Abstract
Background.
The purpose of this study was to determine the maximum tolerated dose (MTD), recommended phase II dose (RPTD), safety, and pharmacokinetics of ABT-414 plus radiation and temozolomide in newly diagnosed glioblastoma. ABT-414 is a first-in-class, tumor-specific antibody-drug conjugate that preferentially targets tumors expressing overactive epidermal growth factor receptor (EGFR).
Methods.
In this multicenter phase I study, patients received 0.5–3.2 mg/kg ABT-414 every 2 weeks by intravenous infusion. EGFR alterations, O6-methylguanine-DNA methyltransferase (MGMT) promoter hypermethylation, and isocitrate dehydrogenase (IDH1) gene mutations were assessed in patient tumors. Distinct prognostic classes were assigned to patients based on a Molecular Classification Predictor model.
Results.
As of January 7, 2016, forty-five patients were enrolled to receive ABT-414 plus radiation and temozolomide. The most common treatment emergent adverse events were ocular: blurred vision, dry eye, keratitis, photophobia, and eye pain. Ocular toxicity at any grade occurred in 40 patients and at grades 3/4 in 12 patients. RPTD and MTD were set at 2 mg/kg and 2.4 mg/kg, respectively. Among 38 patients with pretreatment tumor tested centrally, 39% harbored EGFR amplification, of which 73% had EGFRvIII mutation. Among patients with available tumor tissue (n = 30), 30% showed MGMT promoter methylation and none had IDH1 mutations. ABT-414 demonstrated an approximately dose proportional pharmacokinetic profile. The median duration of progression-free survival was 6.1 months; median overall survival has not been reached.
Conclusion.
ABT-414 plus chemoradiation demonstrated an acceptable safety and pharmacokinetic profile in newly diagnosed glioblastoma. Randomized studies are ongoing to determine efficacy in newly diagnosed (NCT02573324) and recurrent glioblastoma (NCT02343406).
Keywords: ABT-414, antibody-drug conjugate, EGFR, glioblastoma, phase
Importance of the study
Glioblastoma remains an incurable disease with an urgent need for better treatment options. Standard treatment consists of surgical resection followed by radiation plus concomitant and maintenance temozolomide therapy. EGFR is a known driver of glioblastoma growth; however, several trials of EGFR inhibitors have failed to produce meaningful or durable efficacy. ABT-414, an antibody-drug conjugate, utilizes a novel approach to target the aberrant EGFR (amplified, overexpressed, or mutated) expressed on tumor cells. Upon binding to EGFR, ABT-414 delivers the cytotoxin directly into these cells. Conventional EGFR inhibitor toxicity is absent because ABT-414 does not bind to wild-type EGFR present on normal cells, and the parent antibody (ABT-806) has been shown to cross the blood–brain barrier and bind to EGFR-expressing intracranial tumors. In the present study, we report the safety, pharmacokinetics, and promising antitumor activity of ABT-414 in combination with radiation and temozolomide in patients with newly diagnosed glioblastoma.
Glioblastoma is the most common primary malignant brain tumor in adults, with a 5-year survival rate of about 5%.1 Current standard-of-care therapy for newly diagnosed glioblastoma is surgical debulking followed by radiation therapy (RT) plus daily temozolomide (TMZ), and at least 6 months of adjuvant TMZ monotherapy. Despite this multimodal approach, median survival is 1–2 years.2 Recently, tumor-treating fields that deliver low intensity, intermediate frequency alternating electric fields to the shaved scalp of glioblastoma patients have shown to prolong survival when combined with TMZ after initial treatment with chemoradiation.3 However, glioblastoma patients all but invariably relapse, and the median survival at recurrence is about 9 months.4 Thus, there is an urgent need to develop novel therapeutics that can improve outcome in glioblastoma patients.
Frequent abnormalities have been observed in the expression of the epidermal growth factor receptor (EGFR) in glioblastoma.5,6 Approximately 50% of glioblastomas harbor EGFR gene amplification and the majority overexpress EGFR protein. About half of EGFR-amplified tumors also exhibit the constitutively activated EGFR variant III (EGFRvIII) mutation (EGFRde2-7).5–7 The prognostic significance of this mutation in glioblastoma remains controversial. Some studies report this variant to be unrelated to patient outcomes,8,9 while others report it to be associated with shorter10 or longer survival.11 Agents targeting EGFR signaling pathways have displayed limited or no therapeutic efficacy in glioblastoma clinical trials.12–17 Therefore, in order to exploit the aberrant EGFR expression and signaling in glioblastoma, alternative strategies are required.
ABT-414 is an antibody-drug conjugate (ADC) with a humanized recombinant immunoglobulin G (IgG)1κ antibody (ABT-806) linked to a noncleavable maleimidocaproyl linker and a potent microtubule cytotoxin, monomethyl auristatin F (MMAF).18 The antibody binds to a unique conformational epitope of the receptor that is accessible only when EGFR is in an extended or activated conformation (ie, when EGFR is amplified, mutated, or overexpressed in tumor). Preferential binding of ABT-414 to activated EGFR allows for minimal binding to normal tissue, thereby avoiding toxicities typically associated with EGFR inhibitors.19,20
After binding to EGFR, the complex is internalized and intracellular proteolytic enzymes release the toxin, leading to inhibition of microtubule function, disruption of critical cellular processes, and ultimately cell death.21 Thus, ABT-414 acts distinctly from other EGFR inhibitors that utilize the failed approach of reducing EGFR signaling. Instead, ABT-414 bypasses multiple EGFR signaling resistance mechanisms by utilizing EGFR solely as a vehicle for delivering a potent microtubule toxin into the tumor cell.
Additionally, ABT-414 has been shown to cross the blood–brain barrier. Single-photon emission computed tomography imaging with a 111indium-labeled conjugate of ABT-806 (ABT-806i) has demonstrated that the antibody can effectively bind to EGFR-expressing intracranial tumors, both in preclinical orthotopic models and in patients with brain tumors.22 A chimeric form of ABT-806 (ch806) has also shown uptake in patients with glioma.19 Preclinical data have shown that ABT-414 has potent antitumor activity in glioblastoma cell lines and in standard as well as patient-derived xenograft models.18,23,24
Other common genetic alterations in glioblastoma include methylation-induced silencing of the O6-methylguanine-methyltransferase enzyme (MGMT) gene promoter25 and mutations in the isocitrate dehydrogenase gene (IDH1).26 Both alterations have been associated with a positive prognosis in glioblastoma.27 To predict patient outcomes in glioblastoma, the Radiation Therapy Oncology Group (RTOG) established a recursive partitioning analysis model to provide distinct prognostic classes of patients.28,29 Recently, this model has been combined with the Molecular Classification Predictor (MCP) model to take into account the clinical, genetic, and molecular variations in glioblastoma. The resulting model offers better separation of prognostic classes, translating into improved prediction of survival in newly diagnosed glioblastoma patients.30,31
In this study, we describe the safety, tolerability, and pharmacokinetics (PK) of ABT-414 in combination with TMZ and RT in patients with newly diagnosed glioblastoma. The primary objectives were to determine the recommended phase II dose (RPTD) and maximum tolerated dose (MTD) of ABT-414 in combination with RT and TMZ. Secondary objectives included exploring antitumor activity of ABT-414 and assessing biomarkers to predict patient outcomes. Patient tumor samples were studied for EGFR alterations, MGMT promoter methylation, and IDH1 mutations, and the MCP model was used to assign patients to distinct prognostic classes.
Methods
This multicenter, phase I, open-label study was designed to identify the safety, PK, RPTD, and MTD of ABT-414 in a variety of treatment scenarios for diagnoses of glioblastoma. The trial consisted of 3 treatment arms, Arm A (ABT-414 plus RT and TMZ in newly diagnosed glioblastoma), Arm B (ABT-414 plus TMZ after RT in either newly diagnosed or recurrent glioblastoma), and Arm C (ABT-414 monotherapy in recurrent glioblastoma multiforme [GBM]). Herein we report the results of Arm A of the study. Prior to study initiation, the trial was registered with Clinical Trials Registry (NCT01800695) and approved by the independent ethics committee/institutional review board of all participating institutions. Written informed consent was obtained from all patients or their legal representative before enrollment, and the study was performed in accordance with the 1964 Declaration of Helsinki and its later amendments.
Patients
Eligible patients (≥18 y of age) had newly diagnosed supratentorial glioblastoma or subvariants, Karnofsky performance status score of 70 or above, and no significant postoperative hemorrhage. They also had adequate bone marrow, renal, and hepatic function, as follows: neutrophil count ≥1500/mm3; platelets ≥100000/mm3; hemoglobin ≥9.0 g/dL; serum creatinine ≤1.5 times the upper limit of the normal range (ULN); bilirubin, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) ≤2.5 times ULN; prothrombin time/international normalized ratio ≤1.5. Women of childbearing potential and men who had agreed to use adequate contraception prior to, during, and 6 months after completion of therapy were allowed. Patients were considered ineligible if they had received any anticancer therapy within 28 days before the treatment, or had any medical history of major immunologic reaction to any IgG-containing agent or any component of TMZ or to dacarbazine (DTIC). Lactating or pregnant females were not allowed in the study.
Study Design
Arm A of this study was conducted in 2 cohorts, including a dose-escalation cohort and a safety expansion cohort. The exposure-adjusted continual reassessment methodology (EACRM) was used during dose escalation to identify the MTD and RPTD.32,33 The EACRM allowed for continuous enrollment with individualized dosing selected in single-patient cohorts. Dosing was based on the preceding toxicities observed. The first patient was assigned an ABT-414 dose of 0.5 mg/kg and was followed for the entire dose limiting toxicity (DLT) observation period (~10 wk, from day 1 of ABT-414 until 4 wk after the last dose of RT). No dose escalations were allowed until the first patient completed the entire DLT observation period. Subsequently, as each new patient entered screening, a patient-specific dose was determined at the new estimated MTD based on the toxicities observed and time to such events. As a safety measure, no more than 100% (doubling) of the previous dose was allowed and the AbbVie medical monitor reviewed and approved all dose assignments. There was no intrapatient dose escalation.
Enrollment continued until one of the following was reached: change in the estimated MTD was less than 0.20 mg/kg, or the final MTD was estimated, or futility was seen. Futility was to be declared when the estimated DLT rate, at the lowest dose of 0.5 mg/kg, was greater than 33.3% and at least 2 patients were already assigned to that dose in combination with chemoradiation. The MTD was defined as the dose associated with an approximately 33.3% chance of a DLT, and the RPTD was defined as a dose equal to or less than the MTD and was determined based on the safety and PK data. The following events were considered DLTs when related to the study treatment and occurring in the DLT observation period: grade 4 neutropenia or anemia (>7 days), grade 3 or 4 febrile neutropenia, grade 3 or 4 thrombocytopenia (>7 days), grade 3 or 4 nonhematologic adverse events (AEs) (except grade 3 nausea, vomiting, or diarrhea if adequately managed within 48 h), and >14 days delay of treatment due to the failure to recover from attributable toxicity. Other toxicities either within or after the defined period were evaluated on a case-by-case basis.
Subsequent to the identification of RPTD, patients were enrolled in the safety expansion cohort to further evaluate the side effect profile and safety of ABT-414 at the RPTD and explore preliminary efficacy.
Treatment Regimen
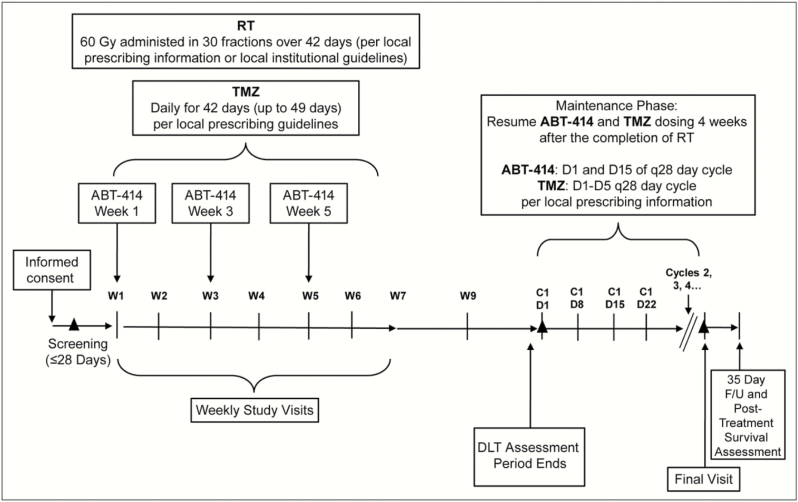
All enrolled patients received standard RT (planned 60 Gy in 30 fractions) with concurrent TMZ (75 mg/m2/day). ABT-414 was administered intravenously over 30–40 minutes on day 1 of weeks 1, 3, and 5 during the RT and TMZ treatment phase (Fig. 1). The ABT-414 dose was escalated or de-escalated to the MTD based on projections from the EACRM. Four weeks following completion of concurrent RT and TMZ, patients received adjuvant TMZ (150–200 mg/m2, days 1–5 of 28-day cycle) plus ABT-414 (days 1 and 15 of 28-day cycle). The dose of ABT-414 used during adjuvant TMZ was assigned as the highest dose that did not surpass the MTD in the concurrent Arm B of the study.23,34
Fig. 1.
Study design: Arm A and expanded cohort A. C, cycle; D, day; DLT, dose-limiting toxicity; F/U, follow-up; RT, radiation therapy; TMZ, temozolomide; W, week; ▲, tumor assessment screening; C1D1, every other cycle post C1D1 and final visit (if not within 3 weeks).
Radiographic assessments for disease progression were performed 4 weeks after the end of RT and thereafter approximately every other month or as clinically indicated. Progression was determined by the local investigator using the Response Assessment in Neuro-Oncology (RANO) criteria.35 ABT-414 continued until disease progression or unacceptable toxicity. Adjuvant TMZ continued for at least 6 months unless disease progression or toxicity was observed in the interim, and continued at the discretion of the local investigator.
Toxicity was assessed among all patients who received at least one dose of ABT-414, and was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.1. A baseline ophthalmology exam was performed for all patients during screening. After observing frequent ophthalmologic toxicities in patients treated at doses of 1.0 mg/kg and above, prophylactic 0.1% dexamethasone eye drops were subsequently administered to all patients at these dose levels. Examinations were administered to patients by an ophthalmologist at baseline and onset of ocular symptoms, and repeated thereafter, as clinically appropriate.
Pharmacokinetic Assessments
Serum samples were collected immediately before and at multiple time points after ABT-414 dose administration on day 1 of weeks 1 and 5 during the chemoradiation phase, and the levels of ABT-414 and total ABT-806 (both ABT-414 and unconjugated antibody) were determined as previously described36 using a validated electrochemiluminescence immunoassay. Plasma samples were also collected at the same time to determine the concentrations of cysteine-maleimidocaproyl MMAF (cys-mcMMAF, a metabolite of ABT-414) using a validated liquid chromatography method with tandem mass spectrometric detection. PK parameters of ABT-414, total ABT-806, and cys-mcMMAF including the peak concentration (Cmax), terminal elimination half-life (t1/2), and area under the serum concentration–time curve (AUC) were determined using noncompartmental methods.
Tumor Protein and Molecular Characterization
EGFR status (amplification, mutation, and total expression), MGMT methylation, and IDH mutations were determined centrally on formalin-fixed, paraffin-embedded (FFPE) archival tumor tissues collected before treatment. Locus-specific amplification of EGFR was detected by fluorescence in situ hybridization (FISH) utilizing 2 FISH probes (Vysis Locus Specific Identifier EGFR SpectrumOrange Probe, Vysis Chromosome Enumeration Probe 7 SpectrumGreen Probe; Abbott Molecular). Total EGFR expression and EGFRvIII mutation were determined by a custom triplex real-time reverse-transcription polymerase chain reaction (qRT-PCR) on RNA extracted from FFPE tissue using the Qiagen FFPE RNA kit (modified manufactured protocol) (Abbott Molecular). Beta-actin mRNA served as an endogenous control and was used to assess RNA integrity. The cutoff values to define positives for these assays are listed in Supplementary Table S1.
MGMT methylation was determined by bisulfite conversion using 1 µg of DNA (Zymo EZ96 DNA methylation kit, Zymo Research). Amplification was performed as previously described,37 generating a 136 bp amplicon derived from positions 131155505-131155619 (RefSeq NM_002412) on chromosome 10. PCR primer sequences were as published38 and real-time PCR was performed using SYBR green (Life Technologies). Reactions were carried out in 15-µL volumes and cycled as follows: 50°C for 2 minutes, 95°C for 10 minutes, and 45 cycles of 95°C for 15 seconds and 62°C for 1 minute. A delta cycle threshold (∆CT) was defined as the difference in the cycle in which the detected amplification curve (on a log-linear plot) crosses an empirically determined threshold (set for each batch based within the geometric region of amplification) between the MGMT amplicon and the reference ACTB amplicon. A ∆CT of less than 8 cycles (>256-fold difference) indicated the presence of promoter methylation.
Mutations of IDH1 were detected by Competitive Allele-Specific TaqMan PCR (castPCR) according to the manufacturer’s instructions (Life Technologies). Assays were performed to detect IDH1C394G(R132G), IDH1G395A(R132H), IDH1C394T(R132C), IDH1G394A(R132S), and IDH1G395T(R132L).
Statistical Analysis
The number of patients in the dose escalation portion was based on the occurrence of DLT events as determined in EACRM. MTD was estimated based on observations to construct a model that considers the time-to-DLT events and hazard of DLTs using a parametric survival regression model. The fitted model was then inverted to estimate the target dose associated with 33.3% chance of DLT or the estimated MTD. Baseline characteristics were summarized using descriptive statistics. The number and percentage of patients having treatment emergent adverse events (TEAEs) were listed by MedDRA system organ class and preferred term.
Progression-free survival (PFS) was defined as the interval from the first day of ABT-414 treatment (also the first day of RT) to RANO-defined disease progression or death from any cause. Overall survival (OS) was defined as the interval from the first dose of ABT-414 to death from any cause. Patients without documented progression or death were censored for survival analyses. PFS and OS were analyzed using the Kaplan–Meier method and were estimated with 95% CI limits.
Results
Patient Characteristics
Between April 2013 and January 2016, forty-five patients (32 men, 13 women; median age 60 y, range 34–79) were enrolled in Arm A of the study: 29 in the dose escalation phase and subsequently 16 in the safety expansion cohort. The baseline characteristics and demographics are shown in Table 1.
Table 1.
Baseline characteristics
| Characteristics | Escalation Cohort | Expansion Cohort | All Patients (N = 45) |
|---|---|---|---|
| (n = 29) | (n = 16) | ||
| Median age, y (range) | 58 (34–79) | 60 (44–74) | 60 (34–79) |
| Male gender, n (%) | 19 (66) | 13 (81) | 32 (71) |
| KPS score, n (%) | |||
| 100 | 8 (28) | 5 (31) | 13 (29) |
| 90 | 12 (41) | 6 (38) | 18 (40) |
| 80 | 7 (24) | 3 (19) | 10 (22) |
| 70 | 2 (7) | 2 (13) | 4 (9) |
| Surgery type, n (%) | |||
| Partial or total resection | 22 (76) | 13 (81) | 35 (78) |
| Biopsy | 7 (24) | 3 (19) | 10 (22) |
| EGFR status, n/Na (%) | |||
| Amplification (amplified/patients tested) | 8/26 (31) | 7/12 (58) | 15/38 (39) |
| EGFRvIII mutation (mutated/patients tested) | 5/27 (19) | 7/14 (50) | 12/41 (29) |
| (EGFRvIII mutated and EGFR amplified)/amplified | 5/8 (63) | 6/7 (86) | 11/15 (73) |
| EGFR overexpression/patients tested | 7/27 (26) | 7/14 (50) | 14/41 (34) |
| MGMT methylation status, n/Na (%) | |||
| Methylated | 5/21 (24) | 4/9 (44) | 9/30 (30) |
| Unmethylated | 16/21 (76) | 5/9 (56) | 21/30 (70) |
| MCP class, n/Na (%) | |||
| 2 | 2/17 (12) | 2/8 (25) | 4/25 (16) |
| 3 | 13/17 (76) | 6/8 (75) | 19/25 (76) |
| 4 | 1/17 (6) | 0/8 (0) | 1/25 (4) |
a n = number of patients with that characteristic, N= total number of patients in that group.
EGFR, epidermal growth factor receptor; KPS, Karnofsky performance status; MCP, molecular classification predictor; MGMT, O-6-methylguanine-DNA methyltransferase.
Safety
The escalation phase included 9 dose groups (29 patients) (0.5 mg/kg, n = 1; 0.7 mg/kg, n = 2; 1.0 mg/kg, n = 1; 2 mg/kg, n = 5; 2.3 mg/kg, n = 3; 2.4 mg/kg, n = 8; 2.6 mg/kg, n = 4; 3 mg/kg, n = 2; 3.2 mg/kg, n = 3). Overall TEAEs (≥25% patients), grade 3/4 TEAEs (≥10% patients), and DLTs are summarized in Table 2. Overall, the most common TEAEs observed were fatigue (73%), blurred vision (64%), nausea (47%), thrombocytopenia (47%), constipation (44%), dry eye (36%), keratitis (33%), photophobia (33%), increased AST (33%), increased ALT (31%), eye pain (27%), and seizure (27%). In aggregate, ocular TEAEs were reported in 40/45 (89%) patients, with multiple symptoms occurring in these patients. The underlying pathology of these ocular events was related to microcystic keratopathy.
Table 2.
ABT-414 treatment emergent adverse events
| Events, n (%) | All Patients (N = 45) |
|---|---|
| All Grades (≥25% patients) | 45 (100) |
| Ocular | 40 (89) |
| Blurred vision | 29 (64) |
| Dry eye | 16 (36) |
| Keratitis | 15 (33) |
| Photophobia | 15 (33) |
| Eye pain | 12 (27) |
| Non-ocular | |
| Fatigue | 33 (73) |
| Nausea | 21 (47) |
| Thrombocytopenia | 21 (47) |
| Constipation | 20 (44) |
| Increased AST | 15 (33) |
| Increased ALT | 14 (31) |
| Seizure | 12 (27) |
| Grades 3/4 (≥10% patients) | 35 (78) |
| Ocular | 12 (27) |
| Keratitis | 6 (13) |
| Grade 3 | 6 (13) |
| Grade 4 | 0 |
| Blurred vision | 5 (11) |
| Grade 3 | 5 (11) |
| Grade 4 | 0 |
| Non-ocular | |
| Lymphopenia | 6 (13) |
| Grade 3 | 5 (11) |
| Grade 4 | 1 (2) |
| Thrombocytopenia | 6 (13) |
| Grade 3 | 2 (4) |
| Grade 4 | 4 (9) |
| Increased ALT | 5 (11) |
| Grade 3 | 5 (11) |
| Grade 4 | 0 |
| DLT (≥1 patient) | 7 (16) |
| Ocular | 4 (9) |
| Keratitis | 3 (7) |
| Blurred vision | 1 (2) |
| Eye pain | 1 (2) |
| Non-ocular | 3 (7) |
| Increased AST | 2 (4) |
| Increased ALT | 1 (2) |
| Increased gamma-glutamyltransferase | 1 (2) |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; DLT, dose-limiting toxicity; GGT, gamma-glutamyltransferase.
Grade 3/4 TEAEs were observed in 78% of the patients and the most common were keratitis (grade 3, 13%), blurred vision (grade 3, 11%), lymphopenia (grade 3, 11%; grade 4, 2%), thrombocytopenia (grade 3, 2%; grade 4, 9%), and increased ALT (grade 3, 11%). Importantly, the ocular toxicities were generally reversible once ABT-414 was stopped. The MTD was set at 2.4 mg/kg and the RPTD was declared at 2.0 mg/kg. Only 1/21 patients (5%), including 16 patients in the expansion cohort, experienced DLT at RPTD, whereas 6/20 (30%) patients treated at a dose of ≥2.3 mg/kg experienced at least one DLT over the entire course of ABT-414 therapy. The safety expansion cohort consisted of 16 patients, all treated at the RPTD of 2 mg/kg. None of these patients experienced a DLT during the observation period.
A total of 42/45 patients (93%) discontinued ABT-414 during the study (Supplementary Table S2). In the expansion cohort, 14/16 patients (88%) discontinued and 28/29 patients (97%), excluding the expansion cohort, discontinued. The majority of patients discontinued ABT-414 due to disease progression (40%) or an AE unrelated to progression (31%).
Pharmacokinetics
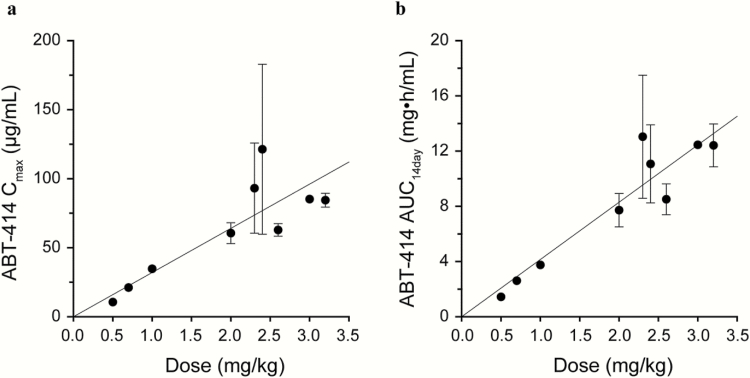
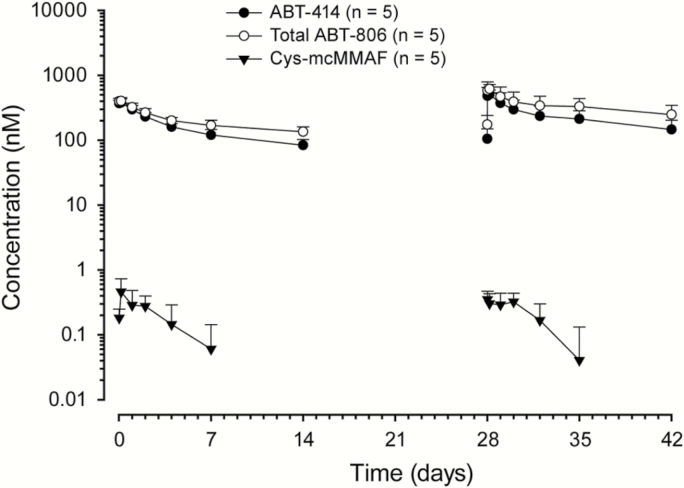
Systemic PK exposures (Cmax and AUC) of ABT-414 and cys-mcMMAF after the administration of ABT-414 via intravenous infusion were approximately dose proportional over the dose range studied (0.5–3.2 mg/kg; Fig. 2 and Table 3). After a single dose of ABT-414, the dose-normalized Cmax and AUCinf (AUC from time 0 to infinity) of cys-mcMMAF were about 1000-fold lower than those of ABT-414 on a molar concentration basis. The observed mean terminal half-lives of ABT-414, total ABT-806, and cys-mcMMAF across all doses studied were 9.0, 11.3, and 4.3 days, respectively. ABT-414 serum exposure was only moderately lower than that of total ABT-806 (Fig. 3), indicating that the level of free (or unconjugated) circulating ABT-806 in serum was low. Thus the drug linker appears to be stable in blood circulation.
Fig. 2.
ABT-414 PK exposure vs dose following ABT-414 dosing in week 1. (A) ABT-414 peak concentration (Cmax) versus ABT-414 dose. (B) ABT-414 area under the concentration–time curve from time zero to 14 days (AUC14 days) vs ABT-414 dose. Values are presented as mean±SD. AUC, area under the curve; Cmax, maximum concentration.
Table 3.
PK parameters of ABT-414 and cys-mcMMAF after week 1 dosing
| Dose | n | Cmax | AUC14 day | AUCinf | t1/2 |
|---|---|---|---|---|---|
| (mg/kg) | (μg/mL) | (mg•h/mL) | (mg•h/mL) | (day) | |
| ABT-414 | |||||
| 0.5 | 1 | 10.6 | 1.44 | 2.23 | 9.5 |
| 0.7 | 2 | 21.1 (19.4, 22.7) | 2.61 (2.56, 2.65) | 3.49 (3.18, 3.8) | 7.2 (6.1, 8.8) |
| 1.0 | 1 | 34.7 | 3.75 | 5.33 | 8.8 |
| 2.0 | 5 | 60.5 ± 7.55 | 7.72 ± 1.21 | 12.5 ± 3.31 | 10.3 ± 1.7 |
| 2.3 | 3 | 93.1 ± 32.7 | 13.0 ± 4.46 | 18.4 ± 4.07 | 8.1 ± 2.7 |
| 2.4 | 5 | 121 ± 61.6 | 11.1 ± 2.83 | 16.3 ± 4.16 | 8.1 ± 2.3 |
| 2.6 | 4 | 62.9 ± 4.54 | 8.51 ± 1.12 | 13.9 ± 4.00 | 9.6 ± 3.5 |
| 3.0 | 2 | 85.2 (79.9, 90.4) | 12.5 (11.1, 13.9) | 20.9 (18.4, 23.5) | 11.0 (10.9, 11.2) |
| 3.2 | 3 | 84.4 ± 5.01 | 12.4 ± 1.55 | 19.1 ± 4.47 | 9.0 ± 3.6 |
| Cys-mcMMAF | |||||
| n | C max | AUC 14 day | AUC inf | t 1/2 | |
| (ng/mL) | (ng•h/mL) | (ng•h/mL) | (day) | ||
| 0.7 | 2 | 0.243 (0.237, 0.249) | 29.7 (22.7, 36.8) | 52.3a | 6.3a |
| 1.0 | 1 | 0.215 | 18.7 | – | – |
| 2.0 | 5 | 0.492 ± 0.278 | 40.0 ± 28.1 | 76.1 (71.7, 80.5)b | 3.7 (3.6, 3.8)b |
| 2.3 | 3 | 0.652 ± 0.169 | 94.6 ± 30.1 | 124 (93.2, 154)b | 3.7 (3, 4.7)b |
| 2.4 | 5 | 0.589 ± 0.235 | 68.7 ± 13.8c | 103 (76.2, 130)b | 4.3 (2.9, 8.5)b |
| 2.6 | 4 | 0.436 ± 0.066 | 56.5 ± 24.9 | 83.7 ± 35.5c | 5.0 ± 1.3c |
| 3.0 | 2 | 0.796 (0.596, 0.996) | 72.8 (64.4, 81.3) | 105a | 5.7a |
| 3.2 | 3 | 0.793 ± 0.104 | 95.2 ± 16.8 | 105 ± 19.5 | 3.8 ± 0.1 |
a n = 1; bn = 2; cn = 3; t1/2 presented as harmonic mean ± pseudo standard deviation; when n = 2, values are presented as harmonic mean (minimum, maximum) for t1/2, and as mean (minimum, maximum) for other PK parameters.
AUC14 day, area under the curve from time 0 to day 14; Cmax, maximum concentration; AUCinf , area under the curve from time 0 to infinity; PK, pharmacokinetics; t1/2 , half life.
Fig. 3.
Concentration–time profiles for ABT-414, total ABT-806, and cys-mcMMAF. The results represent observations following 2 mg/kg ABT-414 dose administration on day 1 of weeks 1 and 5. Values are presented as mean±SD.
Exploratory Antitumor Activity
The median duration of PFS for all patients (n = 45) was 6.1 months (95% CI = 4.5, 9.5); the same PFS of 6.1 months was observed for the expansion cohort patients (n = 16; 95% CI = 2.5, 9.5). The median PFS was 5.9 months (95% CI = 2.5, 11.4) for all EGFR amplified patients (n = 15) and 5.1 months for the subset of EGFR amplified patients in the expansion cohort (n = 7). The median OS has not been reached yet after a median duration of follow-up of 5.8 months (range, 1–21.2).
Biomarker Analysis
Of the 38 patients tested for EGFR status in their tumors, 15 patients (39%) displayed EGFR amplification, and 11 of these 15 patients (73%) also displayed EGFRvIII mutation. Breakdown by cohort and further details are shown in Table 1. Among the 16 expansion cohort patients, 12 were tested for EGFR amplification in their tumors and 7/12 (58%) were found to be EGFR amplified; 14 were tested for EGFRvIII mutation and 7/14 (50%) harbored EGFRvIII mutation (6 of them also showed EGFR amplification and represented 86% of the EGFR amplified patients in the expansion cohort). MGMT methylation status was determined in tumors from 30 patients, of which 9 (30%) were found to have methylation of the MGMT promoter. No mutations of the IDH1 gene were detected. In addition, 25/45 patients (56%) were segregated into distinct prognostic classes (classes 1–4) by the MCP model; the higher the class, the worse the prognosis. In our study, the majority of patients were clustered into class 3 (76%), followed by class 2 (16%) and class 4 (4%) (Table 1).
Discussion
ADCs are a rapidly growing class of anticancer agents that combine the targeting properties of monoclonal antibodies with the antitumor effects of potent cytotoxic drugs. Significant advancements in linker stability and toxin potency are primarily responsible for the improved outcomes and resurgence in ADC development. Distinct advantages of ADCs include their ability to deliver toxic payloads directly to tumor cells allowing enhanced specificity with decreased toxicity as well as their potential to not be affected by downstream resistance mechanisms related to intracellular signaling. These resistance mechanisms have been shown to limit the efficacy of targeted therapies designed to directly inhibit receptor activation, either extracellularly by inhibiting ligand binding or intracellularly through inhibition of tyrosine kinases. Recent examples of clinically relevant, FDA-approved ADCs are brentuximab vedotin (Adcetris) and ado-trastuzumab emtansine (Kadcyla, also called TDM-1). The former is an anti-CD30 ADC, which received FDA approval in 2011 for Hodgkin’s lymphoma and anaplastic large cell lymphoma,39 and the latter is an anti–human epidermal growth factor receptor 2 (HER2) antibody (trastuzumab) conjugated with a toxic drug (DM1), which received FDA approval in 2013 for the treatment of HER2-positive metastatic breast cancer patients who previously received trastuzumab and a taxane.40 ABT-414 is a newer-generation ADC consisting of a humanized recombinant antibody against EGFR tethered to a cytotoxin (MMAF). Given the high frequency of EGFR alterations in this cancer, glioblastoma was considered an attractive indication for ABT-414 development.
Similar to other ADCs, in particular those with mcMMAF drug-linker payload, patients enrolled in the ongoing ABT-414 studies have reported frequent ophthalmologic toxicities, most commonly at the 1.0 mg/kg dose level and above. These AEs can include dry eyes, blurry vision, eye pain, photophobia, watery eyes, and others, and are accompanied by the finding of microcyst development within the cornea. This constellation of ocular symptoms is similar to that reported by other MMAF compounds in development, including SGN-75,41 AGS-16C3F, and AGS-16M8F,42 but are not unique to ADCs. Ocular manifestations of chemotherapy administration have been extensively described. One such chemotherapy, high dose cytarabine, appears to have a common inciting factor as mcMMAF, namely the development of numerous epithelial refractile microcysts within the cornea.43,44 A steroid ophthalmic solution has been used prophylactically prior to and during high dose cytarabine administration to help reduce the incidence and severity of these effects.45,46 Therefore, a comparable strategy was employed in this study given the similarities in the ocular signs and symptoms and the high prevalence of adverse events observed. It is important to note that the impact of steroid eye drops on patients was not meant to be included in a final analysis. Anecdotally, patients have reported some alleviation of symptoms with steroid use and thus dexamethasone, or an equivalent steroid ophthalmologic solution, is recommended to prevent or reduce the ocular side effects for all ABT-414 trials. However, patients would occasionally forget to administer the steroid eye drops, and some did not receive them, which would lead to a worsening of ocular symptoms. Once they started using the eye drops again, the frequency/severity of reported ocular AEs would decrease. These inconsistencies may have led to difficulty in assessing the most accurate number and degree of ocular AEs observed. Another important observation is that once ABT-414 dosing was held or discontinued, ocular symptoms gradually resolved spontaneously in the majority of patients for whom we were able to obtain 3 months of follow-up data. This is likely attributable to the fact that the cornea regenerates itself over a period of 21–28 days, allowing for the microcysts caused by ABT-414 treatment to “slough off”.
Apart from ocular toxicities, other toxicities observed either were common in the population under study (such as seizures) or have been commonly observed with the administration of RT and TMZ (such as thrombocytopenia, fatigue, and liver function test abnormalities). Although a contribution of ABT-414 to these toxicities cannot be excluded, these toxicities have been rare or not at all observed in studies of ABT-414 administered as monotherapy.47 At the assigned RPTD of ABT-414 (2 mg/kg) in the expansion cohort patients, no DLTs were observed. The 2.0 mg/kg RPTD dose of ABT-414 is similar to doses used with other ADCs.40,48 Responses to ABT-414 in recurrent GBM patients have been observed at doses as low as 1.0 mg/kg,34 suggesting that 2.0 mg/kg is sufficient to elicit a response in this population.
Caution should be used in evaluating the efficacy parameters in this phase I study, given the variable doses administered, number of discontinuations of study drug due to toxicities, small sample size, and numerous confounding prognostic factors that can contribute to PFS and OS measurements in this disease. With this in mind, the median duration of PFS (between 5.1 and 6.1 mo) in this study does not compare favorably with the standard-of-care therapy and could reflect the fact that only 56% of patients underwent MCP screening, and of those, the majority were in MCP prognostic class 3. The median duration of survival of glioblastoma patients in MCP class 3 has been reported to be 15 months.31 In our study, the median OS has not been reached yet due to immature follow-up.
Preclinical data suggest that the combination of ABT-414 with TMZ and RT has at least additive properties.18,23 Furthermore, combination with radiation has also been shown to induce EGFR expression,49 which may further enhance the effects of ABT-414. Radiation may also allow for increased drug delivery of ABT-414 to areas of the brain that may be difficult for such a large molecule to penetrate.50 Due to the above hypotheses, as well as the feasibility of safely delivering doses at which tumor responses have been seen, further development of ABT-414 in newly diagnosed glioblastoma is warranted. To this end, a phase II/III study of ABT-414 versus placebo in combination with RT and TMZ in newly diagnosed glioblastoma with EGFR amplification (NCT02573324, RTOG 3508, Intellance 1) is under way, in collaboration with the RTOG Foundation. Another phase II study of ABT-414 is ongoing for patients with recurrent glioblastoma. This study is testing ABT-414 either alone or with TMZ or versus TMZ or lomustine, and is being conducted in collaboration with the European Organisation for Research and Treatment of Cancer (EORTC) (NCT02343406, EORTC 1410-BTG, Intellance 2).
Supplementary Material
Supplementary material is available at Neuro-Oncology online (http://neuro-oncology.oxfordjournals.org/).
Funding
AbbVie provided financial support for this study (NCT01800695) and participated in the design, study conduct, analysis, and interpretation of data as well as the writing, review, and approval of the manuscript. All authors were involved in the data gathering, analysis, review, and interpretation.
Conflict of interest statement. D.A.R. received honoraria from and has a consulting or advisory role with AbbVie, Bristol Myers Squibb, Cavion, Celldex, Inovio, Juno Pharmaceuticals, Merck, Novartis, Roche/Genentech, Amgen, Novocure, Oxigene, Regeneron, and Stemline Therapeutics; is involved in speakers’ bureaus with Roche and Merck; received research funding from Incyte, Midatech, and Celldex.
A.B.L. received personal compensation within the last 12 months from Genentech, Bioclinica, VBI Vaccines, Sapience Therapeutics, Cortice Biosciences, Oxigene, and prIME Oncology; research support from Genentech, Amgen, AbbVie, Novartis, Karyopharm, Celldex, NW Biotherapeutics, Plexxicon, Pfizer, Agenus, Medimmune, Boehringer Ingelheim, Angiochem, Novocure, Stemline, E-Therapeutics, and Millennium.
M.v.d.B. received honoraria from Roche, AbbVie, Celldex, Novocure, Merck Ag, Cavion, Actelion, BMS, and Blue Earth Diagnostics; received research funding from AbbVie and Roche.
P.K. received honoraria for advisory role with AbbVie within the last 12 months.
R.M. is on advisory board for AbbVie.
A.M.S. owns stock in and has a consulting or advisory role with Life Science Pharmaceuticals; received research funding from AbbVie, Daiichi Sankyo, and Avipep; has patents, royalties, or other intellectual property with Life Science Pharmaceuticals, AbbVie, Kalobios, and Ludwig Institute for Cancer Research.
L.F. has no conflict of interest to disclose.
E.P.S. has research funding from AbbVie.
H.K.G. has an investigator-initiated study with AbbVie; received travel support and research funding from AbbVie and MSD; received honoraria from AbbVie, Pfizer, Merck Serono, Novartis, Bristol-Myers Squibb, and Bayer; affiliated with the Ludwig Institute for Cancer Research.
E.G., J.F., H.J.L., W.M., H.X., H.M., L.R.R., P.A., and K.D.H employed by AbbVie and may own AbbVie stock.
Supplementary Material
Acknowledgments
Authors would like to thank the patients and their families, investigators, and their research teams. Medical writing support was provided by Namrata Bhatnagar, PhD, and Mrinal Shah, PhD, employees of AbbVie.
References
- 1. Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17Suppl 4:iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3. Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–2543. [DOI] [PubMed] [Google Scholar]
- 4. Wick W, Brandes AA, Gorlia T, et al. Phase III trial exploring the combination of bevacizumab and lomustine in patients with first recurrence of a glioblastoma: the EORTC 26101 trial. Neuro Oncol. 2015;17 (suppl 5): v1. [Google Scholar]
- 5. Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yoshimoto K, Dang J, Zhu S, et al. Development of a real-time RT-PCR assay for detecting EGFRvIII in glioblastoma samples. Clin Cancer Res. 2008;14(2):488–493. [DOI] [PubMed] [Google Scholar]
- 7. Gan HK, Cvrljevic AN, Johns TG. The epidermal growth factor receptor variant III (EGFRvIII): where wild things are altered. FEBS J. 2013;280(21):5350–5370. [DOI] [PubMed] [Google Scholar]
- 8. Chen JR, Xu HZ, Yao Y, et al. Prognostic value of epidermal growth factor receptor amplification and EGFRvIII in glioblastoma: meta-analysis. Acta Neurol Scand. 2015;132(5):310–322. [DOI] [PubMed] [Google Scholar]
- 9. Weller M, Kaulich K, Hentschel B, et al. Assessment and prognostic significance of the epidermal growth factor receptor vIII mutation in glioblastoma patients treated with concurrent and adjuvant temozolomide radiochemotherapy. Int J Cancer. 2014;134(10):2437–2447. [DOI] [PubMed] [Google Scholar]
- 10. Zhao LL, Xu KL, Wang SW, et al. Pathological significance of epidermal growth factor receptor expression and amplification in human gliomas. Histopathology. 2012;61(4):726–736. [DOI] [PubMed] [Google Scholar]
- 11. Montano N, Cenci T, Martini M, et al. Expression of EGFRvIII in glioblastoma: prognostic significance revisited. Neoplasia. 2011;13(12):1113–IN1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chakravarti A, Wang M, Robins HI, et al. RTOG 0211: a phase ½ study of radiation therapy with concurrent gefitinib for newly diagnosed glioblastoma patients. Int J Radiat Oncol Biol Phys. 2013;85(5):1206–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Uhm JH, Ballman KV, Wu W, et al. Phase II evaluation of gefitinib in patients with newly diagnosed grade 4 astrocytoma: Mayo/North Central Cancer Treatment Group Study N0074. Int J Radiat Oncol Biol Phys. 2011;80(2):347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peereboom DM, Shepard DR, Ahluwalia MS, et al. Phase II trial of erlotinib with temozolomide and radiation in patients with newly diagnosed glioblastoma multiforme. J Neurooncol. 2010;98(1):93–99. [DOI] [PubMed] [Google Scholar]
- 15. van den Bent MJ, Brandes AA, Rampling R, et al. Randomized phase II trial of erlotinib versus temozolomide or carmustine in recurrent glioblastoma: EORTC brain tumor group study 26034. J Clin Oncol. 2009;27(8):1268–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Neyns B, Sadones J, Joosens E, et al. Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann Oncol. 2009;20(9):1596–1603. [DOI] [PubMed] [Google Scholar]
- 17. Raizer JJ. HER1/EGFR tyrosine kinase inhibitors for the treatment of glioblastoma multiforme. J Neurooncol. 2005;74(1):77–86. [DOI] [PubMed] [Google Scholar]
- 18. Phillips AC, Boghaert ER, Vaidya KS, et al. ABT-414, an antibody-drug conjugate targeting a tumor-selective EGFR epitope. Mol Cancer Ther. 2016;15(4):661–669. [DOI] [PubMed] [Google Scholar]
- 19. Scott AM, Lee FT, Tebbutt N, et al. A phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptors. Proc Natl Acad Sci U S A. 2007;104(10):4071–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jungbluth AA, Stockert E, Huang HJ, et al. A monoclonal antibody recognizing human cancers with amplification/overexpression of the human epidermal growth factor receptor. Proc Natl Acad Sci U S A. 2003;100(2):639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gan HK, Burgess AW, Clayton AH, et al. Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer Res. 2012;72(12):2924–2930. [DOI] [PubMed] [Google Scholar]
- 22. Gan HK, Burge ME, Solomon BJ, et al. A phase I and biodistribution study of ABT-806i, an 111indium-labeled conjugate of the tumor-specific anti-EGFR antibody ABT-806. J Clin Oncol. 2013;31(suppl; abstr 2520). [DOI] [PubMed] [Google Scholar]
- 23. Gan HK, Fichtel L, Lassman A, et al. A Phase I study evaluating ABT-414 with concurrent radiotherapy (RT) and temozolomide (TMZ) in glioblastoma (GBM). Presented at Society for Neuro-Oncology, November 13–16, 2014; Miami, Florida 2014. [Google Scholar]
- 24. Gan HK, Fichtel L, Lassman AB, et al. A phase I study evaluating ABT-414 in combination with temozolomide (TMZ) for subjects with recurrent or unresectable glioblastoma (GBM). J Clin Oncol. 2014;32:5s (suppl; abstr 2021). [Google Scholar]
- 25. Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 26. Nobusawa S, Watanabe T, Kleihues P, et al. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009;15(19):6002–6007. [DOI] [PubMed] [Google Scholar]
- 27. Crespo I, Vital AL, Gonzalez-Tablas M, et al. Molecular and genomic alterations in glioblastoma multiforme. Am J Pathol. 2015;185(7):1820–1833. [DOI] [PubMed] [Google Scholar]
- 28. Li J, Wang M, Won M, et al. Validation and simplification of the Radiation Therapy Oncology Group recursive partitioning analysis classification for glioblastoma. Int J Radiat Oncol Biol Phys. 2011;81(3):623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85(9):704–710. [DOI] [PubMed] [Google Scholar]
- 30. Chakravarti A, Wang M, Aldape KD, et al. A revised RTOG recursive partitioning analysis (RPA) model for glioblastoma based upon multiplatform biomarker profiles. J Clin Oncol. 2012;30(suppl; abstr 2001). [Google Scholar]
- 31. Aldape KD, Wang M, Sulman EP, et al. RTOG 0525: Molecular correlates from a randomized phase III trial of newly diagnosed glioblastoma. J Clin Oncol 2011;29 (suppl; abstr LBA2000). [Google Scholar]
- 32. Qi X, Munasinghe W, Hosmane B, et al. Exposure Adjusted Continuous Reassessment Method (EACRM)—an adaptive design incorporating time to toxicity event for phase I dose finding studies. Drug Designing. 2015;4(2):122. [Google Scholar]
- 33. Michael M, Mulcahy MF, Deming DA, et al. Safety and tolerability of veliparib combined with capecitabine plus radiotherapy in patients with locally advanced rectal cancer (LARC): final results of a phase Ib study. J Clin Oncol. 2015;33(suppl; abstr 3571). [Google Scholar]
- 34. Gan HK, Papadopoulos KP, Fichtel L, et al. Phase I study of ABT-414 mono- or combination therapy with temozolomide (TMZ) in recurrent glioblastoma (GBM). J Clin Oncol. 2015;33 (suppl; abstr 2016). 2015. [Google Scholar]
- 35. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 36. Han TH, Gopal AK, Ramchandren R, et al. CYP3A-mediated drug-drug interaction potential and excretion of brentuximab vedotin, an antibody-drug conjugate, in patients with CD30-positive hematologic malignancies. J Clin Pharmacol. 2013;53(8):866–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Esteller M, Toyota M, Sanchez-Cespedes M, et al. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is associated with G to A mutations in K-ras in colorectal tumorigenesis. Cancer Res. 2000;60(9):2368–2371. [PubMed] [Google Scholar]
- 38. Vlassenbroeck I, Califice S, Diserens AC, et al. Validation of real-time methylation-specific PCR to determine O6-methylguanine-DNA methyltransferase gene promoter methylation in glioma. J Mol Diagn. 2008;10(4):332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Younes A, Yasothan U, Kirkpatrick P. Brentuximab vedotin. Nat Rev Drug Discov. 2012;11(1):19–20. [DOI] [PubMed] [Google Scholar]
- 40. Peddi PF, Hurvitz SA. Ado-trastuzumab emtansine (T-DM1) in human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer: latest evidence and clinical potential. Ther Adv Med Oncol. 2014;6(5):202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tannir NM, Forero-Torres A, Ramchandren R, et al. Phase I dose-escalation study of SGN-75 in patients with CD70-positive relapsed/refractory non-Hodgkin lymphoma or metastatic renal cell carcinoma. Invest New Drugs. 2014;32(6):1246–1257. [DOI] [PubMed] [Google Scholar]
- 42. Thompson JA, Motzer R, Molina AM, et al. Phase I studies of anti-ENPP3 antibody drug conjugates (ADCs) in advanced refractory renal cell carcinomas (RRCC). J Clin Oncol 2015;33 (suppl; abstr 2503). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lochhead J, Salmon JF, Bron AJ. Cytarabine-induced corneal toxicity. Eye (Lond). 2003;17(5):677–678. [DOI] [PubMed] [Google Scholar]
- 44. Hopen G, Mondino BJ, Johnson BL, Chervenick PA. Corneal toxicity with systemic cytarabine. Am J Ophthalmol. 1981;91(4):500–504. [DOI] [PubMed] [Google Scholar]
- 45. Matteucci P, Carlo-Stella C, Di Nicola M, et al. Topical prophylaxis of conjunctivitis induced by high-dose cytosine arabinoside. Haematologica. 2006;91(2):255–257. [PubMed] [Google Scholar]
- 46. Cytarabine package insert, APP pharmaceuticals. [Google Scholar]
- 47. Goss GD, Vokes EE, Gordon MS, et al. ABT-414 in patients with advanced solid tumors likely to overexpress the epidermal growth factor receptor (EGFR). J Clin Oncol 33, 2015. (suppl; abstr 2510). 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Brentuximab vedotin, Adcetris® package insert, Seattle Genetics. [Google Scholar]
- 49. Schmidt-Ullrich RK, Mikkelsen RB, Dent P, et al. Radiation-induced proliferation of the human A431 squamous carcinoma cells is dependent on EGFR tyrosine phosphorylation. Oncogene. 1997;15(10):1191–1197. [DOI] [PubMed] [Google Scholar]
- 50. Cao Y, Tsien CI, Shen Z, et al. Use of magnetic resonance imaging to assess blood-brain/blood-glioma barrier opening during conformal radiotherapy. J Clin Oncol. 2005;23(18):4127–4136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.