Abstract
The pericyte, a constitutive component of the central nervous system, is a poorly understood cell type that envelops the endothelial cell with the intended purpose of regulating vascular flow and endothelial cell permeability. Previous studies of pericyte function have been limited to a small number of disease processes such as ischemic stroke and Alzheimer’s disease. Recently, publications have postulated a link between glioma stem cell differentiation and pericyte function. These studies suggest that there may be an important interaction of pericytes with tumor cells and other components of the tumor microenvironment in malignant primary glial neoplasms, most notably glioblastoma. This potential cellular interaction underscores the need to pursue more investigations of pericytes in malignant brain tumor biology. In this review, we summarize the functional roles of pericytes, particularly focusing on changes in pericyte biology during response to immune cells, inflammation, and hypoxic conditions. The information presented is based on the available data from studies of pericyte function in other central nervous system diseases but will serve as a foundation for research investigations to further understand the role of pericytes in malignant gliomas.
Keywords: Alzheimer’s disease, blood-brain barrier, pericytes, glioma, stroke
The blood–brain barrier (BBB) is a well-established and unique component of the CNS. The BBB’s unique ability to selectively permit transport of substances from the vascular supply to nervous system cells is critical to ensure brain homeostasis and prevent toxic injury to the constituent elements of the brain. However, this characteristic of the BBB does create challenges in treating CNS diseases by inhibiting most systemic therapies from reaching their target. Investigations of the BBB have mainly focused on the endothelial cells and their ability to permit and prohibit various drugs and molecules. Astrocytic foot processes and the accompanying basement membrane are also the focus of many BBB investigations. Despite the prevalence of pericytes within the neurovascular unit, their role and function within the CNS is not fully understood.1–3
Anatomically, pericytes wrap around endothelial cells of the cerebral vasculature during brain development (Fig. 1), suggesting that they have an important role in creating and regulating the BBB. The history of pericytes dates back to 1873, when they were discovered by Charles Rouget and first coined as “Rouget cells.” They were later renamed pericytes by Zimmermann in 1923, since they were always identified to be circumscribed “peri, around” endothelial cells “cyte, cell.” This direct contact with endothelial cells is in concert with the discovery that pericytes regulate cerebral blood flow, participate in angiogenesis, and help maintain BBB integrity.4–6 The abundance of pericytes among neural tissue is directly related to the density of endothelial cells within the brain and is often expressed by the endothelium/pericyte ratio compared with other vascular districts. Together with the endothelial cells, basement membrane, and astrocytic foot processes, the pericytes constitute the complex BBB. Yet, despite the recognition of the pericyte in the regulation of BBB function and vascular flow, the role of pericytes in the biology of malignant gliomas is poorly understood.
Fig. 1.
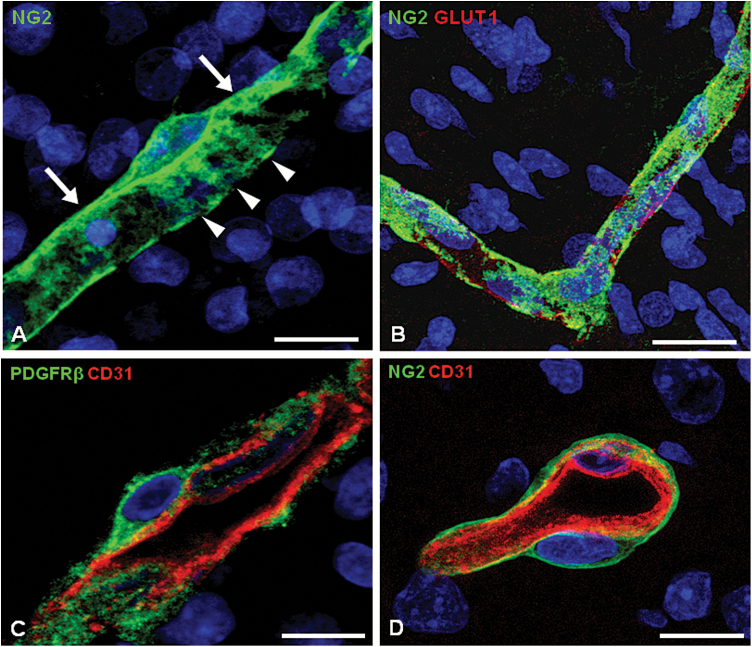
Fetal human brain microvascular pericytes labeled by specific pericyte markers. (A) NG2/CSPG4 expressed by a microvessel pericyte reveals its typical rounded cell body with primary processes oriented according to the longitudinal vessel axis (arrows) giving rise to secondary processes (arrowheads) that embrace the vessel wall. (B–D) BBB endothelial cells appear extensively covered by pericytes revealed by NG2 or PDGFRβ. Nuclear counterstaining TO-PRO3. Bars: (A), 10 µm; (B–D), 15 µm.
Protein expression in pericytes is often defined by 3 common markers: platelet derived growth factor receptor beta (PDGFRβ), alpha-smooth muscle antigen (αSMA), and nerve-glia antigen 2 (NG2)/chondroitin sulfate proteoglycan 4 (CSPG4). PDGFRβ is characteristically expressed in small capillaries and smooth muscle cells. While αSMA is expressed in smooth muscle cells found around large vessels and in perivascular cells,7 pericyte labeling with αSMA is variable and often seen at precapillary arterioles and their branching points, along with postcapillary venules.8 NG2 expression has been linked to early pericyte activations found in the fetal CNS (Fig. 1), whereas in adult brain, NG2 is seen only in disease-activated pericytes (eg, acute injury or degenerative pathologies).9,10
These pericyte markers also assist with cell signaling to astrocytes and endothelial cells for maintenance of neurovascular function and support. PDGFβ acts as an attractant chemokine by signaling to PDGFRβ-expressing pericytes to co-migrate tightly to the vessel wall.11–13 With the absence of PDGFRβ expression on pericytes, Abramsson et al demonstrated that the poor attachment of pericytes to endothelial cells results in vessel diameter enlargement and leakiness.13 Angiopoietin-1 (Ang1) is an angiogenesis factor expressed on varied CNS cells (pericytes, astrocytes, and neurons) which helps regulate vascular remodeling. Combined with its endothelial residing receptor Tie2, Ang1 assists in inhibiting BBB permeability via disruption of crosstalk between pericytes and endothelial cells.14,15 Transforming growth factor-beta (TGFβ) along with Ang1 supports and stabilizes the BBB.16 Yet, when primary human brain pericytes were treated with TGFβ1 to augment TGFβ response, phagocytosis was hindered and the increased inflammatory process aided in disrupting the BBB.17 Additionally, pericyte proliferation and tumor vascularity has been linked to Notch signaling. Specifically, deficiencies and mutations in Notch3 inhibited pericyte expansion, resulting in impaired BBB integrity.18
Pericytes also assist with regulating cerebral blood flow within the neurovascular unit. The composition of pericytes include the mesenchymal intermediate filament protein vimentin, microfilaments that bear resemblance to actin and myosin, and contraction-related proteins of tropomyosin and desmin, which all aid in its contractile nature.3,8,19,20 While wrapped around the endothelial cells, pericytes receive signals from neurons to assist with controlling vessel diameter and ultimately altering cerebral blood flow. Additionally, in vivo imaging in adult mouse brain demonstrated the ability of a potent vasoconstrictor (thromboxane agonist) to induce discrete sectional constriction only at the sites of αSMA- and PDGFRβ-expressing pericytes along capillaries.21 These studies directly correlated the importance of how hemodynamic responses of pericytes correlate to neuronal activation.
Pericyte Role in the Immune System
Pericytes have been shown to play a supportive role in the immune system. Recent studies have demonstrated that pericytes are implicated in brain immune responses at different levels, including (i) expression and secretion of immune-active molecules/receptors; (ii) exhibition of macrophage-like activity and service as antigen presenting cells; (iii) regulation of leukocyte trafficking to sites of inflammation; and (iv) an early role in neutrophil migration. These are all important functions that have been explored in stroke and Alzheimer’s disease (AD) but are inadequately researched in malignant glioma tissue.
Pericytes express receptors of the innate immune system, including toll-like receptor 4 (TLR4), and respond to microenvironmental cues, thus sometimes acting as immune cells.22 Expression of TLR4 activates pericytes by triggering the expression of several common macrophage markers on the pericyte surface. These markers include CD11b (integrin αM), ED2, Fc receptors, scavenger receptors, CR3 complement receptor, prostaglandin, and major histocompatibility complexes I and II, which all aid in facilitating phagocytosis.23,24
In addition, pericytes serve as antigen presenters, paramount to their role in inducing phagocytosis and leading to lymphocyte activation. Balabanov et al demonstrated that with increased expression of cytokine interferon (IFN)-γ (immunostimulatory agent), pericytes present antigens to primed T-lymphocytes. Additionally, these pericytes express cell adhesion molecules (vascular cell adhesion molecule 1 and intercellular adhesion molecule 1 [ICAM-1]) which aid in co-stimulating the neuroimmune system and facilitate leukocyte trafficking to prepare for a robust immune response.24
The priming of immune cells by pericytes seems to also play an important role in leukocyte trafficking. In the initial stages of immune stimuli, pericytes signal pro-inflammatory cytokines TNFα and interleukin (IL)-1β, nitric oxide, reactive oxygen species, and matrix metalloproteinases.19,25 In turn, pericytes induce chemoattraction to circulating neutrophils and elicit an inflammatory response. Intravital imaging has been utilized to demonstrate how pericytes facilitate transmigration of neutrophils.25,26 Using a pericyte-deficient mouse model (pdgfrβ−/−), levels of ICAM1 were significantly upregulated in brain vessels, resulting in an increased number of infiltrating leukocytes within the brain.27 These results suggest that pericytes play an important role in promoting the immune quiescence of brain vessels by limiting leukocyte brain infiltration.
Cerebrovascular disease and neurodegenerative diseases often demonstrate mononuclear cell infiltration, which is likely a reflection of pericyte gaps causing BBB disturbance.28 With this BBB disruption, pericytes signal pro-inflammatory cytokine production. Prolonged pro-inflammatory cytokine exposure leads to scarring of brain parenchyma and pericyte-derived fibrin deposits resulting in cell death and oxidative stress.29,30 High levels of this oxidative stress result in death of pericytes and endothelial cells, ultimately causing neurotoxicity and alterations in cerebral blood flow. This sequelae of abundant leukocyte trafficking within neurovascular tissue, present in both acute and chronic ischemic stroke tissue, is also associated with the presence of amyloid plaques seen with the chronic inflammation of patients with AD.4,30,31
Interestingly, increased mononuclear/neutrophil infiltration within malignant glioma has been associated with worse prognoses.32–34 Higher numbers of neutrophils and macrophages intratumorally and peritumorally have been correlated with a shorter time to tumor progression in malignant glioma.35 This high degree of neutrophil infiltrating immune cells within glioblastoma (GBM) has been previously linked to poor prognosis, establishing a prognostic value in examining the pretreatment neutrophil-to-lymphocyte ratio in GBM tumor tissue. These studies concluded that high neutrophil-to lymphocyte ratios correlated with decreased CD3(+) T-cell infiltration and poor overall survival.36
When tracing NG2 expression within GBM cells, increased expression is seen on tumor cells and angiogenic vessels.37 The NG2-expressing glioma cells are likely derived from glioma stem cells or pericytes.38 Intriguingly, GBM cells with high expression of NG2 can inhibit chemotherapy apoptosis by increased activation of phosphatidylinositol-3 kinase signaling and cell survival promotion.39,40 Additionally, evaluation of patient samples revealed that high NG2 compared with low NG2 expression conferred shorter median survival (8 mo vs 12.5 mo) and a 1.6 increased risk of death in a Cox regression hazard ratio in GBM patients with high NG2 expression on tumor vasculature.41 In this context, it has been postulated that increased neutrophil migration is a marker of systemic inflammation that further drives cancer cell proliferation. This hypothesis was further tested to evaluate responsiveness to radiation treatment in glioma preclinical models, and it was found that high NG2 levels on tumor cells and vasculature correlated with radioresistance secondary to the large proportion of NG2-expressing cells surviving radiation treatment.40,41
This overall poor prognosis may in some part be attributed to the immunosuppressive mediators within the tumor environment. Specifically, in vitro studies demonstrated that isolated GBM pericytes coexpressed stem cell marker CD90 in addition to PDGFRβ, with very high expression on blood vessels comparing GBM with normal brain. When correlating this expression to immune modulation, both leukocyte common antigen expressing leukocytes and CD8-expressing T cells exhibited low expression with increased CD90+ pericyte expression, and these cultured pericyte stem-cell like cells suppressed allogeneic or mitogen-activated T-cell responses. Thus, correlating these specialized pericytes among GBM tumor vasculature may play a much larger role than previously thought in local tumor immunosuppression.37
Collectively, these studies demonstrate the multifactorial role of pericytes to aid in phagocytosis, leukocyte trafficking, and overall immune regulation in progressing neurologic diseases. With these previously noted findings, it may be prudent to further evaluate the relationship of pericyte function among malignant glioma tissue and immune cells. Specific attention should be called to immune cell evasion, T-cell trafficking, and tumor cell proliferation/cell migration.
Pericyte Activation with Inflammation
Inflammation within the CNS occurs with infection, injury, ischemia, demyelination, and rapid cell proliferation. During such inflammation, pericytes react to CNS injury with recognition of endogenously expressed cytokines (IL-1β, TGFβ2, IFNγ), activation of endothelin-1 receptors/PDGFβ, and coordination of chemokine signaling. This chemokine signaling then assists to recruit circulating leukocytes, resulting in additional pro-inflammatory stimuli and eventual leukocyte infiltration.42 Increased vascular endothelial growth factor (VEGF) expression promotes leukocyte infiltration by BBB breakdown among pericytes and endothelial cells. Collectively, pro-inflammatory cytokines, microglia, and infiltrating white blood cells aid in the overall inflammatory state that is evident at times of acute and chronic neurologic diseases.
In the case of ischemic stroke, to compensate for the lack of perfusion insult, ischemic tissue signals to surrounding healthy tissue to initiate the cascade of angiogenesis and vasculogenesis. This signaling induces a rapid VEGF expression within the brain.43 Coordinated regulation of PDGFβ-expressing endothelial cells leads to the activation of PDGFRβ in pericytes, within the peri-infarct region, resulting in enhanced pericyte survival.19,44,45 Concomitantly, ischemic stroke induces a rapid loss of pericytes via caspase-3 activation. In parallel, following ischemic stroke, increased pericyte expression is present within 48 hours of insult. Within hours, multipotent pericytes form stem cells that then develop neural, microglial, and vascular lineage cells at the time of impaired tissue perfusion.46,47 These early progenitor cells express PDGFRβ and stem cell markers and are localized within and around ischemic areas with extension of processes along endothelial cell surfaces.47 Consequently, the pericyte-derived phagocytes increase pro-inflammatory cytokine expression and engulf foreign cellular debris, adding to the inflammatory processes seen in chronically ischemic tissue.17,28
In comparison to ischemic stroke, AD is characterized by neurovascular dysfunction due to tau pathology, and amyloid-beta (Aβ) accumulation BBB breakdown with accompanied inflammation has been reported to occur at the early stages of disease and not during chronic repair such as with strokes. These findings suggest that the early loss of pericytes may contribute to AD pathogenesis.48 Additionally, pericytes have been shown to degrade various circulating proteins in their lysosomes, including immunoglobulins and fibrin.49 Pericytes also function to phagocytize and remove the abnormal Aβ-peptide proteins that can lead to disease progression.5 For instance, under inflammatory conditions, pericytes express the low density lipoprotein receptor–related protein 1 (LRP1), which is a transmembrane protein involved in Aβ processing and clearance through the BBB, thus suggesting an important role of pericytes in Aβ clearance.4 However, the enhanced accumulation of Aβ around brain capillaries induces toxicity to pericytes with resultant poor recovery.50 Preclinical studies revealed that the connection between the loss of pericytes in mice overexpressing Aβ-precursor protein is the result of poor clearance of Aβ from brain interstitial fluid. This failure to eliminate Aβ can also be associated with BBB disruption leading to other conditions with oxidative-induced inflammation.49,51
These findings of pericyte activation and sometimes loss of pericytes during ischemia and Alzheimer’s can be linked to malignant glioma cells. Activation or injury of pericytes can be attributed to phagocytosis and inflammation, but also demonstrates a probable relationship to the tissue insult and multimodal functions of pericytes seen with malignant glioma cell proliferation. At the time of glioma proliferation and invasion, tumor cells require increased vascular supply and growth factor expression.52,53 Accordingly, edema and inflammation are present within proliferating tissue. High pericyte expression has been described within glioblastoma cells.54 Yet, peritumoral pericyte expression was found to be lower, and stem cell marker expression mainly correlated with tumor cell infiltration. In vitro studies demonstrated the expression of macrophage and phagocytic markers on isolated brain pericytes. When co-cultured with GBM cells, the macrophage phenotypes resembled anti-inflammatory M2 tumor associated macrophages.55 These findings suggest a probable connection of pericyte activation intratumorally and stem cells peripherally, but further studies are warranted to directly tie pericytes to differentiation to microglia with an accompanied inflammatory process.
Just as in AD with an accumulation of Aβ causing pericyte toxicity, a better understanding of the relationship of glioma cell proliferation and potential to cause pericyte activation may help to elucidate the biology of local inflammatory responses, peritumoral edema, and BBB dysfunction. Thus, future studies of malignant glioma should reflect the dynamic role of pericytes within the microenvironment. Investigations within the microenvironment would require highlighting of pericyte activation, loss, and transformation to phagocytic cells—as well as evaluating pericyte insult during times of tumor growth/invasion and high inflammatory states pre and post chemotherapy and radiotherapy.
Pericyte Role in Hypoxia
Although only a modest number of investigations have been performed to examine the impact of pericyte activation/survival at times of hypoxia, these studies have uniformly demonstrated the endurance of pericytes during such a level of neuronal stress. Under hypoxic (0.1%–5% oxygen) conditions, in vitro studies of pericytes demonstrate maintenance of their mitochondrial activity and ability to help maintain tight junction barrier integrity tightly adjoined to endothelial cells.56 Specifically, using an in vitro BBB model of pericyte-endothelial cells co-cultured, pericytes assisted direct influence on the permeability of endothelial cells. These studies examined the permeability under severe hypoxic conditions (1% O2 concentration) for 6 hours and found that permeability was unchanged from baseline in normoxic conditions.57 In contrast, in the absence of pericytes, when endothelial cells alone were exposed to hypoxic conditions, there was increased permeability of Evans blue-albumin and sodium fluorescein. Even longer periods of extreme hypoxia (48 h at 0.1% O2) did not impact either astrocyte or pericyte survival, and BBB integrity was maintained as evidenced by poor permeability of sucrose and dextran. Moreover, studies using transendothelial electrical resistance and F-actin organization demonstrated that pericytes play the largest role in maintaining the integrity of the BBB during hypoxic conditions.58 These studies demonstrate the importance of pericytes in the maintenance of the BBB under chronic oxygen deprivation.59 Yet, in CNS disease states which result in pericyte damage or loss from prolonged hypoxia or hypoperfusion, consequences include diminished vascular stability/BBB integrity with accompanied neurotoxicity/neurodegeneration.
In the condition of AD, several studies demonstrate that early vascular disease causes BBB disruption. This increased BBB permeability leads to hypoxia/hypoperfusion, signaling an influx of plasma-derived neurotoxins, and downregulation of the zinc-dependent metalloprotease (neprilysin), which serves a large role in enzymatically degrading Aβ protein accumulation within the brain.5,48,60 This overall BBB dysfunction generates a buildup of Aβ around neurons and vasculature that aids in neuronal degeneration.61 Additionally, the Aβ deposit on vasculature in preclinical models during hypoxic states results in an abundant loss of pericytes. This pericyte loss causes Aβ clearance to be further diminished while the remaining feeble pericytes undergo degeneration from the neurotoxic Aβ. Overall, the pericyte loss and dysfunction triggered by the hypoxic/hypoperfused state and increased BBB permeability in early AD further accelerates AD-like neurodegeneration.5,49
While many investigations have been performed in AD outlining the pathophysiology from hypoxia to neurodegeneration, further studies are needed to understand how pericytes regulate the BBB in the hypoxic areas of rapidly dividing glioma tissue.62 Specifically, rapid growth of malignant glioma tumors increases demand for cellular machinery at the cost of ATP and oxygen availability. Thus, tumor cells continue to proliferate and invade adjacent tissue in anaerobic conditions, due to the presence of hypoxia-inducible transcription factor, which increases glucose uptake during glycolysis through a hypoxia-induced escape.63 The variable permeability of the BBB within the tumor core may be partially due to the increased expression of NG2-expressing pericytes at areas of high cellular proliferation, in addition to these increased transcription factors.39,54 Tumor hypoxic regions also recruit activated pericytes from distant locations to sites of increased glucose production by proposed regulation of hypoxia-inducible transcription factor.62 At the site of these hypoxic tumor cells, glioma cells proliferate, spawn glioma stem cells, and further evade treatment. Additionally, these tumorigenic glioma stem cells can differentiate into pericytes, which can further support BBB integrity, vessel growth, and treatment resistance.38 Additionally, when stem cell–derived pericytes are diminished, permeability increases and tumor growth is disrupted. In all, these studies underscore the need to better understand pericyte activation, recruitment, and loss in and around hypoxic tissue, in an effort to impact tumor vasculature and improve treatment in hypoxic proliferating tumor areas.
Pericyte Role in Glioblastoma Vasculature
Glioblastoma vasculature is characteristically irregular, with areas of hyperpermeability with associated vasogenic edema often further complicated by necrotic tumoral areas within the core. This abnormal and unbalanced vasculature throughout the tumor lends to the aggressive nature of the rapidly proliferating tumor cells and promotes vasculogenesis and angiogenesis.64 Promotion of this aberrant vasculature is secondary to growth factors and chemokines which help regulate the microvascular proliferation as well as the communication of endothelial cells to pericytes. Previous studies have documented that the hyperplasia of αSMA-expressing pericytes aids in the malformed microvasculature that is characteristic of malignant glioma grades versus nontortuous vasculature of normal brain.65 Additionally, when examining GBM cells’ interaction with pericytes, using co-cultured brain slices prelabeled for pericytes, the target pericyte cortex contained tumor-derived cytoplasm.53 These findings all support the role of co-option of GBM cells requiring assistance of pericytes to proliferate and progress.
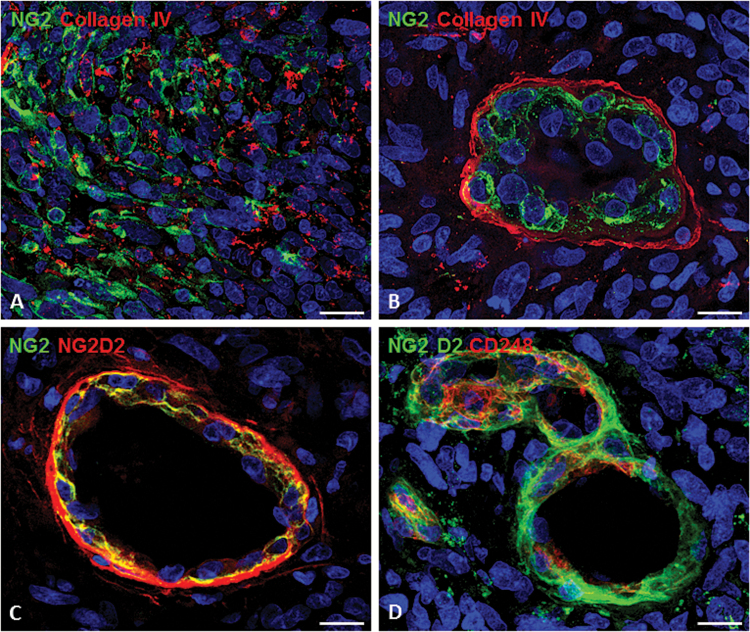
Through crosstalk with endothelial cells, pericytes overexpress the transmembrane receptor, endosialin (CD248, also termed tumor endothelial marker 1), which aids in supporting tumor microvasculature. Yet pericytes have been found to overexpress CD248 only within malignant solid tumors, including high-grade glioma vasculature.38,66 By using a panel of antibodies against tumoral markers of pericytes, including CD248 monoclonal antibodies against the extracellular portion of human NG2/CSPG4, subsets of tumoral pericytes have been revealed together with pericyte-elective NG2/CSPG4 isoforms (ie, not expressed by GBM cells). These CD248 monoclonal antibodies enable the analysis of the pericyte component of the tumoral vessels. The vessels can be characterized by a multilaminar sheet of pericytes, composed of at least 2 phenotypically distinct and spatially separated pericyte populations: the internal (abluminal) and actively dividing versus a more external population (adluminal) surrounded by an additional basal lamina (Fig. 2).67 Specifically within breast tumor vasculature, CD248 overexpression correlates to tumor intravasation leading to distant metastases. Accordingly, a phase I study using a CD248 monoclonal antibody, MORAb-004, in solid tumors, excluding CNS tumors, demonstrated safety and tolerability with minor responses.68 However, no studies have been conducted to evaluate the role of anti-CD248 inhibition of GBM tumor cell proliferation, invasion/associated metastases, and angiogenesis.
Fig. 2.
The pericyte component of the GBM vessels as shown by confocal microscopy and specific tumoral pericyte markers. (A) NG2-reactive tumoral cells in GBM tissue (collagen type IV in red). (B) A tuft-like vessel (garland vessel; collagen type IV in red), whose multilayered pericyte wall is revealed by a pericyte-specific NG2. Double antibodies against different NG2 isoforms (C) as well as NG2 in double labeling with CD248 (endosialin); (D) pericyte subsets. Bars: (A, C), 25 µm; (B, D), 15 µm. Image from Girolamo F et al. PloS ONE. 2013;8(12):e84883; used with permission.
Specifically, endothelial cells of GBM express significantly higher expression of VEGF-A and TGFβ, in comparison to normal brain and low-grade glioma.69 These pro-angiogenic factors play a central role in regulating not only the tumor vascularity, but also the tumor grade and overall prognosis. Pericytes are vital for inducing angiogenesis by increasing VEGF-A production via PDGFRβ signaling.70 In subcutaneously implanted U87 malignant glioma mice, activated pericytes corrected not only the path for invasive endothelial cells but also the ability of pericytes alone to form tubes as an early stage of microvessel development and vessel sprouting.71 Additional studies have detailed the recruitment of these activated pericytes to determine the exact placement of GBM-propagating cells, similar to endothelial cells, which aided in tumor margin expansion.53
Interestingly, when interaction between GBM cells and pericytes is inhibited, tumor growth is impaired and the major role of pericytes changes to phagocytosis. Co-option of pericytes to expand the tumor margin is evident with tumor growth. Once these pericytes are recruited, the GBM cells are able to transfer their “stemness” and alter surrounding vessel morphology by means of cell division cycle 42 (Cdc42)–dependent extensions called flectopodia. They demonstrated that inhibition of these GBM extensions prevented the fusion between pericytes and GBM and thus inhibited tumor progression/angiogenesis. Resultantly, these non–co-opted pericytes are able to transform into hyperactivated macrophages which enable cytotoxic tumor cell death. The conversion of pericytes to microglial cells in rodent brains was first described by Boya in 1976, using electron microscopy in animals with puncture wounds to the cerebral cortex.72 He detailed that pericytes can separate from capillary walls to penetrate into nervous tissue and then transform into voluminous cells with a phagocytic quality very similar to macrophages. Accordingly, while many studies have examined the migration and invasiveness of GBM cells around angiogenesis/vasculogenesis, few studies have evaluated the close switch from pericytes to macrophages within the CNS during times of stress/injury.23,73,74 Thus, further studies are needed to examine the effect of inhibiting GBM-pericyte fusion/crosstalk in vasculogenesis and transformation of pericytes into phagocytes to prevent tumor cell invasion.
Pericytes as Potential Therapeutic Targets
This review outlines the important roles pericytes play in the immune system, inflammation, and hypoxia for a variety of CNS pathologies. However, there is a paucity of data distinguishing the pericyte functionality intratumorally and peritumorally in patients with GBM versus the pericyte’s ability to transform these pro-angiogenic cells into phagocytes which can engulf tumor cells. We propose 3 areas of further research that could assist in further understanding (i) recruitment and activation of pericytes during varied phases of treatment, (ii) the relationship of pericyte role(s) during hypoxic states to vasculogenesis/angiogenesis, and (iii) the efficacy of obstructing crosstalk between pericytes and GBM cells to prevent further growth and invasion (Fig. 3).
Fig. 3.
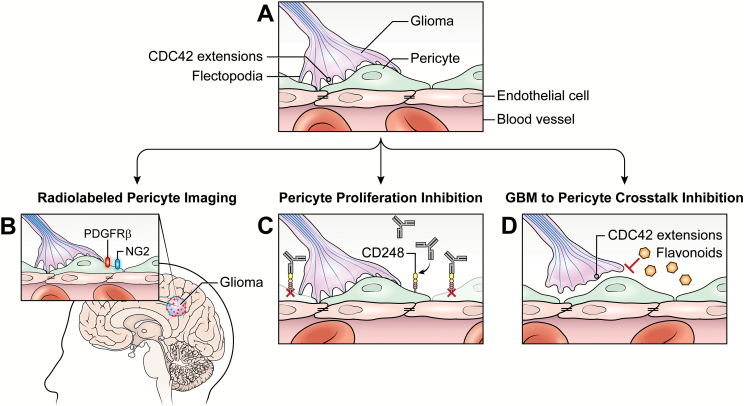
Strategic pericyte targeted therapy for malignant glioma. (A) A simplified glioma pericyte endothelium diagram demonstrating the intimate glioma-to-pericyte interaction while the pericytes envelop tumor endothelium. (B) Radiolabeled NG2 and PDGFRβ pericyte markers would enable tracing of pericyte activation/proliferation during glioma growth, invasion, and recurrence via PET imaging paired with standard of care MRI. (C) Inhibition of CD248-expressing pericytes blocks pericyte proliferation and impacts neovascularization/angiogenesis. (D) Impaired communication between pericytes and glioma cells inhibited by flavonoids blocks Cdc42 flectopodia interaction and tumor cell invasiveness.
Pericyte imaging may provide a non-invasive means to evaluate the function of pericytes at the time of diagnosis, and then after completion of treatment, providing additional insights into mechanisms of treatment response and resistance. To date, preclinical intravital imaging studies have mainly evaluated pericyte expression using mouse-derived glioma models over a short time frame. Yet, we propose clinical studies that are focused on pericyte activation and recruitment during the growth and invasion of malignant gliomas, which may provide important insights into disease biology. Very similar to the preclinical studies that radiolabeled the extracellular domain of PDGFRβ in mice bearing malignant glioma xenografts, a potential clinical trial would investigate the role of pericytes using a radiotracer of CNS PDGFRβ pericytes. By evaluating pericyte radiotracer labeling, real-time imaging of pericyte activation and transformation could be interrogated at the time of diagnosis and then pre and post radiation/chemotherapy. Evaluating either PDGFRβ or NG2 binding in malignant glioma patients, single-photon emission CT or PET imaging would assist in identifying areas of pericyte activation, migration, and proliferation and then provide connections to changes in enhancement with tumor progression and peritumoral edema along with standard brain MRI. This could help investigators focus on pericyte involvement in inflammation, areas of hypoxia, and treatment effect from cytotoxic/immune modulationg agents.75 Use of a radiotracer to correlate the impacts on pericyte changes with tumor growth in patients with GBM may also help discern functional changes in activated pericyte expression and location.
From a therapeutic perspective, pericyte proliferation/activation inhibitors may be synergistic to standard cytotoxic agents alone or in conjunction with immunotherapies in an effort to impact tumor vasculature. Other groups have demonstrated that the CD248 transmembrane glycoprotein is actively expressed at times of hypoxia and angiogenesis on stromal cells and pericytes, detailing the role of CD248-expressing cells in angiogenesis, vascular remodeling, and resultant solid tumor proliferation.76 These studies led to the creation of a monoclonal antibody, MORAb-004, anti-human CD248 antibody Fb5, first tested in lung carcinoma models with high CD248 expression. Use of MORAb-004 resulted in defects in neovascularization characterized by small and shortened microvessels, which in turn significantly impacted tumor growth and metastasis.77 With an attempt to look at the safety and feasibility of the agent, a multi-institutional phase I study using MORAb-004 in patients with solid tumors (excluding patients with CNS malignancies) was completed. The monoclonal antibody was found to be safe and feasible and demonstrated a minor tumor response in patients with hemangiopericytoma, hepatocellular carcinoma, and pancreatic neuroendocrine tumors.68 It is likely that this trial excluded CNS disease patients because the large size of the monoclonal antibody precludes its entry into the CNS, but other agents with smaller molecular weights could be used as inhibitors of CD248. Accordingly, it may be plausible to evaluate the effect of CD248 inhibition by the tyrosine kinase inhibitor imatinib mesylate. Imatinib has been shown to induce defects in sprouting angiogenesis by selective inhibition of PDGF signaling and NG2 expression.78 High NG2 expression of GBM cells and vasculature confers an overall poor survival.53 Thus, imatinib or a similar inhibitor of CD248 and NG2 that targets the role of pericytes in angiogenesis may be a useful adjunctive agent with chemotherapy/immunotherapy to influence the abundant neovascularization/angiogenesis and associated hypoxia that is responsible for GBM proliferation.
Additionally, inhibiting the communication of GBM cells to pericytes, in an effort to inhibit cell migration and accompanied angiogenesis may have a therapeutic benefit. Previous studies described the effectiveness of inhibition of Cdc42-dependent GBM actin extensions, resulting in poor communication with pericytes. This lack of communication led to subsequent transformation of pericytes to macrophage-like cells in animal models. Inhibition of Cdc42 by a flavonoid, luteolin (3′,4′,5,7-tetrahydroxyflavone), significantly impairs malignant glioma cell line migration in in vitro studies.79 Interestingly, the flavones apigenin (4′,5,7,-trihydroxyflavone) and luteolin inhibit not only Cdc42 extensions but also PDGFRβ activity in pulmonary aortic smooth muscle cells, which resulted in markedly reduced migration/invasiveness and VEGF secretion.80 While additional studies are needed to further delineate the efficacy of inhibiting Cdc42-dependent GBM extensions as a potential adjunct to chemotherapy/immunotherapy, future clinical studies that utilize such agents that interfere with the communication of GBM cells and pericytes may impede tumor cell invasiveness with associated vasculogenesis.
This review identifies that the role of pericytes within varied CNS diseases is multifactorial. Pericyte functions in ischemic stroke and AD have been outlined here, but overall, limited studies in malignant glioma models have been performed. Thus, the likely multifaceted role of pericytes in glioblastoma invasion, proliferation, and associated angiogenesis is worth being further investigated. Very few studies have examined the varied tasks of pericytes in GBM, hence additional knowledge of pericyte functionality in such an aggressive tumor could be a game changer in improving therapy delivery, tumor progression, and disease recurrence.
Funding
None.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, NCI.
Conflict of interest statement. None.
References
- 1. Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005;7(4):452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Trost A, Lange S, Schroedl F, et al. Brain and retinal pericytes: origin, function and role. Front Cell Neurosci. 2016;10:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Attwell D, Mishra A, Hall CN, O’Farrell FM, Dalkara T. What is a pericyte? J Cereb Blood Flow Metab. 2016;36(2):451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. ElAli A, Thériault P, Rivest S. The role of pericytes in neurovascular unit remodeling in brain disorders. Int J Mol Sci. 2014;15(4):6453–6474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Winkler EA, Sagare AP, Zlokovic BV. The pericyte: a forgotten cell type with important implications for Alzheimer’s disease? Brain Pathol. 2014;24(4):371–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Krueger M, Bechmann I. CNS pericytes: concepts, misconceptions, and a way out. Glia. 2010;58(1):1–10. [DOI] [PubMed] [Google Scholar]
- 7. Verbeek MM, Otte-Höller I, Wesseling P, Ruiter DJ, de Waal RM. Induction of alpha-smooth muscle actin expression in cultured human brain pericytes by transforming growth factor-beta 1. Am J Pathol. 1994;144(2):372–382. [PMC free article] [PubMed] [Google Scholar]
- 8. Bandopadhyay R, Orte C, Lawrenson JG, Reid AR, De Silva S, Allt G. Contractile proteins in pericytes at the blood-brain and blood-retinal barriers. J Neurocytol. 2001;30(1):35–44. [DOI] [PubMed] [Google Scholar]
- 9. Dimou L, Gallo V. NG2-glia and their functions in the central nervous system. Glia. 2015;63(8):1429–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Virgintino D, Girolamo F, Errede M, et al. An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis. 2007;10(1):35–45. [DOI] [PubMed] [Google Scholar]
- 11. Gaengel K, Genové G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29(5):630–638. [DOI] [PubMed] [Google Scholar]
- 12. Lindblom P, Gerhardt H, Liebner S, et al. Endothelial PDGF-B retention is required for proper investment of pericytes in the microvessel wall. Genes Dev. 2003;17(15):1835–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Abramsson A, Lindblom P, Betsholtz C. Endothelial and nonendothelial sources of PDGF-B regulate pericyte recruitment and influence vascular pattern formation in tumors. J Clin Invest. 2003;112(8):1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hori S, Ohtsuki S, Hosoya K, Nakashima E, Terasaki T. A pericyte-derived angiopoietin-1 multimeric complex induces occludin gene expression in brain capillary endothelial cells through Tie-2 activation in vitro. J Neurochem. 2004;89(2):503–513. [DOI] [PubMed] [Google Scholar]
- 15. Zhang ZG, Zhang L, Croll SD, Chopp M. Angiopoietin-1 reduces cerebral blood vessel leakage and ischemic lesion volume after focal cerebral embolic ischemia in mice. Neuroscience. 2002;113(3):683–687. [DOI] [PubMed] [Google Scholar]
- 16. Persidsky Y, Hill J, Zhang M, et al. Dysfunction of brain pericytes in chronic neuroinflammation. J Cereb Blood Flow Metab. 2016;36(4):794–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rustenhoven J, Aalderink M, Scotter EL, et al. TGF-beta1 regulates human brain pericyte inflammatory processes involved in neurovasculature function. J Neuroinflammation. 2016;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Y, Pan L, Moens CB, Appel B. Notch3 establishes brain vascular integrity by regulating pericyte number. Development. 2014;141(2):307–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dalkara T, Alarcon-Martinez L. Cerebral microvascular pericytes and neurogliovascular signaling in health and disease. Brain Res. 2015;1623:3–17. [DOI] [PubMed] [Google Scholar]
- 20. Dalkara T, Gursoy-Ozdemir Y, Yemisci M. Brain microvascular pericytes in health and disease. Acta Neuropathol. 2011;122(1):1–9. [DOI] [PubMed] [Google Scholar]
- 21. Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci U S A. 2010;107(51):22290–22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guijarro-Muñoz I, Compte M, Álvarez-Cienfuegos A, Álvarez-Vallina L, Sanz L. Lipopolysaccharide activates Toll-like receptor 4 (TLR4)-mediated NF-κB signaling pathway and proinflammatory response in human pericytes. J Biol Chem. 2014;289(4):2457–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thomas WE. Brain macrophages: on the role of pericytes and perivascular cells. Brain Res Brain Res Rev. 1999;31(1):42–57. [DOI] [PubMed] [Google Scholar]
- 24. Balabanov R, Washington R, Wagnerova J, Dore-Duffy P. CNS microvascular pericytes express macrophage-like function, cell surface integrin alpha M, and macrophage marker ED-2. Microvasc Res. 1996;52(2):127–142. [DOI] [PubMed] [Google Scholar]
- 25. Proebstl D, Voisin MB, Woodfin A, et al. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med. 2012;209(6):1219–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang S, Cao C, Chen Z, et al. Pericytes regulate vascular basement membrane remodeling and govern neutrophil extravasation during inflammation. PLoS One. 2012;7(9):e45499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Özen I, Deierborg T, Miharada K, et al. Brain pericytes acquire a microglial phenotype after stroke. Acta Neuropathol. 2014;128(3):381–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Armulik A, Mäe M, Betsholtz C. Pericytes and the blood-brain barrier: recent advances and implications for the delivery of CNS therapy. Ther Deliv. 2011;2(4):419–422. [DOI] [PubMed] [Google Scholar]
- 30. Hurtado-Alvarado G, Cabañas-Morales AM, Gómez-Gónzalez B. Pericytes: brain-immune interface modulators. Front Integr Neurosci. 2014;7:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zenaro E, Piacentino G, Constantin G. The blood-brain barrier in Alzheimer’s disease [published online ahead of print July 15, 2016]. Neurobiol Dis. 2016;pii:S0969–9961(16)30165–6. doi:10.1016/j.nbd.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liang J, Piao Y, Holmes L, et al. Neutrophils promote the malignant glioma phenotype through S100A4. Clin Cancer Res. 2014;20(1):187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bambury RM, Teo MY, Power DG, et al. The association of pre-treatment neutrophil to lymphocyte ratio with overall survival in patients with glioblastoma multiforme. J Neurooncol. 2013;114(1):149–154. [DOI] [PubMed] [Google Scholar]
- 34. Fossati G, Ricevuti G, Edwards SW, Walker C, Dalton A, Rossi ML. Neutrophil infiltration into human gliomas. Acta Neuropathol. 1999;98(4):349–354. [DOI] [PubMed] [Google Scholar]
- 35. Rahbar A, Cederarv M, Wolmer-Solberg N, et al. Enhanced neutrophil activity is associated with shorter time to tumor progression in glioblastoma patients. Oncoimmunology. 2016;5(2):e1075693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Han S, Liu Y, Li Q, Li Z, Hou H, Wu A. Pre-treatment neutrophil-to-lymphocyte ratio is associated with neutrophil and T-cell infiltration and predicts clinical outcome in patients with glioblastoma. BMC Cancer. 2015;15:617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ochs K, Sahm F, Opitz CA, et al. Immature mesenchymal stem cell-like pericytes as mediators of immunosuppression in human malignant glioma. J Neuroimmunol. 2013;265(1-2):106–116. [DOI] [PubMed] [Google Scholar]
- 38. Cheng L, Huang Z, Zhou W, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153(1):139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chekenya M, Enger PØ, Thorsen F, et al. The glial precursor proteoglycan, NG2, is expressed on tumour neovasculature by vascular pericytes in human malignant brain tumours. Neuropathol Appl Neurobiol. 2002;28(5):367–380. [DOI] [PubMed] [Google Scholar]
- 40. Chekenya M, Krakstad C, Svendsen A, et al. The progenitor cell marker NG2/MPG promotes chemoresistance by activation of integrin-dependent PI3K/Akt signaling. Oncogene. 2008;27(39):5182–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Svendsen A, Verhoeff JJ, Immervoll H, et al. Expression of the progenitor marker NG2/CSPG4 predicts poor survival and resistance to ionising radiation in glioblastoma. Acta Neuropathol. 2011;122(4):495–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rustenhoven J, Jansson D, Smyth LC, Dragunow M. Brain pericytes as mediators of neuroinflammation. Trends Pharmacol Sci. 2017;38(3):291–304. [DOI] [PubMed] [Google Scholar]
- 43. Manoonkitiwongsa PS, Jackson-Friedman C, McMillan PJ, Schultz RL, Lyden PD. Angiogenesis after stroke is correlated with increased numbers of macrophages: the clean-up hypothesis. J Cereb Blood Flow Metab. 2001;21(10):1223–1231. [DOI] [PubMed] [Google Scholar]
- 44. Kamouchi M, Ago T, Kuroda J, Kitazono T. The possible roles of brain pericytes in brain ischemia and stroke. Cell Mol Neurobiol. 2012;32(2):159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Renner O, Tsimpas A, Kostin S, et al. Time- and cell type-specific induction of platelet-derived growth factor receptor-beta during cerebral ischemia. Brain Res Mol Brain Res. 2003;113(1-2):44–51. [DOI] [PubMed] [Google Scholar]
- 46. Nakagomi T, Kubo S, Nakano-Doi A, et al. Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells. 2015;33(6):1962–1974. [DOI] [PubMed] [Google Scholar]
- 47. Sakuma R, Kawahara M, Nakano-Doi A, et al. Brain pericytes serve as microglia-generating multipotent vascular stem cells following ischemic stroke. J Neuroinflammation. 2016;13(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14(11):1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sagare AP, Bell RD, Zhao Z, et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun. 2013;4:2932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50. Verbeek MM, de Waal RM, Schipper JJ, Van Nostrand WE. Rapid degeneration of cultured human brain pericytes by amyloid beta protein. J Neurochem. 1997;68(3):1135–1141. [DOI] [PubMed] [Google Scholar]
- 51. Marchesi VT. Alzheimer’s dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: implications for early detection and therapy. FASEB J. 2011;25(1):5–13. [DOI] [PubMed] [Google Scholar]
- 52. Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284(5422):1994–1998. [DOI] [PubMed] [Google Scholar]
- 53. Caspani EM, Crossley PH, Redondo-Garcia C, Martinez S. Glioblastoma: a pathogenic crosstalk between tumor cells and pericytes. PLoS One. 2014;9(7):e101402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lama G, Mangiola A, Proietti G, et al. Progenitor/stem cell markers in brain adjacent to glioblastoma: GD3 ganglioside and NG2 proteoglycan expression. J Neuropathol Exp Neurol. 2016;75(2):134–147. [DOI] [PubMed] [Google Scholar]
- 55. Schober O, Creutzig H, Meyer GJ, et al. 11C-methionine PET, IMP-SPECT, CT and MRI in brain tumors. Rofo. 1985;143(2):133–136. [DOI] [PubMed] [Google Scholar]
- 56. Engelhardt S, Patkar S, Ogunshola OO. Cell-specific blood-brain barrier regulation in health and disease: a focus on hypoxia. Br J Pharmacol. 2014;171(5):1210–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hayashi K, Nakao S, Nakaoke R, Nakagawa S, Kitagawa N, Niwa M. Effects of hypoxia on endothelial/pericytic co-culture model of the blood-brain barrier. Regul Pept. 2004;123(1–3):77–83. [DOI] [PubMed] [Google Scholar]
- 58. Engelhardt S, Huang SF, Patkar S, Gassmann M, Ogunshola OO. Differential responses of blood-brain barrier associated cells to hypoxia and ischemia: a comparative study. Fluids Barriers CNS. 2015;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Al Ahmad A, Gassmann M, Ogunshola OO. Maintaining blood-brain barrier integrity: pericytes perform better than astrocytes during prolonged oxygen deprivation. J Cell Physiol. 2009;218(3):612–622. [DOI] [PubMed] [Google Scholar]
- 60. Iwata N, Tsubuki S, Takaki Y, et al. Identification of the major Abeta1-42-degrading catabolic pathway in brain parenchyma: suppression leads to biochemical and pathological deposition. Nat Med. 2000;6(2):143–150. [DOI] [PubMed] [Google Scholar]
- 61. Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12(12):723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Svensson A, Özen I, Genové G, Paul G, Bengzon J. Endogenous brain pericytes are widely activated and contribute to mouse glioma microvasculature. PLoS One. 2015;10(4):e0123553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yuen CA, Asuthkar S, Guda MR, Tsung AJ, Velpula KK. Cancer stem cell molecular reprogramming of the Warburg effect in glioblastomas: a new target gleaned from an old concept. CNS Oncol. 2016;5(2):101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Charles N, Holland EC. The perivascular niche microenvironment in brain tumor progression. Cell Cycle. 2010;9(15):3012–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sun H, Guo D, Su Y, et al. Hyperplasia of pericytes is one of the main characteristics of microvascular architecture in malignant glioma. PLoS One. 2014;9(12):e114246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Simonavicius N, Robertson D, Bax DA, Jones C, Huijbers IJ, Isacke CM. Endosialin (CD248) is a marker of tumor-associated pericytes in high-grade glioma. Mod Pathol. 2008;21(3):308–315. [DOI] [PubMed] [Google Scholar]
- 67. Girolamo F, Dallatomasina A, Rizzi M, et al. Diversified expression of NG2/CSPG4 isoforms in glioblastoma and human foetal brain identifies pericyte subsets. PLoS One. 2013;8(12):e84883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Diaz LA, Jr, Coughlin CM, Weil SC, et al. A first-in-human phase I study of MORAb-004, a monoclonal antibody to endosialin in patients with advanced solid tumors. Clin Cancer Res. 2015;21(6):1281–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dieterich LC, Mellberg S, Langenkamp E, et al. Transcriptional profiling of human glioblastoma vessels indicates a key role of VEGF-A and TGFβ2 in vascular abnormalization. J Pathol. 2012;228(3):378–390. [DOI] [PubMed] [Google Scholar]
- 70. Raica M, Cimpean AM. Platelet-derived growth factor (PDGF)/PDGF receptors (PDGFR) axis as target for antitumor and antiangiogenic therapy. Pharmaceuticals (Basel). 2010;3(3):572–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ozerdem U, Stallcup WB. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis. 2003;6(3):241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Boya J. An ultrastructural study of the relationship between pericytes and cerebral macrophages. Acta Anat (Basel). 1976;95(4):598–608. [DOI] [PubMed] [Google Scholar]
- 73. Stensaas LJ. Pericytes and perivascular microglial cells in the basal forebrain of the neonatal rabbit. Cell Tissue Res. 1975;158(4):517–541. [DOI] [PubMed] [Google Scholar]
- 74. Rucker HK, Wynder HJ, Thomas WE. Cellular mechanisms of CNS pericytes. Brain Res Bull. 2000;51(5):363–369. [DOI] [PubMed] [Google Scholar]
- 75. Tolmachev V, Varasteh Z, Honarvar H, et al. Imaging of platelet-derived growth factor receptor β expression in glioblastoma xenografts using affibody molecule 111In-DOTA-Z09591. J Nucl Med. 2014;55(2):294–300. [DOI] [PubMed] [Google Scholar]
- 76. Kontsekova S, Polcicova K, Takacova M, Pastorekova S. Endosialin: molecular and functional links to tumor angiogenesis. Neoplasma. 2016;63(2):183–192. [DOI] [PubMed] [Google Scholar]
- 77. Rybinski K, Imtiyaz HZ, Mittica B, et al. Targeting endosialin/CD248 through antibody-mediated internalization results in impaired pericyte maturation and dysfunctional tumor microvasculature. Oncotarget. 2015;6(28):25429–25440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Naylor AJ, McGettrick HM, Maynard WD, et al. A differential role for CD248 (Endosialin) in PDGF-mediated skeletal muscle angiogenesis. PLoS One. 2014;9(9):e107146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cheng WY, Chiao MT, Liang YJ, Yang YC, Shen CC, Yang CY. Luteolin inhibits migration of human glioblastoma U-87 MG and T98G cells through downregulation of Cdc42 expression and PI3K/AKT activity. Mol Biol Rep. 2013;40(9):5315–5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lamy S, Bédard V, Labbé D, et al. The dietary flavones apigenin and luteolin impair smooth muscle cell migration and VEGF expression through inhibition of PDGFR-beta phosphorylation. Cancer Prev Res (Phila). 2008;1(6):452–459. [DOI] [PubMed] [Google Scholar]