, what is the impact of tumor heterogeneity on the validity of clinical genomic results and on the treatments being implemented? Heterogeneity has increasingly been observed across various types of tumors, but the impact of such heterogeneity on the robustness of clinical data has not been formally evaluated.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was supported by Kids’ Brain Tumor Cure Foundation: PLGA Foundation (P.B., K.L.L., R.B., M.W.K., L.C.G.), Stop and Shop Pediatric Brain Tumor Program (P.B., S.N.C., K.D.W., P.E.M., M.W.K.), Path to Cure Foundation (K.L.L.), St Baldrick’s Foundation (R.B., P.B.), Team Jack Foundation (P.B., M.W.K., R.B., L.C.G.), Pediatric Brain Tumor Foundation (P.B., R.B.), Andrysiak Fund for LGG (M.W.K.), Jared Branfman Sunflowers for Life Fund for Pediatric Brain and Spinal Cancer Research (P.B., R.B., S.S.), Sontag Foundation (K.L.L., R.B.), P50CA165962 (K.L.L., M.W.K., C.D.S.), R01 CA188228 (R.B., K.L.L.), P01 CA142536 (K.L.L., L.E.M., C.D.S.), NIH SPORE grant CA165962 (K.L.L., L.E.M., C.D.S., M.W.K.), K08NS087118-03 (S.H.R.), Ian’s Friends Foundation (R.B., K.L.L.), Alex’s Lemonade Stand Foundation (R.B., M.W.K.), Gilmore Fund (K.A.J.), Cure ATRT Now (S.N.C.), NIH K99 1K99CA201592-01A1 (P.B.), NIH T32HL007627 (L.A.R.), Hamilton Low-Grade Glioma Fund (M.W.K., K.L.L., C.D.S., R.B.), Joe Andruzzi Fund (M.W.K., R.B., K.L.L.), Itzkovitz Low-Grade Glioma Fund (M.W.K.), Olivia Caldwell Foundation (R.B., K.L.L.), Lauren’s First and Goal Foundation (M.W.K.). The Gray Matters Brain Cancer Foundation (R.B.).
Conflict of interest statement. The authors declare no conflicts of interest.
Supplementary Material
References
- 1. Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2012. National Cancer Institute. [Google Scholar]
- 2. Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. doi:10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 3. Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417(6892):949–954. doi:10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 4. Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med. 2012;18(3):378–381. doi:10.1038/nm.2658. [DOI] [PubMed] [Google Scholar]
- 5. Bandopadhayay P, Ramkissoon LA, Jain P, et al. MYB-QKI rearrangements in angiocentric glioma drive tumorigenicity through a tripartite mechanism. Nat Genet. 2016;48(3):273–282.doi:10.1038/ng.3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. MacConaill LE, Campbell CD, Kehoe SM, et al. Profiling critical cancer gene mutations in clinical tumor samples. PLoS One. 2009;4(11):e7887. doi:10.1371/journal.pone.0007887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brastianos PK, Taylor-Weiner A, Manley PE, et al. Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat Genet. 2014;46(2):161–165. doi:10.1038/ng.2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ramkissoon LA, Horowitz PM, Craig JM, et al. Genomic analysis of diffuse pediatric low-grade gliomas identifies recurrent oncogenic truncating rearrangements in the transcription factor MYBL1. Proc Natl Acad Sci U S A. 2013;110(20):8188–8193. doi:10.1073/pnas.1300252110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nikiforova MN, Wald AI, Melan MA, et al. Targeted next-generation sequencing panel (GlioSeq) provides comprehensive genetic profiling of central nervous system tumors. Neuro-oncology. 2016;18(3):379–387. doi:10.1093/neuonc/nov289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sahm F, Schrimpf D, Jones DTW, et al. Next-generation sequencing in routine brain tumor diagnostics enables an integrated diagnosis and identifies actionable targets. Acta Neuropathol. 2016;131(6):903–910. doi:10.1007/s00401-015-1519-8. [DOI] [PubMed] [Google Scholar]
- 11. Zacher A, Kaulich K, Stepanow S, et al. Molecular diagnostics of gliomas using next generation sequencing of a glioma-tailored gene panel. Brain Pathol. February 2016. doi:10.1111/bpa.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chi AS, Batchelor TT, Dias-Santagata D, et al. Prospective, high-throughput molecular profiling of human gliomas. J Neurooncol. 2012;110(1):89–98. doi:10.1007/s11060-012-0938-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Craig JM, Vena N, Ramkissoon S, et al. DNA fragmentation simulation method (FSM) and fragment size matching improve aCGH performance of FFPE tissues. PLoS One. 2012;7(6):e38881 doi:10.1371/journal.pone.0038881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ramkissoon SH, Bi WL, Schumacher SE, et al. Clinical implementation of integrated whole-genome copy number and mutation profiling for glioblastoma. Neuro Oncol. 2015;17(10):1344–1355. doi:10.1093/neuonc/nov015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wagle N, Berger MF, Davis MJ, et al. High-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer Discov. 2012;2(1):82–93. doi:10.1158/2159–8290.CD-11–0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris MH, Gold DR, Rifas-Shiman SL, et al. Prenatal and childhood traffic-related air pollution exposure and childhood executive function and behavior. Neurotoxicol Teratol. 2016;57:60–70. doi:10.1001/jamaoncol.2015.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abo RP, Ducar M, Garcia EP, et al. BreaKmer: detection of structural variation in targeted massively parallel sequencing data using kmers. Nucleic Acids Res. 2015;43(3):e19 doi:10.1093/nar/gku1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi:10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McKenna A, Hanna M, Banks E, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–1303. doi:10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Robinson JT, Thorvaldsdóttir H, Winckler W, et al. Integrative genomics viewer. Nat Biotechnol. 2011;29(1):24–26. doi:10.1038/nbt.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reich M, Liefeld T, Gould J, et al. GenePattern 2.0. Nat Genet. 2006;38(5):500–501. doi:10.1038/ng0506-500. [DOI] [PubMed] [Google Scholar]
- 22. Northcott PA, Shih DJH, Peacock J, et al. Subgroup-specific structural variation across 1,000 medulloblastoma genomes. Nature. 2012;488(7409):49–56. doi:10.1038/nature11327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pugh TJ, Weeraratne SD, Archer TC, et al. Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature. 2012;488(7409):106–110. doi:10.1038/nature11329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones DTW, Jäger N, Kool M, et al. Dissecting the genomic complexity underlying medulloblastoma. Nature. 2012;488(7409):100–105. doi:10.1038/nature11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sturm D, Orr BA, Toprak UH, et al. New brain tumor entities emerge from molecular classification of CNS-PNETs. Cell. 2016;164(5):1060–1072. doi:10.1016/j.cell.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schulte SL, Waha A, Steiger B, et al. CNS germinomas are characterized by global demethylation, chromosomal instability and mutational activation of the Kit-, Ras/Raf/Erk- and Akt-pathways. Oncotarget. July 2016. [E-pub ahead of print] doi:10.18632/oncotarget.10392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nielsen SM, Rhodes L, Blanco I, et al. Von Hippel-Lindau disease: genetics and role of genetic counseling in a multiple neoplasia syndrome. J Clin Oncol. 2016;34(18):2172–2181. doi:10.1200/JCO.2015.65.6140. [DOI] [PubMed] [Google Scholar]
- 28. Salgado CM, Basu D, Nikiforova M, et al. Amplification of mutated NRAS leading to congenital melanoma in neurocutaneous melanocytosis. Melanoma Res. 2015;25(5):453–460. doi:10.1097/CMR.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 29. Jones DTW, Hutter B, Jäger N, et al. Recurrent somatic alterations of FGFR1 and NTRK2 in pilocytic astrocytoma. Nat Genet. 2013;45(8):927–932. doi:10.1038/ng.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang J, Wu G, Miller CP, et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet. 2013;45(6):602–612. doi:10.1038/ng.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bar EE, Lin A, Tihan T, et al. Frequent gains at chromosome 7q34 involving BRAF in pilocytic astrocytoma. J Neuropathol Exp Neurol. 2008;67(9):878–887. doi:10.1097/NEN.0b013e3181845622. [DOI] [PubMed] [Google Scholar]
- 32. Zhang L, Chen LH, Wan H, et al. Exome sequencing identifies somatic gain-of-function PPM1D mutations in brainstem gliomas. Nat Genet. 2014;46(7):726–730. doi:10.1038/ng.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zheng H, Ying H, Wiedemeyer R, et al. PLAGL2 regulates Wnt signaling to impede differentiation in neural stem cells and gliomas. Cancer Cell. 2010;17(5):497–509. doi:10.1016/j.ccr.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. doi:10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 35. Loius D, Ohgaki H, Wiestler O, et al. WHO Classification of Tumors of the Central Nervous System. Revised. Fourth Edition Lyon, France: IARC. [Google Scholar]
- 36. Cho Y-J, Tsherniak A, Tamayo P, et al. Integrative genomic analysis of medulloblastoma identifies a molecular subgroup that drives poor clinical outcome. J Clin Oncol. 2011;29(11):1424–1430. doi:10.1200/JCO.2010.28.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012; 123(4):465–472. doi:10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kool M, Korshunov A, Remke M, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012; 123(4):473–484. doi:10.1007/s00401-012-0958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cryan JB, Haidar S, Ramkissoon LA, et al. Clinical multiplexed exome sequencing distinguishes adult oligodendroglial neoplasms from astrocytic and mixed lineage gliomas. Oncotarget. 2014; 5(18): 8083–8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 114(2):97–109. doi:10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Northcott PA, Korshunov A, Witt H, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011; 29(11):1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pajtler KW, Witt H, Sill M, et al. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell. 2015; 27(5):728–743. doi:10.1016/j.ccell.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Korshunov A, Ryzhova M, Hovestadt V, et al. Integrated analysis of pediatric glioblastoma reveals a subset of biologically favorable tumors with associated molecular prognostic markers. Acta Neuropathol. 2015; 129(5):669–678. doi:10.1007/s00401-015-1405-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.